Immune System
Christine Ruehl-Fehlert1, George A. Parker2, Susan A. Elmore3* and C. Frieke Kuper4, 1Bayer AG, Wuppertal, Germany, 2Charles River Laboratories, Inc., Hillsborough, NC, United States, 3National Toxicology Program/NIEHS, Research Triangle Park, NC, United States, 4TNO, Zeist, The Netherlands
Abstract
Cells of the immune system are found in every organ, from the classic lymphoid organs to tissues such as liver, mucosae, and omental adipose tissue. Toxicity to the immune system may be from a direct or indirect injury to lymphoid organs. The morphological responses range from lymphocyte depletion to proliferation, and may result in an increased susceptibility to infections and in the development of tumors. Toxicity may also be caused via the generation of a specific immune response against the compound or by deregulation of immunological responses, leading to allergy/hypersensitivity or autoimmune disease. The damage can be found at multiple sites of the body, depending on the specificity of the hypersensitivity or autoimmune response. Special attention is needed to evaluate toxicity to the immune system in sensitive groups; responses may be modified by age, pregnancy, genetic make-up, and compromising conditions.
Keywords
Thymus; spleen; lymph nodes; mucosa-associated lymphoid tissue (MALT); tertiary lymphoid tissues; omental adipose tissue; liver; neuroendocrine network; antigen processing; innate immunity; adaptive immunity; hypersensitivity; allergy; direct toxicity; indirect toxicity; autoimmune disease; host resistance; lymphoma; leukemia; lymphoid depletion; lymphoid proliferation; enhanced histopathology; developmental immunotoxicity testing (DIT); interspecies differences; stress reaction
Introduction
The primary concern of immunotoxicology is to assess the undesired interactions of substances with the immune system. Toxic responses may occur when the immune system is a passive target of chemical insults, leading to altered immune function. This can result in an increased susceptibility to infection or to the development of allergy, autoimmune disease, or neoplasia. Alternatively, toxicity may arise when the immune system responds to the antigenic specificity of a substance, which can lead to substance-specific allergy (hypersensitivity) or autoimmune disease. The immune system may be especially sensitive during immune development, as in the fetus and neonate, but also in adult life during pregnancy, glucocorticoid-related stress, and aging.
To interpret undesired alterations of the immune system, comprehension of the histophysiology of the system is required. Table 12.1 gives an overview on histophysiology and compartments of the thymus, spleen, lymph nodes, mucosa-associated lymphoid tissue (MALT), tertiary lymphoid tissues, and lymphoid cells in omental adipose tissue. In addition, the immune histophysiology of nonlymphoid organs is described, exemplified by the liver. For the blood and bone marrow, the reader is referred to Chapter 13, Hematopoietic System. T- and B-lymphocytes of lymphoid organs reside in different compartments (Table 12.2).
Table 12.1
Compartments, Cells, and Functions of Lymphoid Tissues
| Organ | Function | Organization | Compartments/subcompartments | Main type of resident cells | Main type of mobile cells |
| Thymus | Mechanical stability | Capsule and trabeculae (forming two lobes divided into lobules) | Fibrocytes | ||
| Proliferation and maturation of T-cells | Cortex (containing epithelium-free areas) | TEC | Immature T-cells | ||
| Corticomedullary junction/zone | TEC, IDC | T-cells in transition | |||
| Medulla | TEC (can form Hassall’s bodies); IDC | T-cells (and B-cells) | |||
| Spleen | Mechanical stability | Capsule | Fibrocytes | ||
| Contraction | Trabeculae | Smooth muscle cells | |||
| Blood storage, filtration of blood and hematopoiesis | Red pulp | Sinuses | Endothelium | Lumen: blood components, wall: macrophages | |
| Splenic cords | FRC | Macrophages | |||
| Ellipsoids (nonrodent) | FRC | Macrophages | |||
| Immune responses to blood-borne antigens and generation of antibodies | White pulp (lymphoid tissue) | Follicles | FRC, FDC | B-cells | |
| PALS—inner part | FRC, IDC | T-cells | |||
| PALS—outer part | FRC | B-cells, T-cells | |||
| Marginal zones | FRC | Specialized macrophages (rodent: marginal metallophils), B-cells, T-cells in marginal zone bridging channels | |||
| Lymph nodes | Mechanical stability | Capsule | Fibrocytes | ||
| Filtration of lymph | Vascular spaces | Sinuses | Endothelium | Macrophages | |
| Recirculation of lymphocytes | HEV of interfollicular areas and paracortex, pigs mostly via normal vasculature | Endothelium | Lumen: blood components, wall: migrating lymphocytes | ||
| Immune reactions related to local stimulation of tributary and generation of antibodies | Lymphoid tissue arranged in lobules | Cortex—follicles | FRC, FDC | B-cells | |
| Paracortex/interfollicular cortex | FRC, IDC | T-cells | |||
| Medullary cords | FRC | B-cells, macrophages | |||
| Malt | Immune reactions related to mucosal Antigens | Lymphoid tissue | Follicles | FRC, FDC, | B-cells |
| Interfollicular areas | FRC, IDC | T-cells | |||
| Specialized covering epithelium | M (microfold)-cells | Migrating lymphocytes | |||
| Recirculation of lymphocytes | Vasculature | HEV of interfollicular areas | Endothelium | Lumen: blood components, wall: migrating lymphocytes | |
| Tertiary lymphoid tissue | Response to continued exposure to local antigen | Lymphoid tissue | Follicles | FRC, FDC | B-cells |
| Interfollicular areas | FRC, IDC | T-cells | |||
| Recirculation of lymphocytes | Vasculature | HEV of interfollicular areas | Endothelium | Lumen: Blood components, wall: Migrating lymphocytes | |
| Omental adipose tissue | Restoration of peritoneal tissue integrity, reaction to immunogenic stimuli in the abdominal cavity | Adipose tissue with high content of diverse immune cells | Clusters of immune cells (milky spots) | Fat cells | Mixture of lymphocytes, macrophages, and other immune cells |
| Liver | Immune reactions, phagocytosis, and waste sink for immune cells and molecules, extrathymic T-cell generation, hematopoiesis | Associated with liver sinuses and oriented at lobular organ structure | Sinusoidal endothelial cells, stellate (Ito) cells | Pit (NK) cells, KCs, T-cells, aggregates of mixed immune cells |

FDC, follicular dendritic cell; FRC, fibroblastic reticulum cell; HEV, high endothelial venules; IDC, interdigitating cell (derived from hematopoietic progenitor cells but act as fixed cells in lymphoid organs); TEC, thymic epithelial cell.
Table 12.2
Anatomical Structure and Distribution of T- and B-Cells in Compartments of Lymphoid Organs
| Organ | Structure of lymphoid tissue | T-cell region | B-cell region |
| Thymus | Cortex, medulla | Cortex, medulla | Not present but a specific B-cells population exists in medulla |
| Bone marrow | Not applicable | Not present but T-cells proven | Not present but B-cells proven and lymphoid follicles may develop |
| Spleen | White pulp | PALS | Follicles, marginal zones |
| Lymph nodes | Lymphoid lobules | Paracortex, interfollicular cortex | Follicles, medullary cords |
| MALT | Follicles and interfollicular areas | Interfollicular areas | Follicles |
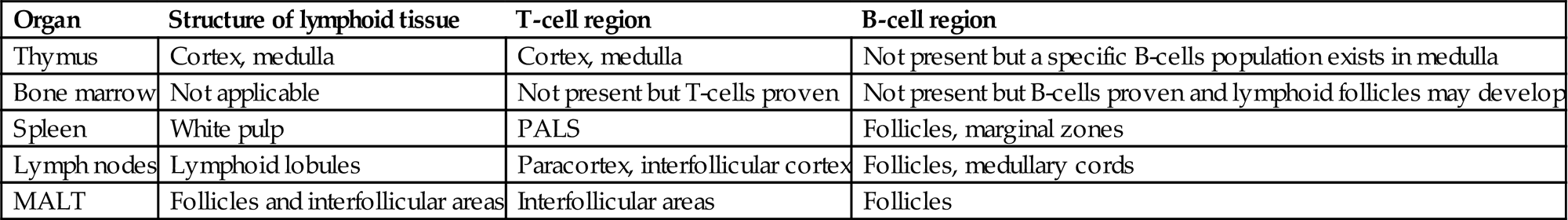
MALT, mucosa-associated lymphoid tissue; PALS, periarteriolar lymphoid sheath.
A schematic presentation of antigen processing and recognition is given in Figure 12.1. The antigen is ingested and degraded by immature antigen-presenting (dendritic) cells, which reside in the targeted tissue (Figure 12.1A,B). The antigen is then carried to local/draining lymph nodes, where the dendritic cells (DCs) present the antigen to T-lymphocytes.
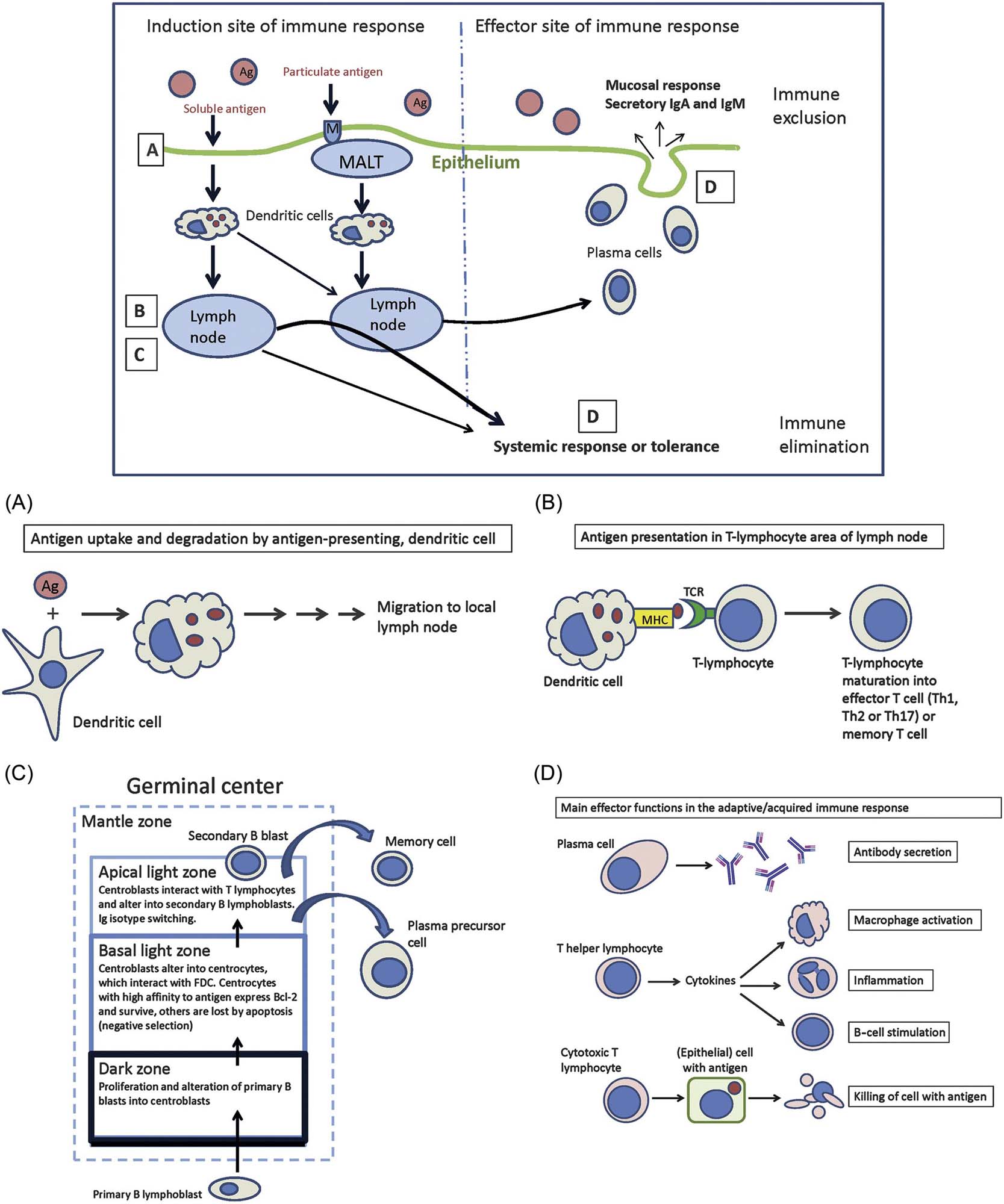
By autophagy, cells can eliminate intracellular pathogens by lysosomal degradation of cell constituents. Autophagy moderates inflammation by contributing to antigen presentation via proinflammatory cytokines or by suppressing inflammasome activation. Autophagy also enhances major histocompatibility complex (MHC) class II antigen presentation and is required for homeostasis of T- and B-cell populations. Defective autophagy can be associated with tissue inflammation and autoimmune disease. Drugs like rapamycin and 3-methyl-adenine target autophagy by influencing central mechanisms. Aberrant or foreign protein structures undergo proteasomal degradation leading to antigenic peptides, which are finally presented together with MHC-I molecules on the cell surface.
After antigen presentation, and activation of T-cells, a choice of effector reactions (response) enables the optimal destruction or inactivation (“immune elimination” and “immune exclusion”) of the antigen/pathogen (Figure 12.1C,D). In the antibody (immunoglobulin)-mediated reaction, activated T-cells induce B-cells to become antibody-producing plasma cells (Figure 12.1C). In the cellular effector reaction, T-cells activate cytotoxic (Tc) cells and other effector T-cells. The immune response needs to be downregulated properly, to prevent unnecessary tissue and organ destruction.
DCs play a prominent role in immune responses as interface between innate and adaptive immunity. Different populations have been recognized in humans and mice regarding their provenience—either from bone marrow (conventional DCs, plasmacytoid DCs, monocyte-derived DCs) or yolk sac (Langerhans cells)—and function. Conventional DCs are involved in the selection of T-cells in secondary lymphoid organs and have a short half-life. Monocyte-derived DCs develop in the context of inflammation. Nonphagocytic, follicular DCs of B-cell areas have to be differentiated from phagocytic DCs. Follicular DCs interact with B-cells by long-term presentation of complement-fixed antigens and production of iccosomes (immune complex-coated bodies).
The immune system aims at a continuous state of homeostatic balance, whereas the introduction of an antigen/pathogen disturbs this balance due to activation of antigen-specific T- and B-lymphocyte clones. The system not only allows the proliferation and amplification of relevant clones to cope with the antigen, but also searches for (and reaches) a state of newly defined homeostasis.
The communication with other homeostatic mechanisms in the body is an important aspect of immunoregulation. Communication and cooperation of the immune system with the neural and endocrine systems is particularly important. Their interaction is mediated by a network of hormones, cytokines, and receptors (Figure 12.2).
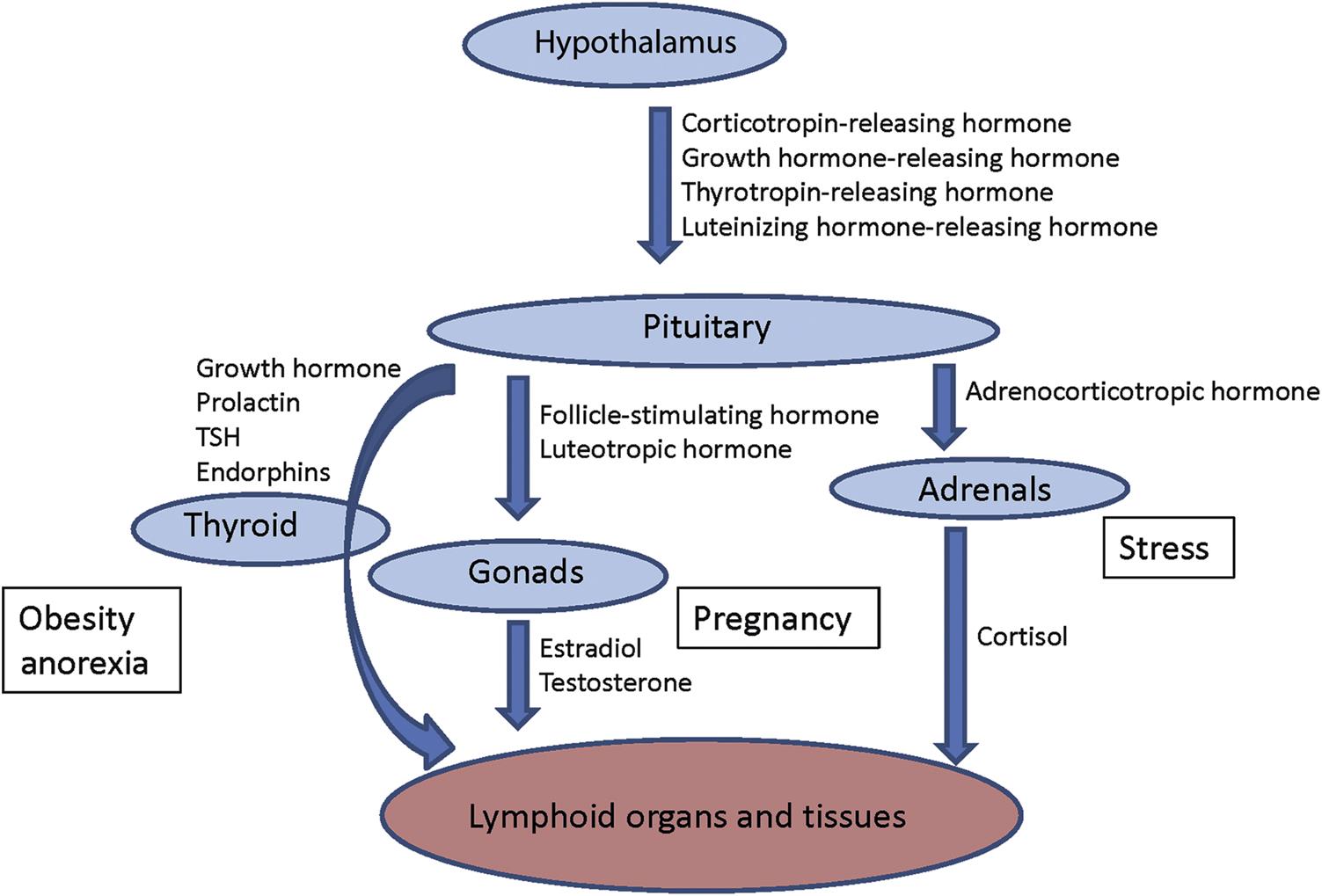
Structure and Physiology of Lymphoid Organs and Tissues
Components of the immune system are present throughout the body (Figure 12.3). The lymphoid organs can be divided into primary or central (lymphocyte generation from progenitor or stem cells), secondary or peripheral (interaction with antigen and activation and development of naive lymphocytes into mature T-lymphocytes and plasma cells) such as lymph nodes (Table 12.3), and tertiary lymphoid organs and tissues. As the immune system is ubiquitously represented throughout the organism, classification of organs and tissues into lymphoid and nonlymphoid organs/tissues is somewhat artificial. The liver plays an important role in fetal lymphopoiesis. Later, it is active in the clearance of immune complexes by Kupffer cells (KCs), removal of immunologic signaling and effector molecules by sinusoidal endothelial cells, removal of activated T-cells, in IgA transport, and in the regulation of immune responses in the intestines (see Chapter 8: Liver and Gall Bladder). Although the liver is often not perceived as an immune organ, it is the organ that is most commonly affected by xenobiotics, and is mentioned here to demonstrate its importance in immune function.

Table 12.3
Areas Drained by Lymph Nodes in Rodents
| Lymph node (group), rata/mouseb | Area drained | Efferent drainage |
| HEAD AND NECK | ||
| Superficial cervical/(accessory) mandibular | Tongue, nasolabial lymphatic plexus, brainb | Posterior cervical lymph nodes |
| Facial/superficial parotid | Head: ventral aspect and sides of neck | Posterior cervical lymph nodes |
| Internal jugular | Pharynx, larynx, proximal part of esophagus | Posterior cervical lymph nodes |
| Posterior cervical/cranial deep cervical | Superficial cervical, facial and internal jugular nodes, pharynx, larynx, proximal part of esophagus, NALTc | Cervical duct |
| UPPER AND LOWER EXTREMITY, TRUNK | ||
| Brachial/proper axillary | Upper extremities, shoulders, chest | Axillary nodes |
| Axillary/accessory axillary | Upper extremities, trunk, brachial nodes | Subclavian duct |
| Inguinal/subiliac | Thigh, flanks, scrotum, lateral tail | Axillary nodes |
| Popliteal/popliteal | Foot, hind leg | Lumbar, inguinal nodes |
| Gluteal/external iliac | Tail | Caudal, lumbar, inguinal and popliteal nodes |
| THORAX | ||
| Parathymic/cranial mediastinal | Peritoneal cavity, liver, pericardium, thymus, lung | Mediastinal duct |
| Posterior mediastinal/tracheobronchial; caudal mediastinal | Thoracic viscera, pleural space, pericardium, thymus | Mediastinal duct |
| Paravertebral | Diaphragm, thoracic viscera | Posterior mediastinal nodes |
| PELVIS AND RETROPERITONEUM | ||
| Caudal | Ventral tail, anus, rectum, gluteal nodes | Iliac nodes |
| Iliac/medial iliac | Pelvic viscera, popliteal, gluteal, caudal nodes | Renal nodes |
| Para-aortic/lateral iliac; lumbar aortic | Pelvic viscera, popliteal, gluteal, caudal nodes | Renal nodes |
| Renal/renal | Kidney, suprarenal, lumbar lymphatics | Renal duct to cistern chyli |
| External lumbar | Fat pad, psoas muscles, pelvic viscera | Lumbar lymphatics |
| ABDOMEN | ||
| Splenic | Splenic capsule and trabeculae | Posterior gastric nodes |
| Posterior gastric/gastric | Distal esophagus, stomach, pancreas, splenic node | Portal nodes |
| Portal | Liver, splenic, posterior, gastric nodes | Portal duct to cistern chyli |
| Superior mesenteric/jejunal | Duodenum, small bowel, cecum, ascending and transverse colon, PP | Superior mesenteric duct to cisterna |
| Inferior mesenteric/colic | Descending and sigmoid colon | Inferior mesenteric duct to cisterna |
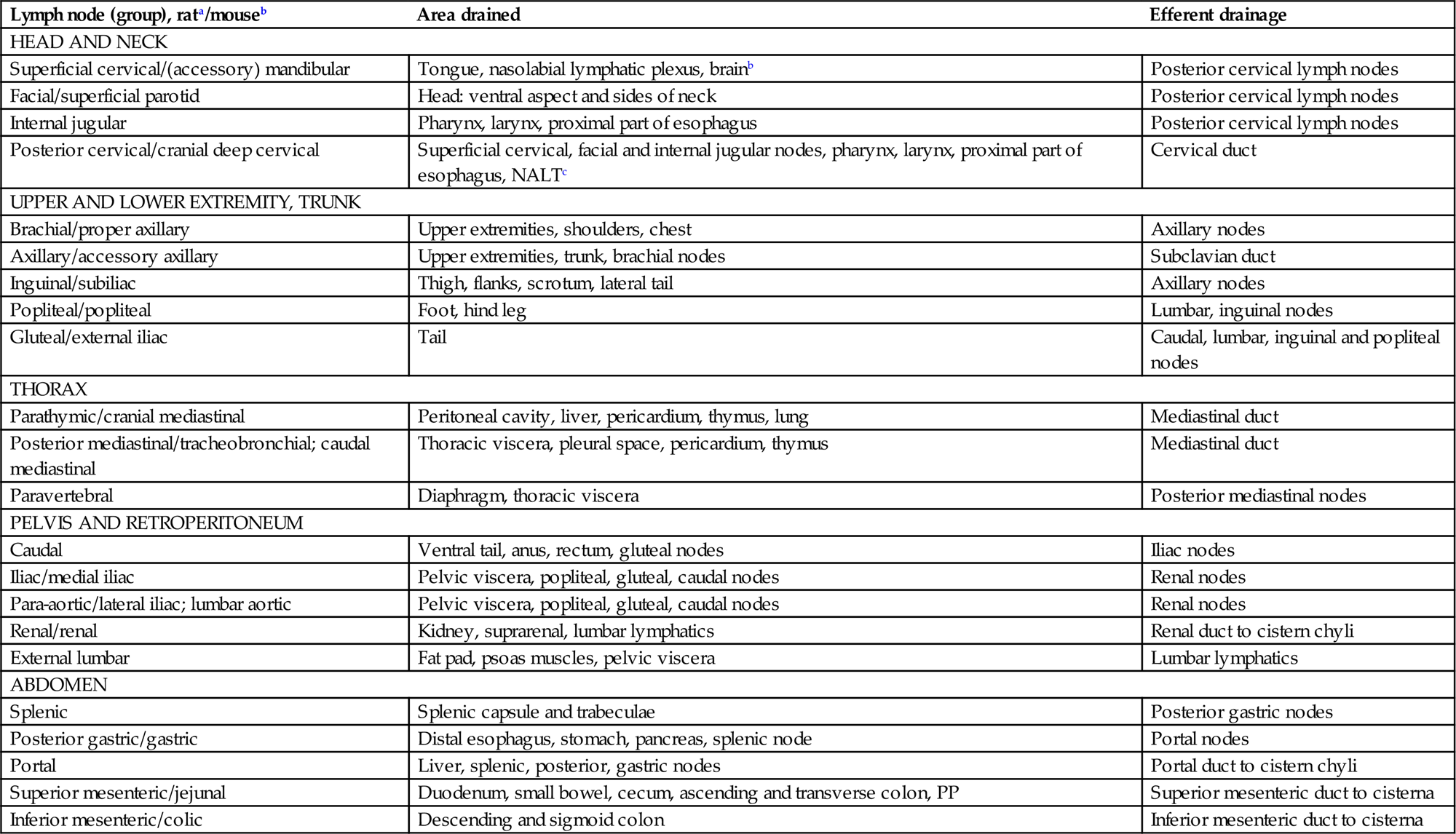
aNomenclature according to Tilney (1971).
bNomenclature according to Van den Broeck et al. (2006).
Thymus
The thymus is a primary lymphoid organ assigned to the development of a repertoire of immunocompetent T-lymphocytes (Figure 12.4). Its organization into the compartments cortex and medulla is highly consistent throughout mammalian species. During embryo-fetal development (Figure 12.5A,B) the thymus quickly matures to its final “adult” morphology with a highly cellular cortex and less densely populated medulla (Figure 12.5C). In particular in young animals the thymic cortex has a high level of cellular proliferation (Figures 12.5C and 12.6) that is most pronounced in the subcortical zone. In active thymuses of certain strains of rats areas free of epithelium with high proliferative activity may be observed as a normal finding in the outer cortex (Figure 12.7A). Physiologically reduced activity of the thymus is encountered in the context of aging (Figure 12.5E); activity is also altered during pregnancy and stress.

T-cell progenitors are formed in the bone marrow, and then migrate to the thymic cortex. Here, they undergo extensive proliferation and selection processes to form single positive CD4+CD8− and CD4−CD8+ T-cells (Figure 12.4). Cells that fail to recognize self-MHC (major histocompatibility genes; in humans designated HLA or human leukocyte antigens) or have an excessively high affinity with self-MHC, and cells that have an affinity for self-antigens, are eliminated by apoptosis. It is estimated that 95%–99% of immature T-lymphocytes undergo apoptosis due to negative selection. The population that survives migrates to the medulla, where maturation continues. Maturation of T-cells involves an orchestrated movement of cells from the entry point of venules at the corticomedullary junction, then outward to the subcapsular region, and subsequently exit from the medulla of the thymus.
While the thymus is considered to be a “T-cell organ,” it is not uncommon to encounter clusters of B-cells or plasma cells in the thymus of various animal species. The B-cell population tends to be more plentiful in aged animals.
Spleen
The structure of the spleen is complex due to its hematopoietic, storage, and defensive functions. The red pulp consists of venous sinuses lined by endothelium and cords containing a mixed population of immune cells including highly active macrophages filtering the blood and removing worn erythrocytes. In dogs and pigs, the red pulp is a major blood reservoir, which is reflected by a thick splenic capsule and strong trabecular muscle. The splenic compartments are outlined in Tables 12.1 and 12.2.
The spleen’s main immunological function is to guard the body’s vascular compartment by generating, among others, T-cell-independent IgM antibody responses to bacterial polysaccharides and by exerting an enormous phagocytic power. Together with the hepatic phagocytic system, splenic macrophages synthesize the majority of components involved in the complement cascade. The marginal zone plays a key role in the antibody responses of the spleen, via trapping of blood-borne antigens/pathogens by marginal zone macrophages and marginal metallophilic macrophages (MOMA-1+ cells; interferon alpha/beta-producing cells). Following antigen trapping, marginal zone B-cells proliferate and IgM antibodies against the pathogen are produced. The marginal zone also retains B-lymphocyte memory. In the fetus, the spleen is an important site of hematopoiesis. This function is maintained in rats and mice throughout adulthood, but in other species it is resumed only in certain diseases. Figure 12.8A shows a periarteriolar lymphoid sheath (PALS) from the rat spleen with adjacent marginal zone embedded in the red pulp. Figure 12.8B–D give an overview of the pig spleen, which has prominent ellipsoids containing macrophages ensheathing vessels of the marginal zones. Ellipsoids are also seen in dogs, pigs, and nonhuman primates, but not in rodents.

Lymph Nodes
Lymph nodes are widely distributed throughout the mammalian body, and are typically located at points where there is confluence of lymphatic vessels from a major anatomic area such as a limb. Patterns of lymphatic drainage in the laboratory rat have been systematically investigated (Table 12.3). Lymph nodes are composed of a variable number of contiguous lymphoid lobules, each of which consists of a cortex, paracortex, and medulla (Tables 12.1 and 12.2; Figures 12.9, 12.10, and 12.11).



Following antigenic stimulation, the primary follicles of the lymph node enlarge to form secondary follicles (Figure 12.12). Each lobule of the lymph node drains a specific region, and therefore different lobules within a lymph node commonly exhibit different levels of reactivity. This is particularly noticeable in mesenteric lymph nodes of rodents, which form a long chain of contiguous lymphoid lobules, often with striking variation in histomorphology. High endothelial venules (HEVs) are postcapillary venules and the site of lymphocyte migration from blood into the lymph node (Figure 12.13). HEVs serve as a storage pool for lymphocytes entering the lymph node, and in pigs HEVs may also be exit sites for lymphocytes. Lymphocytic migration from blood into stimulated lymph nodes can increase lymphocyte exiting 10-fold over that seen with nonstimulated lymph nodes. The increase in lymph node cellularity due to local lymphocytic proliferation and “lymphocyte trapping” from the blood results in the “reactive” lymph nodes that are commonly seen in association with inflammatory lesions.


Lymph nodes have a filtering function, and provide a matrix that allows the immune system to interrogate and, when appropriate, respond to incoming pathogens and antigenic epitopes. Lymph nodes and other secondary lymphoid organs as, for example, the inner PALS of the spleen have a specific population of (myo)fibroblastic reticular cells that limit the circulating lymphocyte population by controlling the production of lymphocyte survival factors such as IL-7. The chain of events that results in lymph node stimulation and expansion of specific T-cell populations is initiated by DCs. Upon stimulation by antigen, the B-lymphocytes undergo clonal expansion, forming a secondary follicle with a germinal center (GC). Selective processes result in apoptosis of a large percentage of the proliferating cells. Surviving antigen-specific cells form memory B-cells and plasma cells. Plasma cell precursors migrate to medullary cords, where they produce (predominantly IgM) antibody that is secreted into the efferent lymph (Figure 12.14). Of note, plasma cells producing high-affinity antibodies elocate to the bone marrow.
Mucosa-Associated Lymphoid Tissue
Organized lymphoid tissues are found in the mucosae of the gastrointestinal, respiratory, and urogenital tracts as well as the conjunctiva; they are referred to as MALT (Figure 12.15). MALT in the oronasal region in man, domestic animals, and minipig is quite extensive (Waldeyer’s ring); the larger lymphoid nodules in the pharyngeal region are called tonsils and represent lympho-epithelial organs. In the mouse and rat it is limited to nasopharynx-associated lymphoid tissue (NALT) (Figure 12.16). In addition, larynx-associated (LALT), bronchus-associated (BALT), and gut-associated (GALT) lymphoid tissues are observed in rat. In other species, like mice and humans, BALT is present when stimulated or induced (iBALT). Peyer’s patches (PP, small intestinal GALT) and tonsils in primates and dogs often feature prominent follicles with GCs. Numerous single T-lymphocytes are disseminated in the covering epithelium (intraepithelial lymphocytes) and lamina propria (lamina propria lymphocytes or LPL) of the gastrointestinal tract; in fact, these lymphocytes form the body’s largest single T-lymphocyte pool.
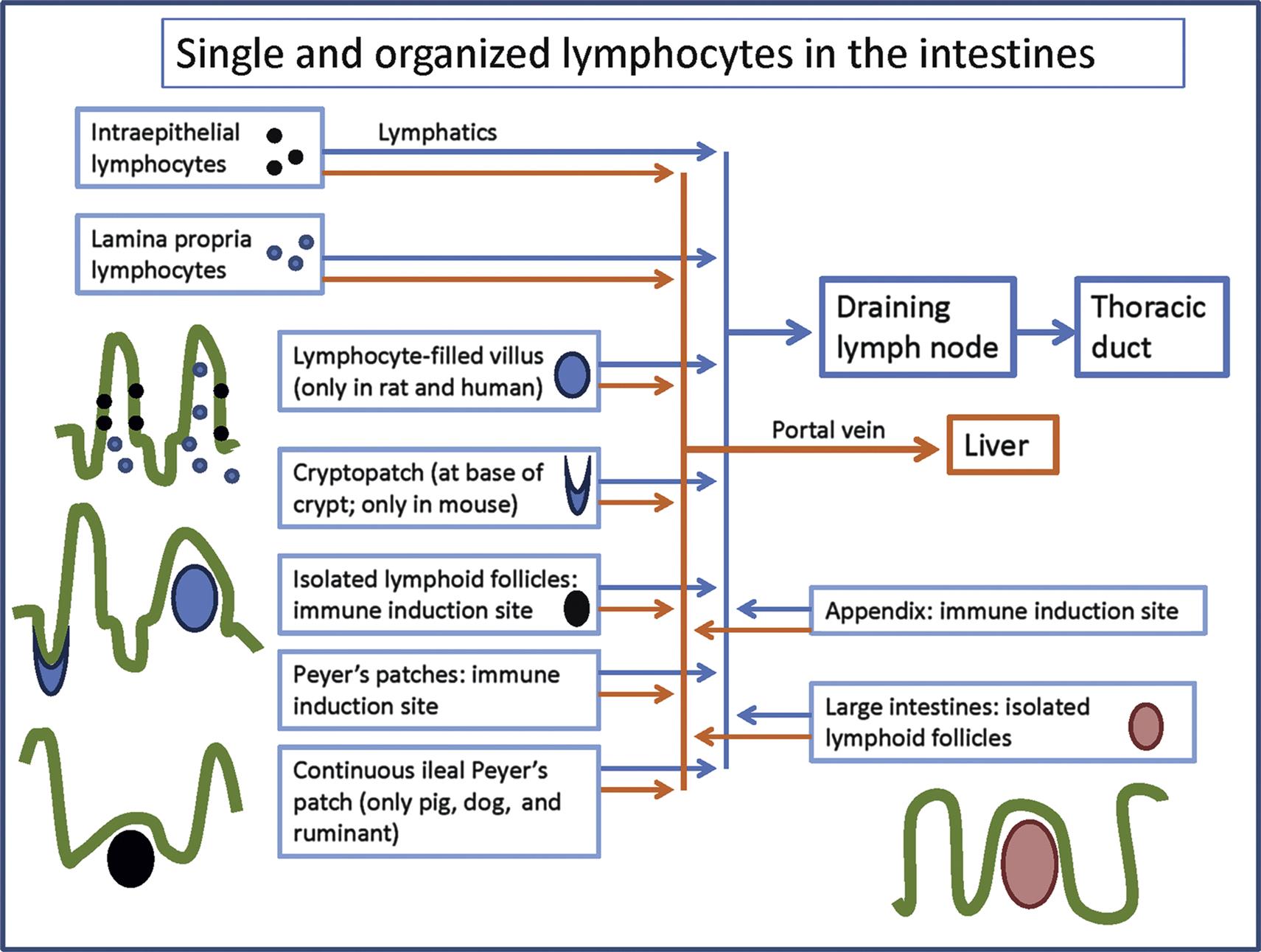

The epithelial surfaces of the body form the major route of entry for antigens. Upon antigen contact an immune response is generated in MALT, which is passed on to the draining lymph nodes, such as the mesenteric lymph nodes of the gastrointestinal tract. Nonspecific lymphocyte-like killer cells in the epithelium have been found to kill pathogens, presumably without prior sensitization. In mice, these cells have been characterized as T-cells with a specific receptor, T cell receptor (TCR)gd, which, in contrast to TCRab Tc cells, kill targets in an MHC nonrestricted manner. They may serve also as initiators of TCRab T-mediated responses.
The immune response in MALT differs from that at other sites of the body, being especially devoted to the generation of an (secretory) IgA-antibody response. The main function of secretory IgA is to prevent the entry of potentially pathogenic substances into the body, a specific antigen exclusion function whereby the epithelium is coated with “antiseptic paint” that prevents binding and uptake.
Tertiary Lymphoid Tissue
Tertiary lymphoid structures are ectopic-organized lymphoid structures that typically consist of follicles often with active GCs and surrounded by T-cell aggregates with HEVs. The microarchitecture of the GCs resembles that of GCs in lymph nodes and spleen (Figures 12.1C and 12.12). Like MALT, tertiary lymphoid structures are not supplied by afferent lymph vessels and are not encapsulated, indicating that they are directly exposed to antigens from the surrounding environment. Tertiary lymphoid tissues can form at any time and lack site specificity (Figure 12.17).

Tertiary lymphoid structures are the result of lymphoid neogenesis in the course of chronic inflammation. Many of the same signaling pathways that are involved in inflammation, for example, the tumor necrosis facto-α family of cytokines and receptors, are involved in the organogenesis of lymph nodes, spleen, and MALT. As with the formation of lymph nodes, formation of tertiary lymphoid tissues involves collaboration between lymphoid tissue initiator cells of bone marrow origin and lymphoid tissue organizer cells that are derived from adipocyte precursors. Once formed, further cytokines and chemokines are produced along with antibodies directed against specific antigens in the inflamed tissue. In some cases the selection process in the GCs is disturbed and negative selection does not function properly (as evidenced by a low number of apoptotic bodies in the basal light zone; Figure 12.1C). This has the potential to lead to the generation of detrimental antibody responses, including unwanted responses against self-antigens. Tertiary lymphoid structures can keep a chronic infection in check, but in autoimmunity they can exacerbate deleterious inflammatory changes.
Omental Adipose Tissue
The omentum connects visceral organs like the spleen and the stomach. It is enriched in macrophages, B and T-lymphocytes, DCs, and natural killer (NK) cells. At certain locations, the leukocytes are organized into clusters (milky spots). Omental milky spots collect fluids, particulates, bacteria, and cells from the peritoneal cavity and can induce an immune response or support immune suppression. The omentum helps to restore tissue integrity in the peritoneum by connecting tissue repair with immunological defense. Upon intraperitoneal immunization, follicles and GCs can be formed.
Liver (See Also Chapter 8: Liver and Gall Bladder)
The liver contains a substantial population of immunologically active cells, including T- and B-lymphocytes, KCs, liver-adapted NK-cells (pit cells), NKT cells, stellate cells, and DCs. Hepatic T-cells are phenotypically different from T-cells in blood, lymph nodes, or spleen. There may be a concentrated population of highly active KCs in the region of the lobule that is the first point of contact for incoming, potentially pathogenic microorganisms. A liver-specific population of NK-cells, also known as pit cells, comprises intrasinusoidal cells that are defined morphologically as large granular lymphocytes (LGLs) and functionally as liver-associated NK-cells. Pit cells are located inside sinusoidal lumina, where they adhere to endothelial cells and KCs. Pit cells and KCs have a symbiotic relationship, with each cell producing cytokines that potentiate the effects of the other cell. Hepatic stellate cells (“Ito cells” or “perisinusoidal fat-storing cells”) were first described by Kupffer in 1876, and have historically been known for their ability to store lipoid materials, including vitamin A. Activated stellate cells produce mononuclear cell and neutrophil chemoattractants, complement protein C4, and a plethora of chemokine and immunomodulatory molecules that participate in both innate and acquired immune functions.
The liver is the primary hematopoietic organ during the fetal stage (Figure 12.18). Hematopoiesis undergoes constant reduction in rats until PND 21 (Figures 12.19 and 12.20) but can be reactivated in case of demand even during adult life. The postnatal liver retains immunologically important functions. Major hepatic contributions to innate and adaptive immunity are summarized in Table 12.4.


Table 12.4
INNATE (NONSPECIFIC) IMMUNITY INVOLVEMENT
Production of acute phase proteins
Nonspecific phagocytosis
Nonspecific cell killing
Disposal of waste molecules of inflammation and nonspecific immunity
Nonspecific immunoregulation
ADAPTIVE (SPECIFIC OR ACQUIRED) IMMUNITY INVOLVEMENT
Deletion of activated T-cells
Induction of tolerance to ingested and self-antigens
Extrathymic proliferation of T-cells
Disposal of waste molecules of specific immunity
Specific immunoregulation
From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.4, p. 1818, with permission.
Developmental and Aging Changes
Developmental Changes
The immune system of rodents develops from a population of pluripotential hematopoietic stem cells (HSCs) generated early in gestation from uncommitted mesenchymal stem cells in the intraembryonic splanchnoplure surrounding the heart. HSCs give rise to all circulating bone marrow cell lineages, including cells of the innate and adaptive immune system. Definitive hemopoietic progenitors have been shown to emerge in the aorta–gonad–mesonephros region before the fetal liver develops. Methodological and interspecies/strain differences, however, lead to a certain variation in the reported time points of pre- and postnatal development of immune organs and functions. Nevertheless, subsequent stages of development of the immune organs follow a chronological sequence, which is highly similar in laboratory species and man (Table 12.5).
Table 12.5
Time Sequence of Immune Organ Development in Rodents and Human
| Development phase | Rat/mouse | Human |
| Critical window of HSC formation | GD 7 to 11 | GW 5 |
| Critical window of cell migration to liver and thymus—liver as predominant hematopoietic organ—precursor T-cells in thymis | GD 10 to 16—start GD 12—start GD 9 to 10 | GW 5 to 7—start GW 5—start GW 7 |
| Sequential development of lymph nodes | GD 9 to 17 | GW 15 to 38 |
| Formation of bone marrow vascular mesenchyme | Start GD 15 to 18 | Start GW 20 |
| Critical window of colonization of thymus and bone marrow | GD 11 to 16 | GW 16 |
| Differentiation of thymic cortical and medullary epithelia | GD 15 | GW 16 |
| IgM-expression by hepatic B-lymphocytes | Start GD 16 to 17 | Start GW 10 to 12 |
| Formation of PP | GD 17 | GW 11 |
| Thymus: morphological distinction of cortex and medulla | GD 20 | GW 16 |
| Demarcation of splenic architecture (red and white pulp) | PND 6 | GW 26 |
| Compartments in lymph nodes discernible | PND 3 | Until GW 38 |
| Development of NALT | PNW 1 to 2 | At birth |
| Critical window of developed immunoreactivity—occurrence of GCs in follicles | Postnatal—PND 21 to 28 | Prenatal—GD 270 |
| Critical window of establishment of immune memory | PND 30 to 60 | PNY 1 to 13 |
GD, gestational day; GW, gestational week; PND, postnatal day; PNW, postnatal week; PNY, postnatal year.
Aging changes
Aging leads to a progressive reduction in weight and size of the thymus (Table 12.9), the delineation between thymus cortex and medulla becomes indistinct, proliferative areas in the outer cortex vanish, and the lymphocyte population is replaced by univacuolar adipocytes. Remnants of thymic tissue can still be detected in old animals. Secondary lymphoid organs do not undergo related involution but a functional shift from naïve to memory T-cells takes place. The altered functional status of secondary lymphoid organs is accompanied by histologic changes that are less spectacular than age-related thymic involution, but nevertheless important in histopathological evaluation of immune system organs. The bone marrow gradually releases increased numbers of myeloid cells at the expense of B-lymphocytes. In mice, it has been shown that total numbers of peripheral B-cells remain constant, related to the increased lifespan of B-cells and the expansion of existent antigen-experienced cell populations, in particular of CD5+ B1 cells. Immunosenescence leads to production of antibodies with low affinity and polyspecificity, as well as to increased autoantibodies, resulting in a proinflammatory status (inflammaging), which contributes to the pathogenesis of various diseases such as atherosclerosis, diabetes, and Alzheimer’s disease. Spontaneous inflammatory lesions of old animals may thus be triggered by age-related changes of B-cell subpopulations. It should also be noted that B-cell lymphoid follicles are frequently encountered in the thymic medulla of healthy beagle dogs and older rats without any evidence of a pathological background. Lymphoid nodules are also seen with some frequency in the bone marrow of cynomolgus monkeys (Macaca fascicularis). Figure 12.5 illustrates the profound age-related changes of rat thymus from fetal life to old age.
Mechanisms and Evaluation of Toxicity
Mechanisms of Toxicity
The primary aim of the immune system is to recognize exogenous agents; therefore, it interacts with many compounds in an antigen-specific fashion. Usually, immune responses to exogenous agents are beneficial to the host. Undesired, exaggerated responses are allergies/hypersensitivities and autoimmune diseases (antigen-specific toxicity). Compounds also can affect the immune system in an antigen-nonspecific fashion either via direct toxicity to components of the immune system, which can lead to malfunctioning of the system, or by indirect toxicity, namely via other organ systems, especially the nervous and endocrine systems. Compound-specific allergy, autoimmunity, and nonspecific toxicity often are set wide apart (Table 12.6). Yet compound-specific allergy and autoimmunity can result in malfunctioning of the immune system, which in turn can lead to exaggerated responses to nonrelated substances (Figure 12.21). Examples of immune-modulating compounds are presented in Table 12.7.
Table 12.6
Deranged Immune Reactions, Immunity, and Toxicity
| Condition | Mechanism | Antigen source | Result |
| Allergy/hypersensitivity | Immunologic | Foreign | Disease |
| Autoimmunity | Immunologic | Self (altered) | Normal: homeostasis and regulation of immunity Deranged: disease |
| Immunity | Immunologic | Foreign | Prophylaxis |
| Nonimmune toxicity | Toxic | Not applicable | Adverse changes/disease |
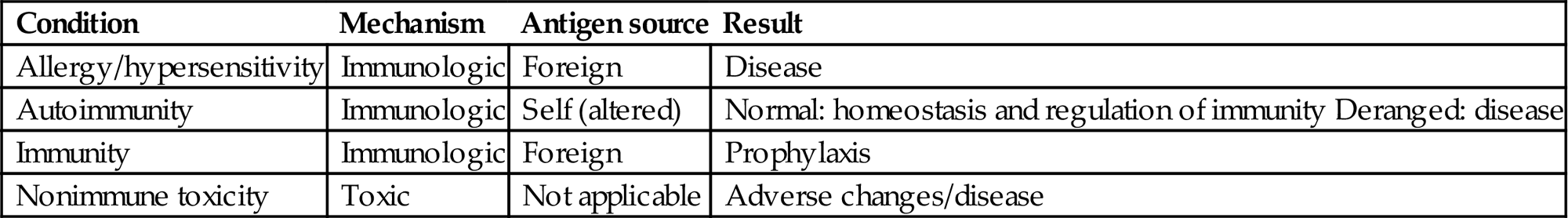
From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.5, p. 1822, with permission.
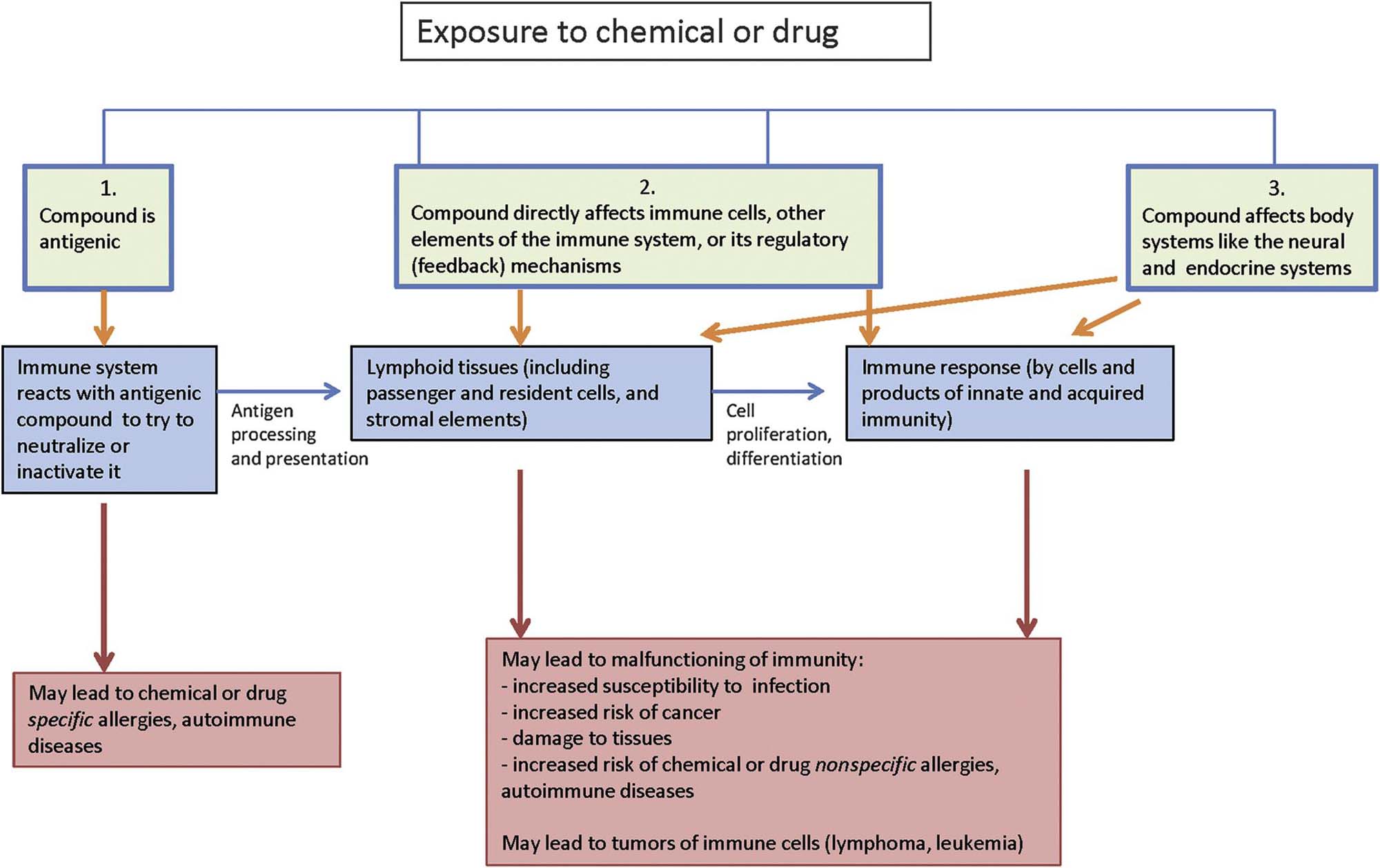
Table 12.7
Examples of Compounds and Agents That Can Affect the Immune System

From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.6, pp. 1824–1825, with permission.
Antigen-Specific Toxicity
Adverse-specific responses of the immune system to compounds are consequences of inadvertent sensitization. The classification system of Gell and Coombs distinguishes four types of hypersensitivity, although in practice the distinction is often less strict than suggested. New classifications are suggested because drug allergies do not easily fit into one of the three categories; these are (1) pseudoallergic reactions, (2) primarily antibody-mediated reactions, and (3) cell-mediated reactions.
Nonantigen-Specific Direct Toxicity
The dynamic and complex nature of the immune system renders it vulnerable to toxic compounds, in particular the “passenger” cells (lymphocytes, macrophages, DCs of bone marrow and thymus). The framework constituents of the lymphoid organs, on the other hand, appear relatively resistant to toxic substances. Immature T-cells (thymocytes) are quite susceptible, due to their fragile composition and to their development, which involves gene amplification, transcription, and translation. The disappearance of lymphoid cells from blood and tissue is often the first sign of this type of toxicity. The stromal framework constituents may respond to this disappearance by degeneration, ending in atrophy and fibrosis. It should be kept in mind that suppression of one immune cell type can result in overall immune enhancement—for instance, when T suppressor cells are affected and immune responses are subsequently not properly checked or regulated. The immune cells can be stimulated as well—for instance, by compounds that act as adjuvants (nonspecific enhancers of the immune system). Adjuvants are often used in vaccines to help generate a protective immune response. The immune system has a great regenerative capacity, and it occurs in a relatively short time: depleted lymphocyte populations can be restored within weeks. Regeneration following destruction of the white blood cell system does not occur when stem cells in the bone marrow are affected—for example, after sublethal irradiation.
Indirect Toxicity
Compounds can have an effect on the immune system via effects on other organ systems—for instance, via induction of acute phase proteins like C-reactive protein and transforming growth factor beta as a result of liver injury. The immune system is especially sensitive to imbalances in the endocrine and neural systems, because these three systems are so closely intertwined with each other. This is exemplified by the profound influence of sex and stress hormones on immune reactivity. Autoimmune diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis of humans are linked to sex steroid hormone balance. They occur more frequently in females, and the course of the disease may be exacerbated when the sex hormone ratio shifts toward a higher estrogen to androgen ratio.
Animal models of autoimmune disease, such as (NZB-NZW)F1, B/W, and MLR/lpr mice, have provided further evidence for this sex (hormone)-dependent intrinsic disturbance of the immune system. This autoimmunity mainly concerns (auto) antibody formation, since cell-mediated effector immune reactions can be depressed in females or in males with enhanced estrogen levels. Estrogen receptors are present in the thymus, both on thymocytes and epithelial cells; thymocytes also have androgen receptors. Glucocorticosteroid hormones have a pivotal position in the homeostasis of the immune system and their increased synthesis often leads to suppression of the immune system with lymphopenia (see “Stress” section, later in this text, for a description of the histology). The toxicologic pathologist should be aware of stress-associated thymic involution in rodents and other species in order to correctly interpret the effects of xenobiotics in short-term high-dosage toxicity studies.
Immune Derangements and Neoplasia
The risk of lymphoma is significantly increased in congenital or acquired immunodeficiency and certain autoimmune diseases, in particular collagen vascular diseases. The relationship with immunodeficiency is based on the “immune surveillance” theory that assumes a continuous guarding function for the immune system against, and elimination of, potentially neoplastic cells in the body. However, the association between neoplasia and immunodeficiency cannot be explained solely by reduced immune surveillance. Some viruses may act as neoplastic agents, and a defective host resistance facilitates the oncogenic potential of viruses. Epstein–Barr virus of humans is a well-known example, producing polyclonal B-lymphocyte activation that shifts from polyclonal into oligoclonal and monoclonal B-cell malignancies. Cytomegalovirus can also manifest oncogenic potential in such situations. Finally, a shared pathway of expression (e.g., DNA repair mechanisms in human ataxia telangiectasia) may underlie both, immunodeficiency and cancer, without necessarily a causative relation between the two phenomena.
Evaluation of Toxicity
Biomarkers
Biomarkers help in the translation of results from experimental animals to humans, and to monitor human exposure and immune reactions to biopharmaceuticals, which have high species specificity. An array of methods to assess immune function is available in humans, but most of these assays pertain to blood analysis (Table 12.7).
Morphologic Evaluation
Conventional histopathology enables evaluation of the effects of xenobiotics on main cell subsets by assessing their distinct cytomorphology or tissue location. In this way, the effects on lymphocytes of T and B lineage, or on components of the supporting stroma, can be investigated. The sensitivity of histopathologic assessment can be increased by combination of immunohistochemistry with quantitative methods such as morphometry, cell counts, and flow cytometry to investigate subpopulations. As histopathological slides represent a static time point, the dynamic events of the immune system should be carefully considered in the assessment of immunotoxicity. For example, histologic structure of lymph nodes is highly dependent on (local) antigenic stimulation. Normally, lymph nodes draining antigen-rich tributaries are in a state of chronic stimulation indicated by prominent secondary follicles with GCs, well-developed paracortex, considerable numbers of macrophages in the sinuses and paracortex, and plasma cells in the medullary cords. Histologic assessment of MALT such as gut-, bronchus-, nose-, larynx-, skin-, vascular-, and eye-associated lymphoid tissue gives information concerning the mucosal, secretory immune system. Splenic histology reflects the systemic immune system, which is directly associated with blood. When evaluating hematoxylin and eosin (H&E)-stained slides, “enhanced histopathology” provides higher accuracy and sensitivity of histopathological diagnostics. The core points of this structured assessment are that the compartments of lymphoid organs should be evaluated individually as they support specific immune functions and that a semiquantitative descriptive (rather than interpretative) terminology should be used (Table 12.8).
Table 12.8
Terminology of Enhanced Histopathology

From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.8, p. 1830, with permission.
Immunotoxic compounds may have an effect on one compartment while leaving other compartments unaffected. This is of interest for the evaluation of the mode of action of a compound because distinct compartments within a lymphoid organ reflect one or more specific functions, and each houses lymphoid and nonlymphoid cells of different lineages and in different ratios. One of the problems in the detection of alterations in cell numbers is discrimination from “normal” morphology, since the range of normal appearance for lymphoid organs may be wide. Once the normal range is established, tissues from treated animals can be compared with those of control animals. This requires that “blind scoring” of tissue sections not be done at the beginning of the morphologic evaluation, although it can be useful at a later stage of the evaluation to confirm subtle changes. Enhanced histopathology may not be helpful in chronic studies due to the variability of normal aging changes that occur in long-term studies. Though procedures such as enhanced immunohistopathology are valuable in accurately defining specific effects on individual immune system organs, the overall interpretation of immunomodulation or immunotoxicity should be based on integration of the observations in the whole animal or, preferably, the group of animals.
Animal Models
Immunotoxicity of a compound is generally assessed in the framework of standard, guideline-driven toxicity studies. In this way, effects on the immune system can be evaluated against effects on other organ systems. Histopathology of lymphoid organs is crucial in these studies, but immune function tests are needed to interpret the consequences of observed histopathological changes on immune function. Special attention should be paid to juvenile immunotoxicity because of proven high sensitivity as well as different modes of reactivity, latency, or longer persistence of effects in the developing immune system. A test strategy for juvenile exposure is implemented in guidelines for reproductive and developmental toxicity and called developmental immunotoxicity testing (DIT). Within the critical developmental windows gestation, postnatal development, and weaning, there are specific critical windows of immune system development (Table 12.5). Their sequence shows similarities across mammalian species but may vary with respect to the time point in intrauterine or postnatal life. DIT should address all windows of immune development. Principal transferability of DIT to human has been shown for compounds such as methylmercury, ethanol, arsenic, or polychlorinated biphenyls (PCB). Besides for chemical entities in the environment, DIT is used in investigational drug development, depending on the causes of concern, or in pediatric drug development. When using mouse models, a completely anatomically intact morphology can be found on PND42 with maturation of T-cell compartments prior to B-cell compartments. Importantly, histological signs of maturity have to be distinguished from functional maturity.
Most immunotoxicity studies are performed in rodents, usually the mouse or the rat. Other species, like monkeys, minipigs, and fish, are used less often, but may be valuable or even better alternatives in some cases. Specialized studies looking at a specific arm of the immune system have used spontaneously occurring mutants or artificially constructed laboratory animals such as the congenitally athymic (nude) rodents. In athymic animals, lacking production of T-lymphocytes, T-cell compartments are devoid of lymphocytes. In addition, single-gene animal models of immunodeficiency are now available to extend such studies for the evaluation of effects on specific arms of the immune system. An interesting model of immunodeficiency in this respect is the mouse with the severe combined immunodeficiency (SCID) mutation, which results in severe combined immunodeficiency. The lack of immunocompetent T and B-cells permits engraftment of tissues from other species without being rejected. This extends also to cells of the human immune system allowing the investigation of human immune reactions specifically in a model organism without functional T, B, and NK-cells (SCID-hu mouse).
Genetically modified animals are another generation of laboratory animal constructs. Examples are mice expressing frequent Caucasian HLA-alleles. Histopathological evaluation of toxic effects in lymphoid organs of these animal models requires a thorough knowledge of their histophysiology. A major challenge is the safety and immunotoxicity testing of monoclonal antibodies (mAbs). The primary toxicity of mAbs lies in their ability to block or enhance the activities of the target molecule on the target cells, or target molecules on nontarget cells or crosslinking with nontarget molecules, which may lead to cytokine/eicosanoid “storms,” immune suppression, and autoimmune diseases. Unfortunately, these effects are often highly species-specific, and animal models may either exaggerate (reactions against foreign, namely human, proteins; induction of neutralizing antibodies) or severely underestimate the effects in humans. Formation of neutralizing antibodies, though not per se a toxic effect, may lead to sights of immune activation including GC formation in follicles, lymphocyte proliferation or increased compartment sizes. Routine toxicity studies are thus often inadequate to detect the side effects of MAbs and often a valid second species normally required in toxicological investigations may not be available. Main reasons are absence of the intended human target in animal species, neutralizing antidrug antibodies directed to foreign protein structures and inherent differences between the immune systems of humans and selected test species. An example of the complexity of changes in the immune system that can be induced is natalizumab, a humanized mAb against human α integrin. In monkeys, due to its intended pharmacological mechanism in humans, trafficking of leukocytes was likewise impeded in this closely related species. In addition, immunogenicity leads to individually differing amounts of neutralizing antibodies associated with immune stimulation, and variable lowering of serum concentrations of the test article.
Typically, tissue cross-reactivity studies are needed to identify target structures in animal tissues in comparison to human. In the conduct of studies, monitoring of antidrug antibody formation is required and high levels may cause an increase of dose or even termination of the study. In general toxicity studies, enhanced histopathology is recommended. In the case of positive results, immune phenotyping is recommended for further characterization of the effect. In long-term studies in nonhuman primates, activation of endogenous viruses such as lymphocryptovirus, cytomegalovirus, Epstein–Barr virus, and polyomavirus may occur. Virus activation can be monitored serologically and may eventually lead to lymphoma.
Embryo-fetal and peri-/postnatal development toxicity studies are required for immunomodulatory MAbs for treatment of women with child-bearing potential and include investigation of the fetal immune system. Positive results have been reported for an antiCD11a surrogate antibody of efalizumab, which caused prolonged immune function effects in the treated offspring of CD-1-mice and reduced CD11a-expression. Also Rituximab, an anti-CD20 antibody, showed prolonged B-cell depletion in the offspring of treated cynomolgus monkeys.
With nanoparticles immune compatibility is a main issue and often the immune system is affected at lower doses than other target organs. Small particle sizes seem to be particularly prone to elicit immune reactions. As there is only partial correlation of in vitro and in vivo studies, in vivo investigations including histopathology are indispensable and contribute to the investigation of organ distribution, clearance, disposition, or degradation of nanoparticles. As with other classes of potentially immunomodulating entities, a combination of functional (e.g., macrophage function tests) and structural investigations is needed. An example of immunotoxicity is the intravenous administration of silver nanoparticles in rats in a 28-day repeat-dose study with accumulation of particles in liver, spleen, and lymph nodes associated with a suppression of splenic NK-cell activity. In children exposure to diesel exhaust particles has been associated with pronounced allergic airway reaction.
Response to Injury
The immune system represents multiple organs but one system. Hematological investigations, weighing of selected lymphoid organs, histopathology of the immune organs, and bone marrow cytology belong to the core investigations when screening for immunotoxicity in standard repeat-dose studies. The focus of these investigations is on immunosuppression, although signs of immunostimulation can be detected as well (see also “Autoimmune Diseases and Hypersensitivity Reactions” section).
Nonneoplastic Changes
Lymphoid Organ Weight and Gross Pathology
Assessment of lymphoid organ weight and gross changes at necropsy give a first indication of immune effects although there may be influences by a variety of unspecific factors including feeding status, aging, and stress phenomena near the maximum tolerated dose (Table 12.9). These have to be differentiated from immunotoxicity. Figure 12.22A–D shows normal lymph node morphology and examples of alterations.
Table 12.9
Effects of Aging, Feed and Stress on Organ Weights, Immune Functions, and Histology of Lymphoid Organs
| Species | Organ weights | Immune cells/functions |
| AGING | ||
| Rat | Spleen ↑, thymus ↓ | Proliferative response to mitogens ↓, lymphokine production ↓ |
| Mouse | Spleen ↑, thymus ↓ | B-cells: proliferative response to mitogens ↓, affinity response ↓, monoclonal gammopathies ↑, autoantibodies ↑T-cells: age-related shift of subsets depending on strain, bone marrow production of pro-T-lymphocytes ↓NK cell function ↓ |
| Hypocaloric status/feed restriction | ||
| Rat | Spleen ↓ | Proliferative response to mitogens ↑, IL-2 receptors on splenic T-lymphocytes ↓, white blood cells/granulocytes ↓ |
| Mouse | Spleen ↓ | NK cell function ↑, B-cell subpopulations ↑, T-precursor cells ↑ |
| STRESS | ||
| Rat | Spleen and thymus (most sensitive parameter) ↓ (along with deteriorated condition or reduced body weight gain) | Granulocytes ↑ |
| Mouse | Spleen and thymus↓ (along with deteriorated condition or reduced body weight gain) |
Granulocytes ↑, lymphocytes (T:CD4+ and CD8+) ↓, B-cells expressing MHC class II protein (most sensitive parameter) ↓, spleen cell number ↓ |

From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.9, p. 1835, with permission.

Stress
Under physiological conditions, glucocorticoids support thymic homeostasis. Death by neglect of CD4+CD8+ thymocytes is mediated by glucocorticoids (Figure 12.4). Hence, in adrenalectomized mice increased numbers of CD4+CD8+ and CD4+CD8− thymocytes could be found in the thymus. However, the mechanism of glucocorticoids’ role in thymocyte apoptosis is still not clarified. Investigations in mice with glucocorticoid receptor deficiency did not show changes in thymic lymphoid cellularity. Any stress reaction leading to an increase of corticosterone (in rodents, or closely related glucocorticosteroids in other species) causes increased apoptosis of CD4+CD8+ thymocytes; these particular thymocytes are most susceptible to apoptosis due to abundant expression of glucocorticoid receptors. The acute stress response is very fast. Within a few days, histological cortical changes in thymus progress from increased apoptosis of cortical thymocytes with increased amounts of tingible body macrophages (Figure 12.23A, minipig) to lymphocyte depletion (Table 12.9), which can be such that a reversed pattern of lymphocyte density is observed in the medulla (Figure 12.23B, minipig). Under experimental conditions such as administration of dexamethasone to rats or mice, significant apoptosis of cortical lymphocytes is underway in the thymus at 6 hours postdosing, and by 24 hours postdosing the thymic cortex is largely depleted of lymphocytes. B-cells are also influenced by glucocorticoids and show reduced expression of MHC class II. In 28-day studies in mice, investigations of habituation to different stressors have shown that tolerance to stress depends on the nature of the stressor. The magnitude of the response after habituation correlates well with respective corticosterone levels. In moribund animals, the splenic red and white pulp can become severely depleted (Figure 12.24A,B). Indications of stress imply, besides decreased thymic weight and decreased lymphoid cellularity, increased adrenal weight, increased glucocorticoid levels, and adrenocortical hypertrophy.

Histopathology
Thymus
The thymus is often the first organ to be affected by immune-modulating compounds (Table 12.7; Figure 12.25A–C). Sensitivity of cortical thymocytes to immune-modulating compounds differs between the stages of T-lymphocyte development in the thymus. Immature thymocytes of the thymic cortex are very susceptible to toxic injury and prone to apoptotic cell death, which can be distinguished from necrosis by presence of apoptotic bodies and immunohistochemically by cleaved caspase 3-positivity or TdT-mediated dUTP-Biotin nick end labeling (TUNEL) staining. With necrosis, contiguous cells are often affected; karyolysis, pyknosis, and karyorhexis, cell swelling with disruption of the cell membrane, and release of cytoplasm into the surrounding tissue are observed. Apoptosis and necrosis can occur simultaneously when apoptosis mediators are depleted.

Signaling pathways leading to chemically induced thymocyte apoptosis often involve the FAS/FAS ligand. Aromatic hydrocarbons such as dioxins are ligands of AhR. They cause apoptosis of thymocytes and affect all subsets, probably by activation of the FAS/FAS ligand system. TCDD toxicity of the thymus of mice showed apoptosis only up to 12 hours after treatment. Thymic atrophy started at 72 hours after treatment. This indicates that investigations of early time points after exposure have to be taken into account since transient apoptosis may be missed due to rapid clearance of apoptotic cells by thymic macrophages. Cytostatic agents such as azathioprine and 5-fluorouracil as well as irradiation affect DNA synthesis and hence diminish cell proliferation in the outer cortex. In such cases, reduced cellularity may be not accompanied by a clear increase of tingible body macrophages. Administration of xenoestrogens, such as diethylstilbestrol, genistein, or methoxychlor, caused lymphocyte depletion of the thymic cortex. An arrest early in thymocyte development was observed at the stage of CD4−CD8−CD3−CD44+CD25−, which is mediated by estrogen receptors α or β, both present in the thymus. Thus, estrogen antagonists abolished changes mediated via estrogen receptors. Some organotin compounds caused thymic atrophy at doses, which did not lead to overt toxicity in other organs. Perfluorated compounds such as perfluorooctane sulfonate exerted immunomodulating effects as ligands of peroxisome proliferator-activated receptor (PPAR)-α. As a consequence, thymic and splenic atrophy have been observed. The cellular decreases in the thymic cortex affected, in particular, CD4+CD8+ thymocytes. Ultrastructurally, lipofuscin granules were observed in thymic lymphocytes. Structural changes similar to aging have been induced in the thymus and less obviously in the spleen of mice treated with D-galactose, a compound known to accelerate the aging process by formation of advanced glycation end-products. Cytotoxicity to cells in the bone marrow may reduce the flow of progenitor cells from the bone marrow into the thymus. The immune-modulating sphingosine 1-phosphate agonist FTY720 reduced the entrance of thymic progenitor cells and led to reduced numbers of early double negative thymocytes in mice after short-term treatment. The cross-talk between epithelial and lymphoid cells in the thymus may lead to changes in epithelial cells when primarily lymphoid cells are affected and vice versa. Staining for the different cytokeratins allows the identification of medullary and cortical thymic epithelial cell (TEC) subpopulations and Hassall’s bodies (rodents: CK5 and CK14 in medullary TEC, CK8 in cortical, and medullary TEC including Hassall’s bodies). With flow cytometry, or with electron microscopy, TECs were found to be reduced in mice treated with different immunosuppressants, including dexamethasone, cyclophosphamide, and cyclosporine A. The depleted TECs included those producing the autoimmune regulator, AIRE. The depletion of AIRE-producing cells compromises immunological self-tolerance. The changes were reversible within a few weeks.
Induced cortical atrophy may be accompanied by a high cellularity of the thymic medulla, leading to an “inverse” appearance (Figure 12.25B), where the cortex has fewer lymphocytes than the medulla. When assessing medullary cellularity, however, one must consider whether the observed increases are absolute—due, for example, to increased transition of T-lymphocytes into the medulla—or to decreased emigration of thymocytes from the thymus because of arrest of T-cells in the thymic medulla. Increases in medullary cellularity can be relative as well, where absolute medullary cellularity remains constant but cortical cellularity is decreased. An increased ratio of cortex–medulla lymphocytes seen after treatment with cyclosporine in rats is a rare finding (Figure 12.5C), since generally cortical thymocytes are most susceptible to induced apoptosis. Effects on the thymus such as this can lead to reduced lymphocyte emigration into the periphery, whereupon T lymphocyte-dependent areas in peripheral lymphoid tissues are reduced in cellularity and size. Immune-modulating compounds such as cyclosporine, cyclophosphamide, corticosteroids, and dioxins influence regulatory T-cells either in vivo or in vitro. Unwanted effects are difficult to predict in preclinical studies, and even if manipulation of function of regulatory T-cells is the therapeutic goal, investigation in preclinical studies is challenging. If physiological tolerance is disrupted, for example, inflammation in response to normally tolerated microflora of the gastrointestinal tract will result, and changes in the thymus in response to this can be seen by the pathologist. Xenobiotic-related alterations in the intestinal microbiome may have far-reaching effects such as the known interactions between the intestinal microbiome, cytokine production, and development of Candida albicans infections in the oral cavity.
Peripheral Lymphoid Organs: Spleen and Lymph Nodes
Reduced generation, maturation, and/or emigration of thymocytes to the periphery subsequently lead to depletion of T-cell–dependent areas of peripheral lymphoid organs. The spleen has the highest frequency of lymphocyte recirculation; therefore, reduced lymphocyte cellularity secondary to reduced thymic lymphocyte output is commonly seen in splenic white pulp. In lymph nodes, which have generally lower rates of lymphocyte recirculation, atrophy/reduced cellularity of T-cell regions can be less pronounced or even absent.
Compounds associated with suppression of T-lymphocytes can cause, as a secondary effect, morphological changes in B-cell areas as well. Short-term treatments of organotin compounds in mice showed marked decreases of peripheral lymphocytes within a few hours after injection. Besides a decrease in peripheral T-lymphocytes, a marked decrease in B-lymphocytes has also been observed. Histological findings related to immunosuppression are decreased lymphoid cellularity of splenic white pulp follicles and marginal zones, lymph node cortex and medullary cords, and follicles of PP. Often the development of secondary follicles with active GCs from primary follicles is decreased, although at very low doses GC development can be increased. This may be due to an early effect on T suppressor cells, which leads to an overall stimulation of the immune response. As an example of B-cell responses to injury, cannabinoids have an immunosuppressive potential mediated by interaction with cannabinoid 2 (CB2) receptors found on various types of immune cells, including B-lymphocytes. CB2 agonists and antagonists influence the retention of immature B-cells in bone marrow sinuses. Thus, CB2 antagonism with SR144528 reduced the number of B-lymphocytes in bone marrow sinuses and splenic marginal zones. A specific effect on the shuttling of marginal zone B-cells in the spleen has been described for FTY720, an immunosuppressing sphingosine 1-phosphate receptor agonist which prevents egress of lymphocytes from lymphoid organs, and of plasma cells from secondary lymphoid organs, to blood and bone marrow. In the spleen, a displacement of marginal zone lymphocytes to splenic follicles was observed. A similar displacement of marginal zone lymphocytes was reported to occur after LPS treatment. Marginal zone B-cells represent a specific subset, which produces high levels of soluble IgM and expresses the complement receptors CD21 and CD35. Antigens and chemically inert substances, which enter the body by chance or are administered on purpose, can lead to increased cellularity of peripheral lymphoid organs (Figure 12.26A,B). Substances migrate with the lymph with different velocities depending on their size. For example, in lymph nodes, unbound small molecules reach the tributary lymph node within a few minutes, but antigens transported by DCs need several hours to travel the same distance. In the subcapsular sinus, specialized macrophages are the first immune cells to encounter unbound antigenic material. They are located in the sinus wall, and their cytoplasm contacts the subcapsular sinus as well as the underlying cortical B-cell region. Capture of antigens is followed not by degradation but by transport along the cell body into the B-cell area. Small antigens, which are not taken up by macrophages, enter the lymphoid tissue through so-called conduits, tracts of collagenous fibers ensheathed by projections of stromal cells. Circulating antigen evokes lymphocyte trapping, which is increased recruitment of lymphocytes through HEVs combined with reduced release of lymphocytes through efferent lymphatics. This leads to gross enlargement and weight increase of lymph nodes.

Microscopically, prominent HEVs with increased transmigration of lymphocytes as well as increased cellularity of lymph node compartments are visible. Within a few days, the immune response to T-cell–dependent antigens causes formation of GCs in follicles. As a consequence of stimulation, an increase in plasma cells is observed in the medulla of lymph nodes. Immunostimulatory effects can occur even after exposure to compounds without obvious immunogenicity, but which nevertheless cause malfunction of the immune system. Vanadium pentoxide inhalation over 12 weeks in mice caused increased spleen weights and GCs, which could become very large and not clearly delimited. Antigen challenge showed that high-affinity antibodies were diminished in treated mice when compared to control. Oncostatin M (OM), a member of the IL6 family, caused thymic atrophy and had a paracrine effect as well. It supported T-cell maturation from hematopoietic progenitor cells to mature T-cells in lymph nodes, which then functioned as primary lymphoid organs. Both effects of OM—thymic atrophy and enhanced T-cell maturation in the lymph nodes—have been detected in OM-transgenic mice. The extrathymic T-cells in these mice were, however, functionally different from thymic T-cells because of higher responsiveness to antigen stimulation and increased susceptibility to apoptosis. Histopathologically, the lymph nodes of OM-transgenic mice showed an increased number of HEV. This angiogenic effect seemed to be necessary for extrathymic T-cell development under OM influence, and was induced by cyclooxygenase-2. Increased weight and lymphoid cellularity of lymphoid organs mediated by increased macrophage activity were seen after treatment with hexachlorobenzene (HCB) in rats. The effect seemed to be mediated by increased recirculation of lymphocytes via HEVs (Figure 12.27) to peripheral lymphoid organs. Functional tests revealed no clear evidence of immunostimulation in this investigation in Wistar rats, whereas in Brown Norway rats, T-cell activation was observed. HCB toxicity demonstrates that effects on cells of the innate immune response have to be taken into account when effects on lymphocytes are observed.
Peripheral Lymphoid Organs: Malt
Reports about chemically induced changes of the immune system of the mucosae with its MALT are relatively scarce. This is remarkable, since it houses the majority of the body’s immune cells and is particularly complex with MALT and large pools of diffuse, single lymphoid cells. Moreover, the mucosae (together with the skin) are the main contact sites of the body with the environment. The cause of this discrepancy is likely the predominance of tolerance induction by MALT; however, MALT and the single intraepithelial and LPL are also more difficult to examine critically. Even inherited immunodeficiency related to IgA secretory function of PP as observed in the wasted mutant mouse do not cause morphological or morphometric differences when compared to wild-type controls. The different MALT tissues share structural and functional features, but differences in cellular composition regarding the T-cell/B-cell ratio and CD4+/CD8+ T-cell ratio are known to exist; these are as yet relatively undefined. They are thought to reflect adaptation to the anatomic location. For example, in the intestinal PP (part of the GALT), M cells continuously perform transcytosis of bacteria, particulate matter, and macromolecules, which they deliver to macrophages of the dome area. Pathogens can lead to inflammation and macrophage aggregates in the PP. Although pathogenic bacteria are uncommon in toxicity studies, their potentially confounding effects have to be taken into account when assessing the effects of potent immunosuppressive compounds. In immunosuppression, there could even be pathogenicity of normal mucosal microbiota.
The immune response of MALT, and in particular GALT, is mainly the formation of secretory IgA. IgA does not interact with complement, and thus has only low proinflammatory potential. However, divalent secretory IgA has a critical role in the incapacitation of microbes in the intestinal lumen. Reduced GALT tissue is reported, for example, in specific pathogen-free animals after parenteral nutrition, administration of oral antibiotics, in immunodeficient animals such as SCID and nude phenotypes, and in aging animals. Reference compounds for the investigation of immunotoxic effects such as cyclosporine or HCB induced changes in the PP concomitantly with similar changes in other secondary lymphoid organs. Some compounds (azaspiracid, nivalenol, dimethyl sulfoxide) have been shown to cause degeneration, necrosis, and subsequent mineralization of PP in toxicity studies in rodents. The specialized M cells covering all MALT structures can be affected directly as well.
Inhalation of ozone induced increased vacuolation of these cells in BALT, together with increased proliferation of BALT lymphocytes. BALT hyperplasia is a common observation subsequent to chronic inflammatory stimuli such as infection by constitutive androstane receptor bacillus or mycoplasma species in rodents. BALT increase, especially in subacute and subchronic studies, can be a physiological response or direct evidence of immunotoxicity. Since lymphocytes from MALT migrate to their draining lymph nodes, these nodes should be evaluated together with MALT.
Preneoplastic Changes and Neoplasia
Tumors of Resident Cells
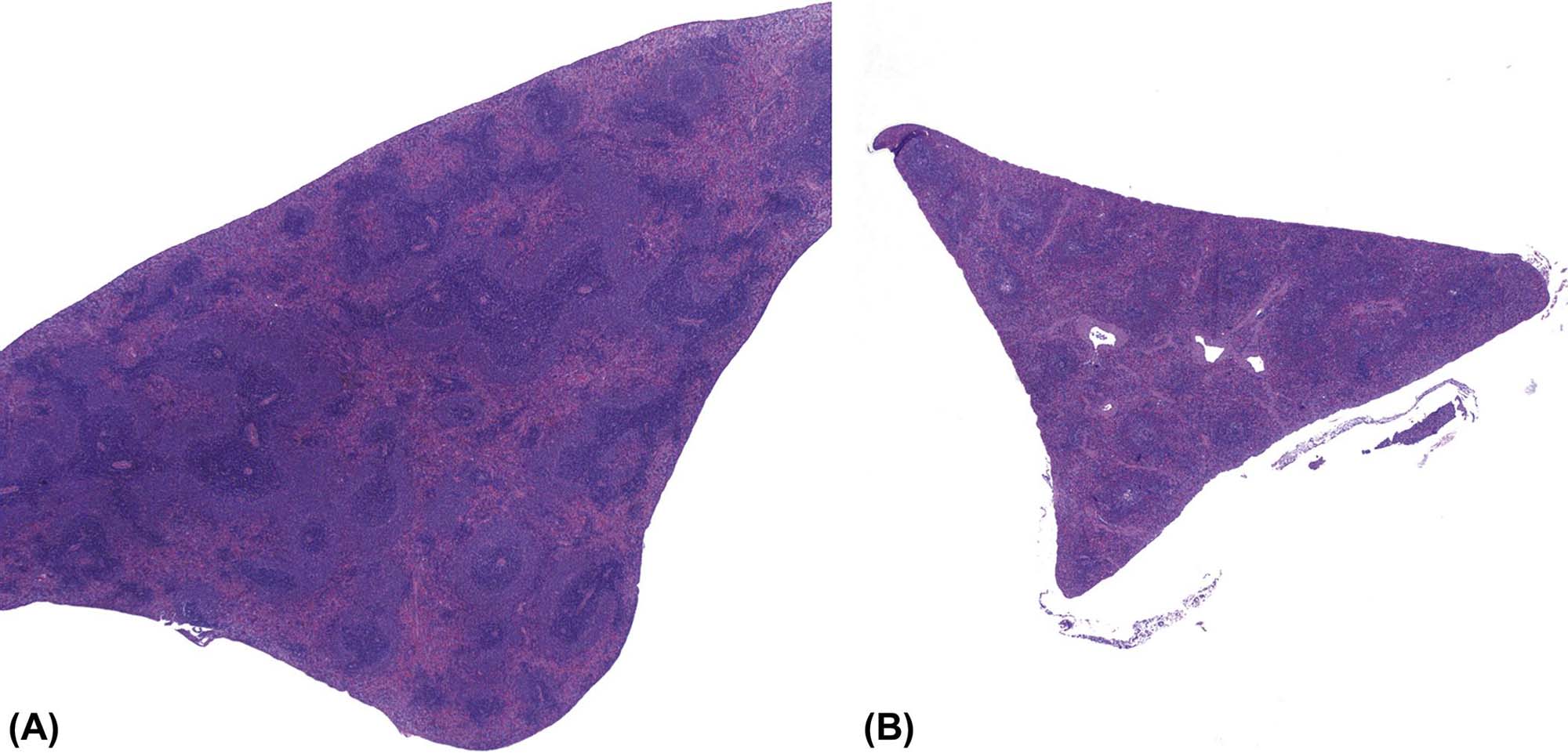
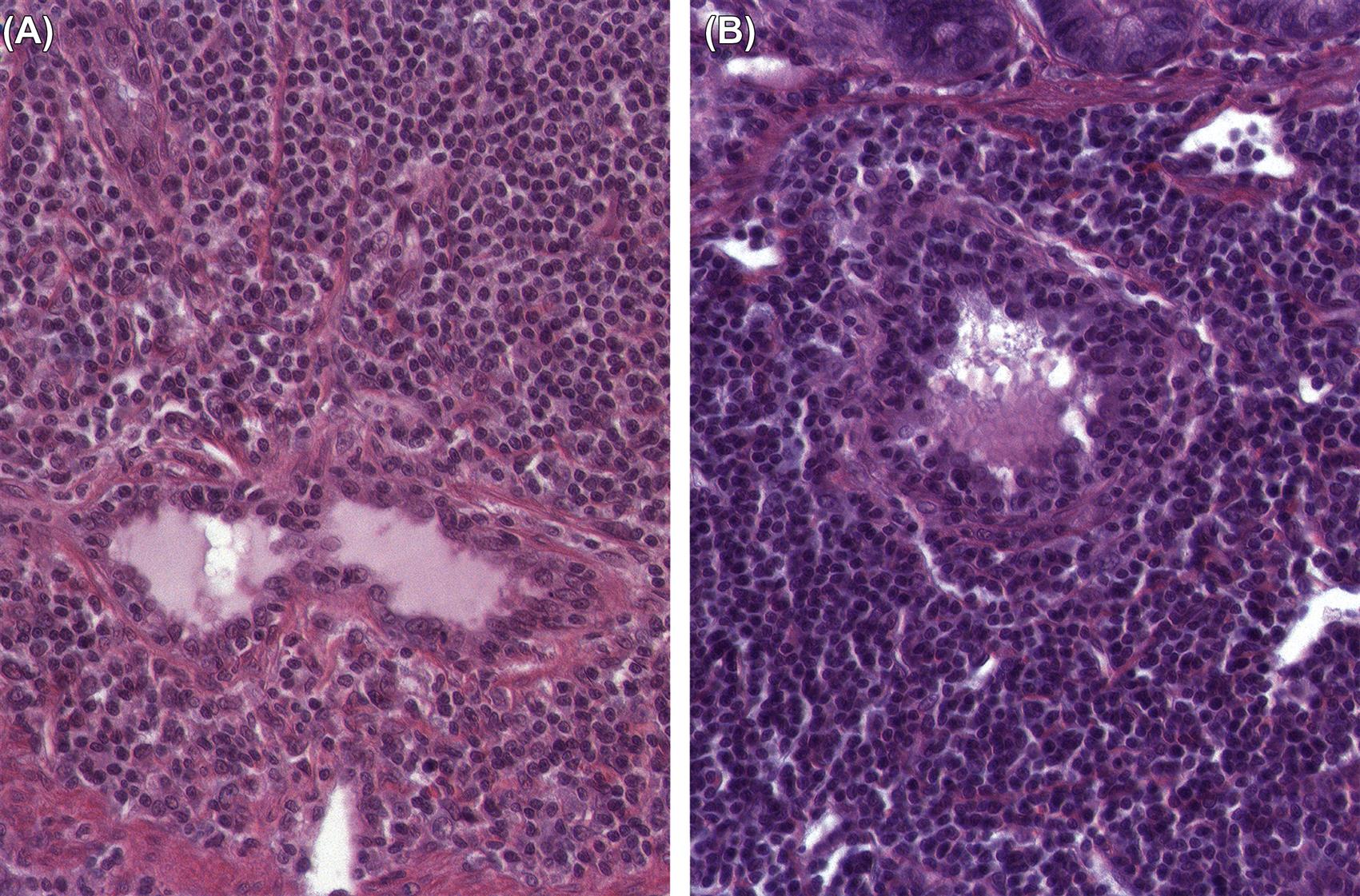
The incidence of tumors derived from resident mesenchymal and vascular cells of lymphoid and hematopoietic tissues is generally low in rodents, but unanticipated increases may be seen depending on genetic background. Reactive proliferations involving the stromal and vascular compartments of lymphoid organs or bone marrow and blood are more common, and often related to circulatory disorders in aging rodents. In the splenic red pulp, long-term treatment with hematotoxic compounds such as aniline leads to stromal fibrosis, fibroma, and fibrosarcoma. Hyperplasia and neoplasia of TECs (thymoma) have a strong interconnection with the proliferation, maturation, and release of lymphoid cells from the thymus. The spectrum of thymomas ranges from cases with low proportions of proliferating epithelial cells and high number of thymocytes, thus resembling T-cell lymphoma, to purely epithelial tumors resembling squamous cell carcinoma (Figure 12.28A–D). In rodents, females are generally more prone to thymoma than males. Thymomas with high amounts of lymphocytes often lead to concomitantly increased lymphoid cellularity in secondary lymphoid organs. As a differential diagnosis, epithelial hyperplasia of branchial remnants forming tubules, cords, and cysts has to be taken into account. The latter finding is common in both aging rats and mice, and more frequently seen in females. This finding does not influence the lymphoid compartment. In aging female mice of some strains (in particular CD-1), a B-cell hyperplasia is frequently observed and has to be differentiated from thymoma or lymphoma (Figure 12.29A–C).

Tumors of Hemopoietic Cells
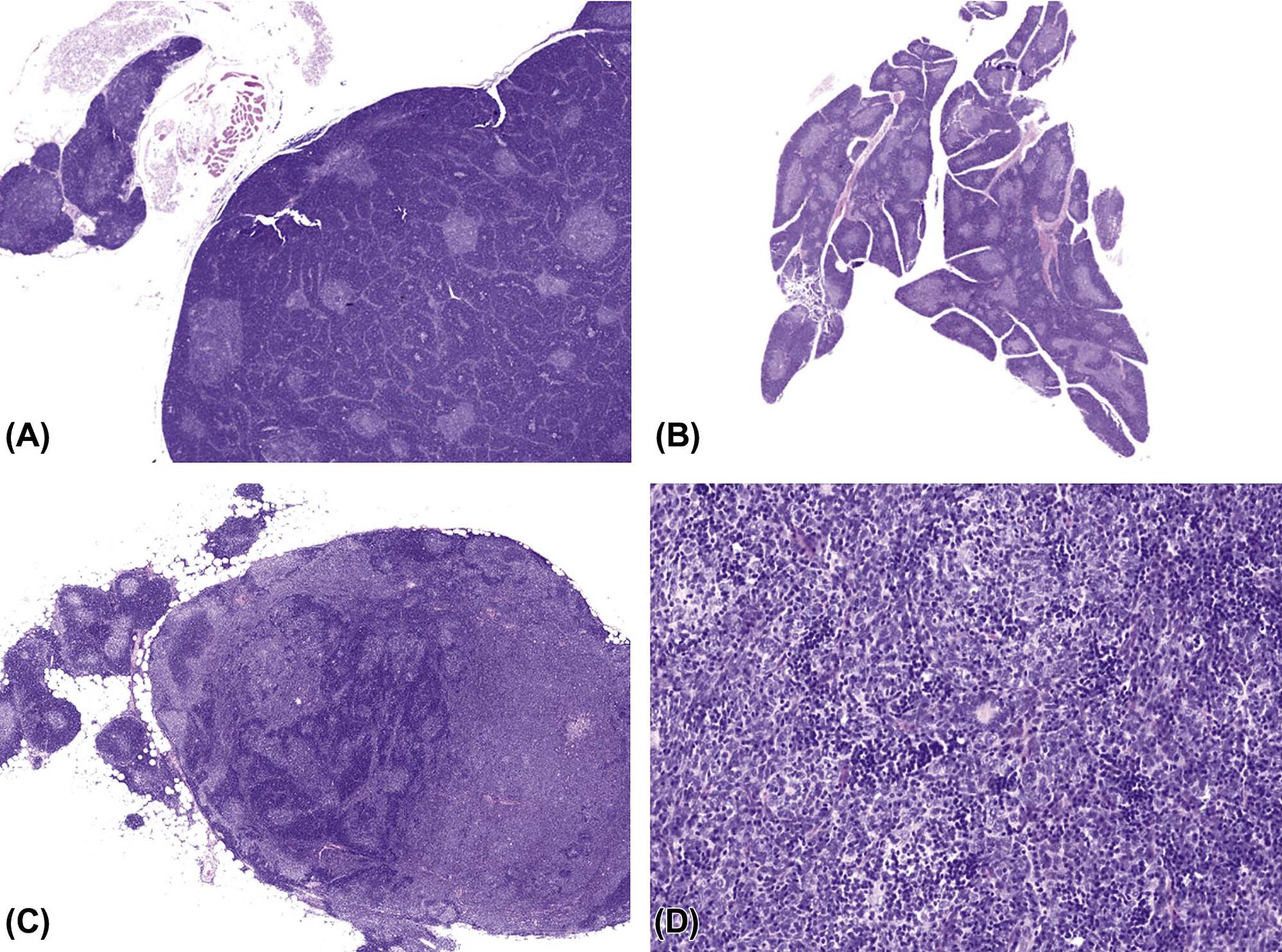
Hemopoietic cell tumors include myeloid leukemia, lymphoma, and histiocytic sarcoma. They represent frequent spontaneous and induced tumor entities in rodents. The spontaneous background incidences of these tumor entities are highly strain-specific. Induced tumors in rodents have been observed due to viruses, irradiation, and chemical carcinogens (Table 12.10).
Table 12.10
| Causative agent | Mouse | Rat | Relevance for humansa |
| ONCOGENIC VIRUSES | |||
| Lymphoma, erythroleukemia | Low susceptibility | Various leukemias | |
| RADIATION | |||
| Myeloid leukemia and lymphoma | Low susceptibility | Various leukemias | |
| IMMUNOSUPPRESSION AND ACTIVATION OF LATENT VIRUSES | |||
| Lymphoproliferative disorder and lymphoma (mostly B-cell type) | Lymphoproliferative disorder and lymphoma (mostly B-cell type) | ||
| ANTICANCER DRUGS | |||
| Lymphoma | Known human carcinogen (HC) | ||
| Lymphomab | Known HC | ||
– N-Ethyl-N-nitrosurea compoundsc |
Lymphoma, myleoid leukemiad | Lymphoma, myeloid leukemia | Known HC |
| Lymphoma, leukemia | Lymphoma | Anticipated HC | |
| Lymphoma | Known HC | ||
| OTHER DRUGS | |||
| Lymphoma, granulocytic leukemiae | –f | ||
| Lymphomab | –f | ||
| Lymphoma, leukemia | –f | ||
| Lymphoma, histiocytic sarcoma | Anticipated HC | ||
| HORMONALLY ACTIVE COMPOUNDS | |||
| Uterine histiocytic sarcomab,g,h | Not investigated | Known HC | |
| Lymphoma | Known HC | ||
| CHEMICALS WITH EXPOSURE VIA NUTRIENTS | |||
| Lymphomab | Mononuclear cell leukemiae | –f | |
| Lymphomab | –f | ||
| Not investigated | Lymphoma, leukemia | Anticipated HC | |
| Histiocytic sarcomag,i | Not investigated | Anticipated HC | |
| Urethane | Lymphoma | Anticipated HC | |
| CHEMICALS WITH OCCUPATIONAL EXPOSURE | |||
| 1,3-Butadiene | Lymphoma | Known HC | |
| 2,4,6-Trichlorophenol | Lymphomae, leukemiae | Anticipated HC | |
| 3,3′-Dimethoxybenzidine-4,4′-diisocyanate | Leukemia, lymphoma | Anticipated HC | |
| 4,4-Methylenedianiline dihydrochloride | Lymphomab | Anticipated HC | |
| Benzene | Leukemia, lymphoma | Known HC | |
| Benzylbutylphthalate | Mononuclear cell leukemiab | –f | |
| Coal tar | Histiocytic sarcomag | Not investigated | Known HC |
| Dimethyl methylphosphonate | Mononuclear cell leukemiae | Possible HC | |
| Ethylene oxide | Lymphomab | Known HC | |
| Formaldehyde | Known HC, increased lymphomaj | ||
| Furan | Mononuclear cell leukemia | Anticipated HC | |
| Glycidol | Histiocytic sarcomae,k | Anticipated HC | |
| σ-Nitroanisole | Mononuclear cell leukemia | Anticipated HC | |
| Tetrachloroethylene | Mononuclear cell leukemia | Anticipated HC | |
| VAT yellow 4 | Lymphomae | –f | |

aKnown or suspected carcinogens according to NIEHS data (Report on Carcinogens, 12th edition, 2010).
bIn females.
cHemopoietic tumors also induced in rodents by other carcinogens of this chemical class.
dNUP98-HOXA9 transgenic mice.
eIn males.
fInadequate knowledge, no positive information.
gOnly females investigated.
hCBA mice.
iP53-knockout mice.
jUnder debate, because of lack of plausible mode of action.
kp16Ink4a/p19Arf tumor suppressor-gene haplo-insufficient mice.
From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.11, p. 1848, with permission.
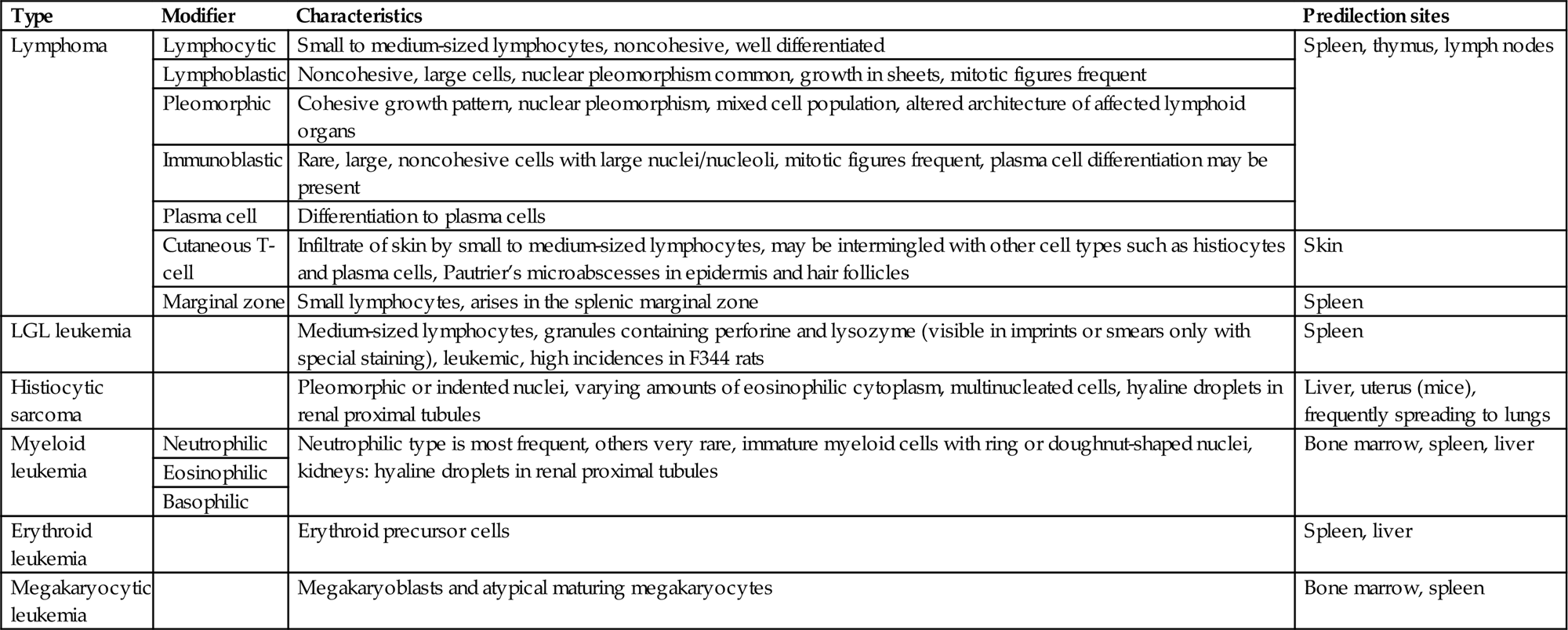
For nonclinical research in mice, the Bethesda proposals for classification of lymphoid neoplasms in mice in 2002 parallel the structure of the WHO classification of 2001 for humans. For rodent carcinogenicity studies, the IARC classification can be used in order to standardize nomenclature and diagnostic criteria in regulatory studies (Table 12.11). Figures 12.30–12.33 illustrate the morphology of lymphoma and histiocytic sarcoma in rodents. For further differentiation, the application of immunohistochemical markers is recommended. The interpretation of immunohistochemical results may be challenging due to issues such as varying fixation periods, variations in the expression of markers, coincidence of systemic tumors, and mixtures of neoplastic and nonneoplastic cells in certain tumor entities.
Table 12.11
IARC Nomenclature of Tumors of the Immune System in Rodents
| Type | Modifier | Characteristics | Predilection sites |
| Lymphoma | Lymphocytic | Small to medium-sized lymphocytes, noncohesive, well differentiated | Spleen, thymus, lymph nodes |
| Lymphoblastic | Noncohesive, large cells, nuclear pleomorphism common, growth in sheets, mitotic figures frequent | ||
| Pleomorphic | Cohesive growth pattern, nuclear pleomorphism, mixed cell population, altered architecture of affected lymphoid organs | ||
| Immunoblastic | Rare, large, noncohesive cells with large nuclei/nucleoli, mitotic figures frequent, plasma cell differentiation may be present | ||
| Plasma cell | Differentiation to plasma cells | ||
| Cutaneous T-cell | Infiltrate of skin by small to medium-sized lymphocytes, may be intermingled with other cell types such as histiocytes and plasma cells, Pautrier’s microabscesses in epidermis and hair follicles | Skin | |
| Marginal zone | Small lymphocytes, arises in the splenic marginal zone | Spleen | |
| LGL leukemia | Medium-sized lymphocytes, granules containing perforine and lysozyme (visible in imprints or smears only with special staining), leukemic, high incidences in F344 rats | Spleen | |
| Histiocytic sarcoma | Pleomorphic or indented nuclei, varying amounts of eosinophilic cytoplasm, multinucleated cells, hyaline droplets in renal proximal tubules | Liver, uterus (mice), frequently spreading to lungs | |
| Myeloid leukemia | Neutrophilic | Neutrophilic type is most frequent, others very rare, immature myeloid cells with ring or doughnut-shaped nuclei, kidneys: hyaline droplets in renal proximal tubules | Bone marrow, spleen, liver |
| Eosinophilic | |||
| Basophilic | |||
| Erythroid leukemia | Erythroid precursor cells | Spleen, liver | |
| Megakaryocytic leukemia | Megakaryoblasts and atypical maturing megakaryocytes | Bone marrow, spleen |
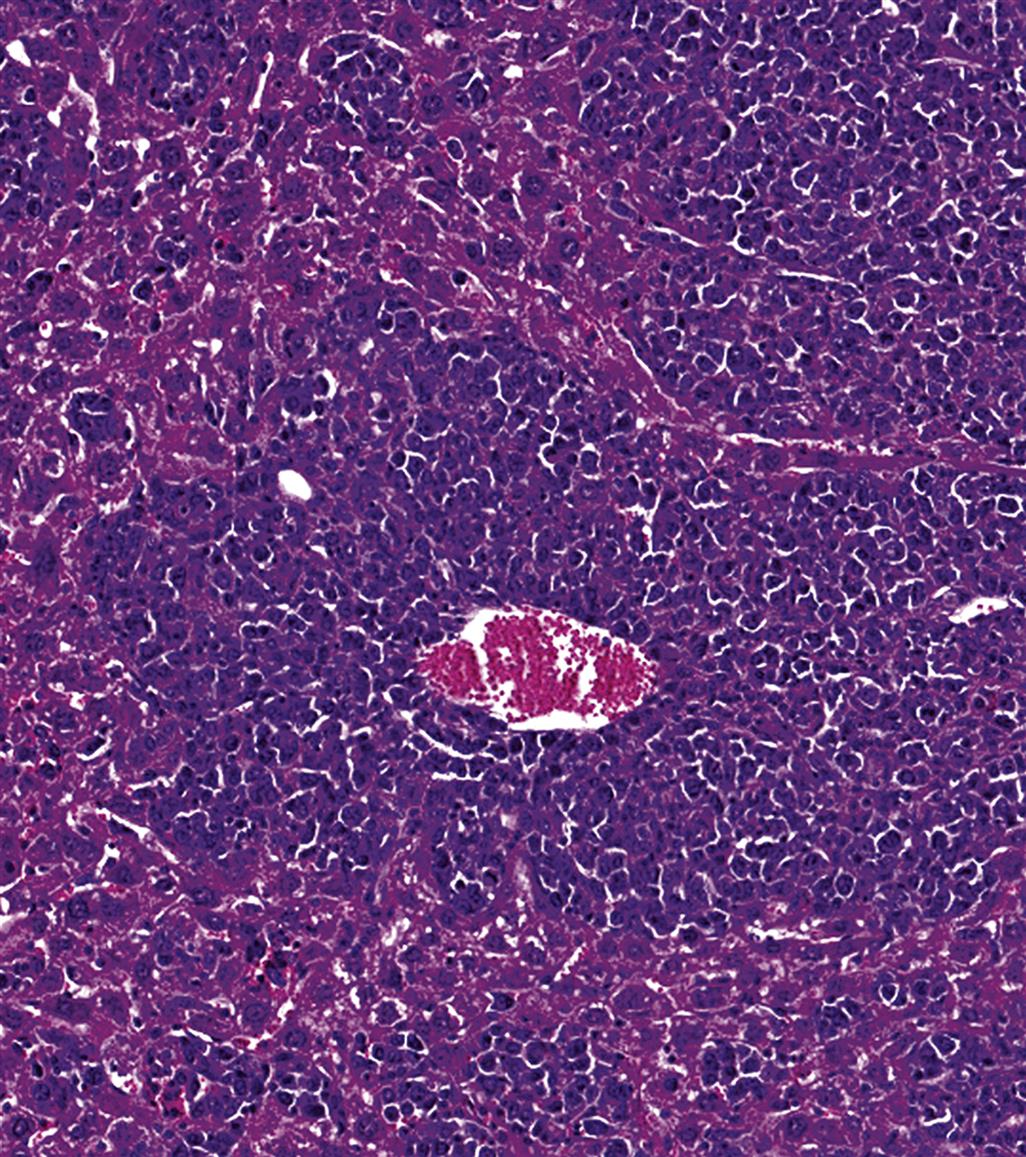

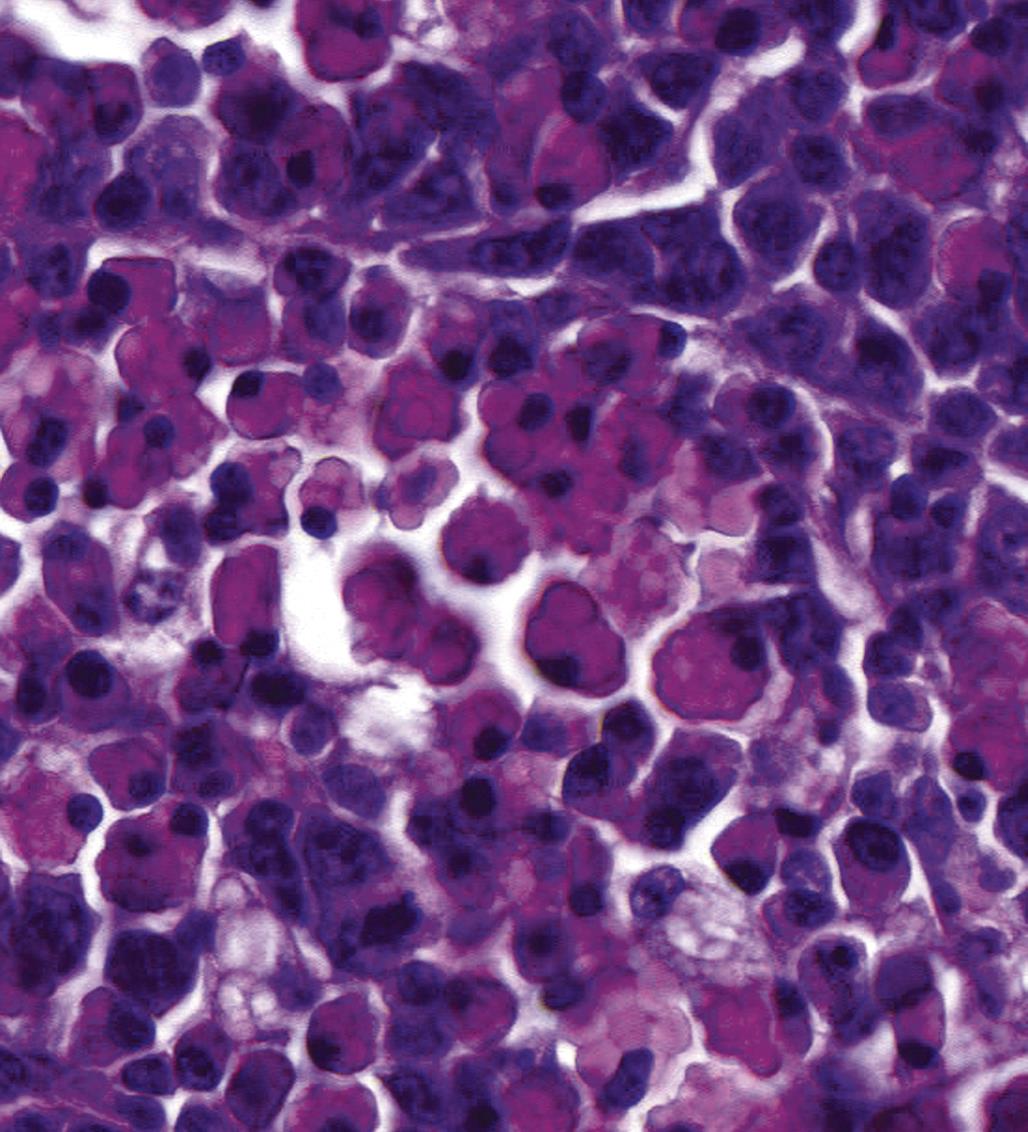

Autoimmune Diseases and Hypersensitivity Reactions
Target Organs
Antigen-specific deranged processes like autoimmune disease and allergy lead to tissue damage, protein (immune) complex deposits, and/or inflammatory cell infiltrates in predominantly nonlymphoid, target organs. Well-known targets are vasculature, kidneys, synovial membranes, thyroid (Figure 12.34), skin, liver, and lungs. Figure 12.35 depicts an allergy-related mononuclear cell infiltrate in rat nasal passages, similar to allergy-related inflammation in the lungs. The morphological hallmark of autoimmune (-like) disease and allergy is inflammation. Unfortunately, there are at present no morphological criteria to identify antigen-specific inflammatory responses. Antigen-specific inflammation in nonlymphoid organs includes changes such as granulocytic and lymphocytic cell infiltrates, granuloma, necrosis, and fibrosis. These infiltrates are also common in nonspecific inflammation, and therefore their interpretation is difficult.


Typical changes like vasculitis, inflammation at dermal–epidermal interfaces, fibrinoid necrosis (Figure 12.36), and expansion of extracellular matrix are suggestive of an allergic- or autoimmune-related inflammation. In subacute and subchronic toxicity studies signs of full-blown autoimmune diseases are rarely encountered, because the time of exposure is too short and the number of animals per group is small, and relatively insensitive animal species or strains may be used. Therefore, in most cases only early indicators of such a disease may be present—for instance, lymphocytic infiltrates in the pancreatic islets as a symptom of early diabetes. The histology of clinically manifest, antigen-specific inflammatory reactions depends on the type of immune reaction, which can be categorized according to the Gell and Coombs classification. It should be kept in mind that an antigen often induces more than one type of reaction, and this is reflected by the morphology of the inflammation.
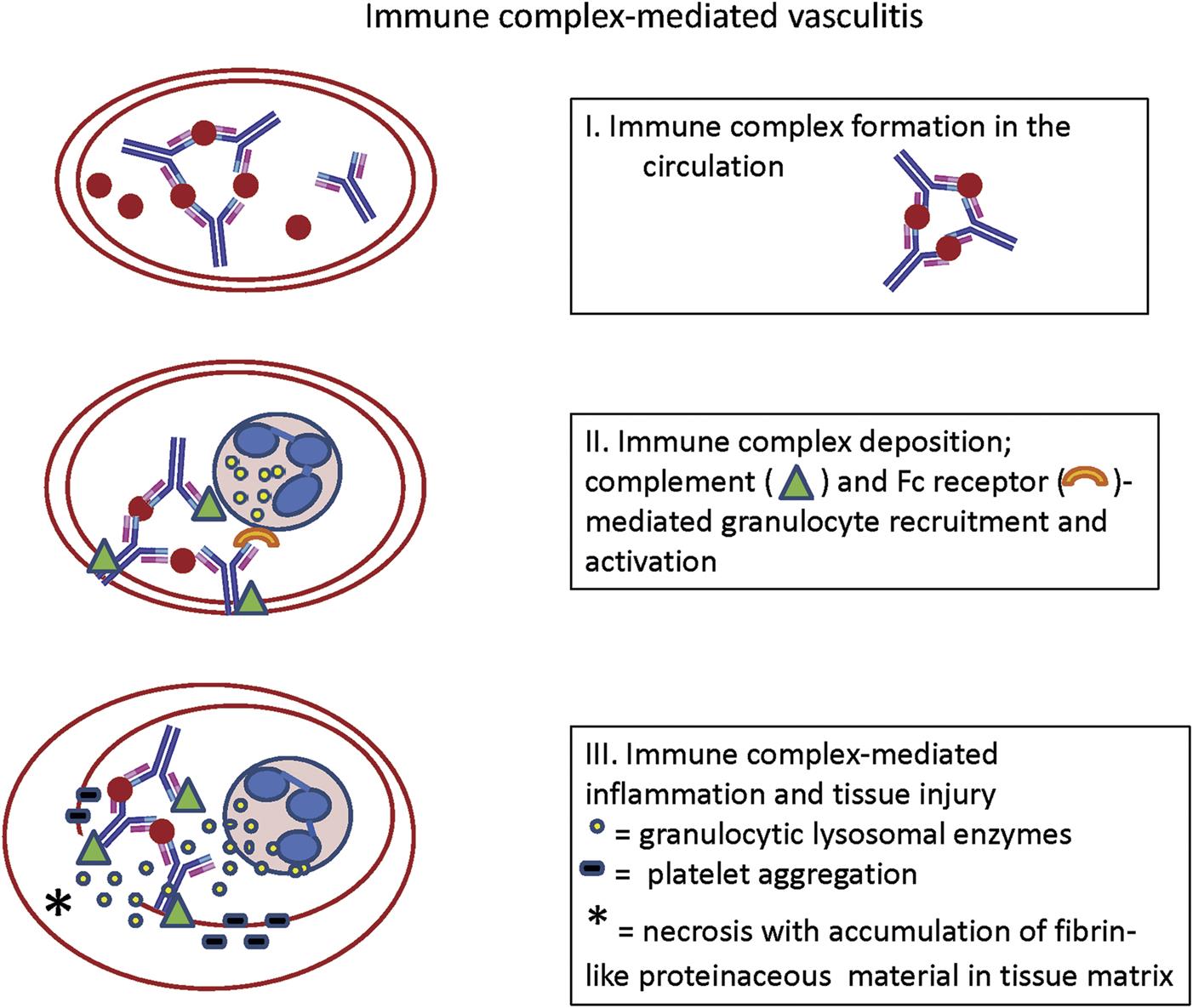
Figure 12.34 presents an example of lymphoplasmacytic infiltrates in the thyroid of the mastomys (Mastomys natalensis), which in this case occurred spontaneously. The infiltrates were associated with autoantibodies to colloid and were therefore considered to represent autoimmune thyroiditis. Histologic changes of this type are also seen with some frequency in the thyroid glands of beagle dogs. Another example of antigen-specific inflammatory reactions is beryllium-induced granulomatous inflammation in the lungs as the result of persistent antigenicity of inhaled beryllium. In dogs, beryllium induces so-called immune granulomas, while in rats nonspecific irritation by beryllium induces so-called foreign-body granulomas. The immune granulomas induced in dogs are complex, discrete, and highly organized, with a central region of epithelioid cells surrounded by significant numbers of lymphocytes and a few multinucleated giant cells of Langham’s type. Coinfiltrating lymphocytes are beryllium-specific T-lymphocytes. The foreign-body granulomas in rats are simpler and more loosely organized than the immune granulomas in dogs, and contain monocytes, enlarged, and vacuolated macrophages, and multinucleated foreign-body giant cells. Few to no lymphocytes are present in the lesions. In a third example, inhaled crystalline silica may continuously be mobilized from macrophages and granulomas, in the lung and draining lymph nodes, even long after initial exposure to the silica, producing progressive lesions. In these lesions, alterations in extracellular matrix were the characteristic features. Silica may disseminate via lymph and blood, leading to granulomas in organs outside the respiratory tract and its local lymph nodes. The continuous stimulation and degradation of inflammatory cells by silica has been associated with autoimmune (-like) diseases such as antineutrophil cytoplasmic antibodies (ANCA)-associated glomerulonephritis and vasculitis. Recently, it has been suggested that “frustration” of macrophages by partial ingestion of long nanotubes (Figure 12.37) may eventually lead to induction of autoimmune phenomena.
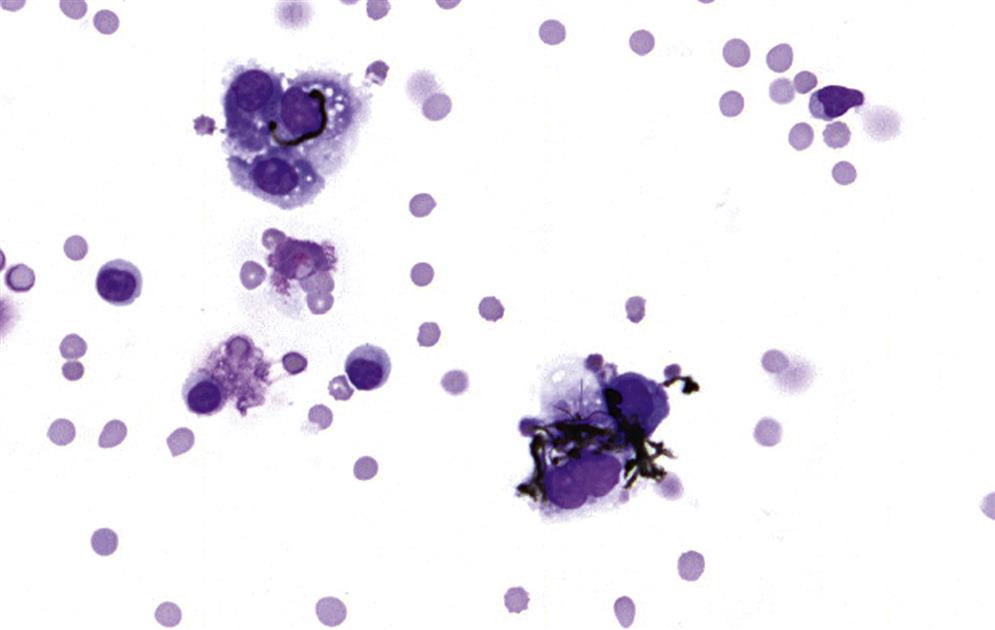
Lymphoid Organs
Changes in lymphoid organs can alert the pathologist to potential autoimmune effects (Table 12.12). Alterations in lymphoid organs often accompany autoimmune inflammation at the nonlymphoid target sites; the most well-known examples in humans are thymoma and thymic follicular hyperplasia in myasthenia gravis. Comparable thymic proliferative changes are found in the autoimmune disease-prone mastomys and (NZB-NZW)F1 mouse. Mercuric chloride induces autoimmune disease in Brown Norway rats, with concomitant follicular hyperplasia in the thymus. The effect of exposure to mercuric chloride depends on the genetic make-up of the animal (and individual), because it induces immunosuppression in Lewis rats.
Table 12.12
Lymphoid Organ Alterations in a Selection of Animal Models With Autoimmune (-Like) Inflammation
| Animal species and strain | Autoimmune (-like) inflammation | Lymphoid organ alterations |
| BB rat | Pancreatic islets inflammation (diabetes mellitus); thyroiditis | Blood lymphopenia; thymus epithelial defects; liver increased apoptosis of recent thymic emigrants |
| Mercuric chloride in BN rat | Nephropathy; Sjögren syndrome-like adenitis | Thymus follicle formation |
| (NZB×NZW)F1 mouse | Chronic glomerulonephritis; vasculitis; SLE | Thymus decreased cellularity and follicle formation; lymph nodes and spleen lymphocyte proliferation; spleen follicular hyperplasia, erythropoiesis and hemosiderosis |
| NZB/Lac mouse | Chronic glomerulonephritis; vasculitis | Thymus decreased cellularity; lymph nodes plasma cell accumulations and increased cellularity; spleen increased cellularity, erythropoiesis, and hemosiderosis |
| NOD mouse | Pancreatic islets inflammation (diabetes mellitus); Sjögren syndrome-like adenitis | Thymus decreased cellularity, plasma cell infiltration and epithelial cell defects |
From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. Haschek and Rousseaux’s Handbook of Toxicologic Pathology, third ed. Academic Press (Elsevier), San Diego, CA, Table 49.14, p. 1859, with permission.
Alterations in lymphoid organs are not only associated with overt manifestation of autoimmune disease but have also been found to precede it; for example, follicular hyperplasia in the NZB mouse is observed before the onset of SLE or SLE-like disease. Moreover, thymectomy or cyclosporine-induced partial thymus depletion in very young animals can result in autoimmune (-like) disease. Therefore, follicular hyperplasia and certain forms of lymphoid organ depletion can be early indicators of autoimmune disease.
Summary
The inherent characteristics of the immune system are its ubiquitous distribution (multiple sites but one system) and multifaceted constituents jointly interacting within the organism. Under physiological conditions, the immune system aims at a status of homeostatic balance, which is constantly challenged by a variety of external influences.
Changes in lymphoid organs and in immune function can be related to beneficial effects like protection against pathogens by vaccination and intended suppression of immune function to try to cure autoimmune disease. Immune changes can also be related to adverse effects leading to an increased rate of infections or even tumors, to allergy and autoimmune disease.
Investigation of the immune system in toxicology needs a specific understanding of its structure and physiology. Moreover, species- and age-related changes and the interaction with the endocrine and nervous systems have to be taken into account. Histopathology has a pivotal role in the detection of immune changes in nonclinical toxicology studies, but additional assays are commonly required for elucidation of underlying immunological mechanisms. For interpretation of immune-related findings, a synopsis of findings in immune and nonimmune-related organs, hematological and immunological biomarkers is necessary. Furthermore, mechanistic studies in animal and in vitro models represent a relevant tool to investigate the translatability of immune findings to humans.