Special Senses
Special Senses—Eye
Leandro Teixeira and Richard R. Dubielzig, University of Wisconsin-Madison, Madison, WI, United States
Abstract
The “Eye” chapter of the third edition of the Fundamentals of Toxicologic Pathology brings a comprehensive description of spontaneous and toxicant-induced pathological processes affecting the ocular tissues in the most commonly used laboratory animals and their correlations with toxic diseases of humans. Also presented are detailed descriptions of the structure, function, and response to injury of the different ocular tissues, known mechanisms of ocular toxicity, the most advanced techniques applied in the toxicological evaluation of the eye, and eye-specific techniques of tissue processing.
Keywords
Ocular; toxicology; pathology; eye; toxicity
Introduction
The principal means by which most animals are made aware of their surroundings is the reflection or emission of light by external objects and the reception of this light by special cells in the eye called photoreceptors. However, reception of light is only the initial step of vision. Visual perception is the ability to interpret the surrounding environment by processing information contained in the visible light, and for that to be accomplished the whole visual system (eye, optic nerve, and cerebral cortex) needs to be involved. It is reported that humans obtain over 80% of all external information from vision. Visual impairment can have devastating health and socio-economic consequences, so risk assessment for toxicity of new chemicals and drugs with respect to the visual system is extremely important.
The evaluation of ocular tissues for efficacious or toxic effects has many challenges that are shared with nonocular tissues while others are eye-specific. One must understand the special requirements for tissue sampling, trimming, fixation, and histologic processing for eye specimens in general, and for the eyes of different species in particular, in order to obtain histologic sections of suitable quality. From the biologic and anatomic perspectives, one needs to be aware of the differences in ocular anatomy among species in order to differentiate toxicant-induced changes from normal anatomical variations. Because of the accessibility of the intraocular structures to clinical examination and detection of lesions in vivo, the accurate identification and diagnosis of microscopic findings requires an awareness and understanding of clinical ocular findings. On the same note, there is an almost complete overlap between the terminology in clinical ophthalmology and ocular pathology, so familiarization with clinical and diagnostic terms is essential for accurate communication. Once identified, ocular findings need to be proper classified as treatment-related, spontaneous, or iatrogenic. A multinational effort involving the Society of Toxicologic Pathology (STP), British Society of Toxicological Pathology (BSTP), European Society of Toxicologic Pathology (ESTP), the Japanese Society of Toxicologic Pathology (JSTP), and the Registry of Industrial Toxicology Animal data (RITA) in Europe is developing standardized nomenclature for classifying microscopic lesions observed in laboratory rats and mice in toxicity and carcinogenicity studies. This project, referred to as the International Harmonization of Nomenclature and Diagnostic (INHAND) Criteria for Lesions in Rats and Mice, has resulted in a list of accepted terms for “Nonproliferative and Proliferative Lesions of the Rat and Mouse Special Sense Organs (Ocular [eye and glands], Olfactory and Otic),” which may be accessed at https://www.toxpath.org/inhand.asp. These terms now are required in product registration packages submitted to the U.S. Food and Drug Administration (FDA) to ensure compliance with the “Standard for Exchange of Nonclinical Data" (SEND) guidance recently implemented by this agency.
Structure and Function
The eye is a highly specialized organ that originates from multiple embryological layers, especially the neural crest, mesoderm, neuroectoderm, and surface ectoderm. This embryological tissue diversity becomes evident in the large spectrum of cellular differentiation as well as the complexity and specialization of the ocular tissues (Figure 22a.1). Table 22a.1 offers timelines for the comparative embryological development of the dog, mouse, and human eyes. The main function of the eye is through the cone and rod photoreceptors located in the retina, which transform light energy into electrical impulses (phototransduction) that are transmitted via the optic nerve to the optical centers of the brain. In order for impulses to be generated in the eye, the light needs to be effectively focused on the retina; proper focusing depends on structural characteristics like size and rigidity of the globe, corneal surface and lens curvature, and locations and optical clarities of the various ocular tissues. These properties are acquired during development and must be maintained throughout life if the function of the eye is to remain intact.
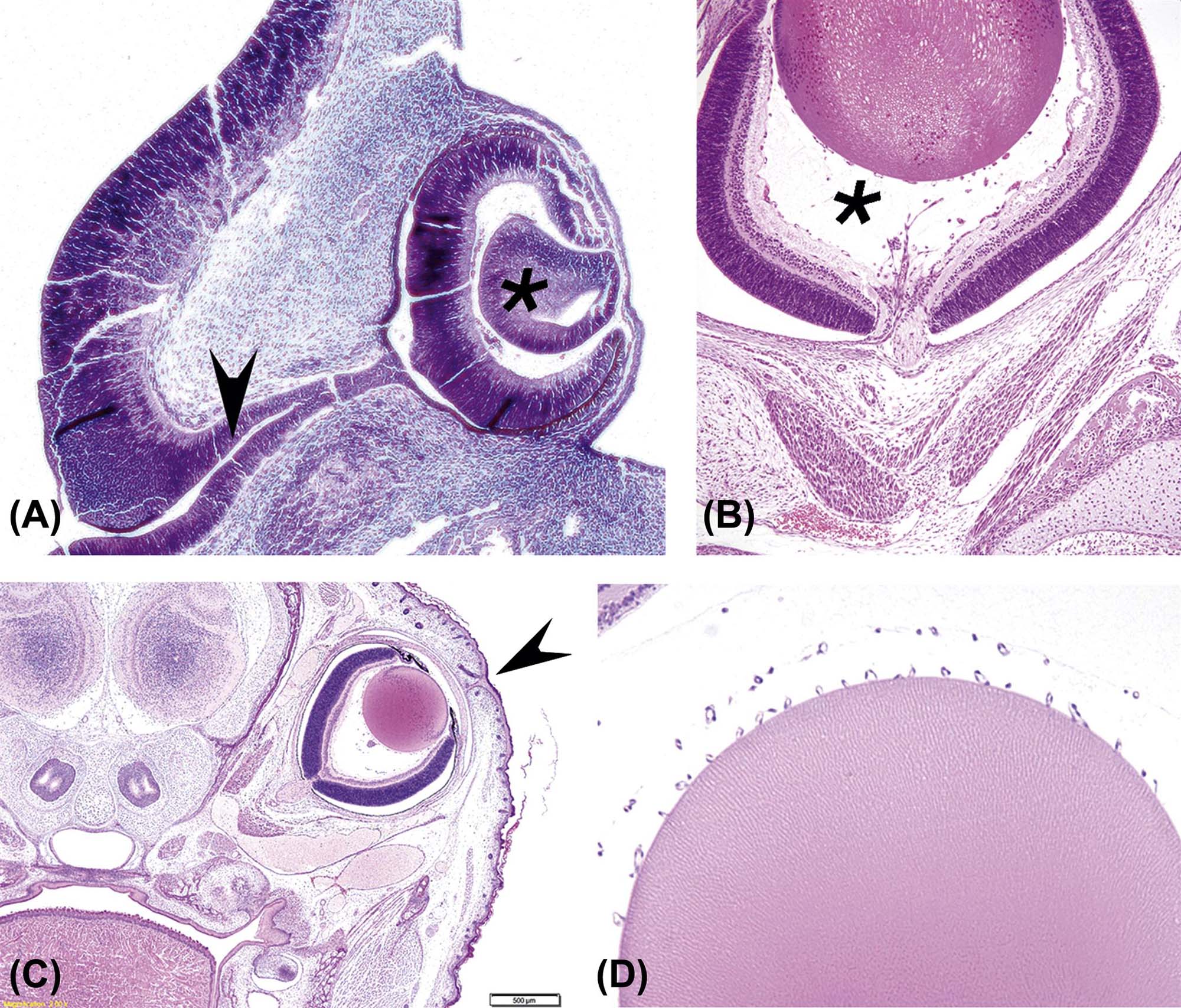
(A) Developing eye from a mid-term (gestational day 128) equine embryo showing the developing neuroretina [thick C-shaped layer lining the deep portion of the globe and encircling the lens vesicle (*)] and the optic stalk [or primitive optic nerve (arrowhead)]. (B) Near-term mouse fetus showing the primary vitreous (*) between the lens (pale eosinophilic orb at the top) and retina (thick multilaminar layer at the bottom). (C) Near-term mouse fetus showing the fused eyelids; the arrowhead points to the cleft where the palpebrae (eyelids) will eventually separate. (D) Lens from a near-term mouse fetus showing the tunica vascularis lentis [as small, regularly spaced capillary cross-sections along the posterior (upper) edge of the lens]. (A) Periodic acid-Schiff (PAS) stain, ×100; (B) H&E, ×200; (C) H&E, ×40; (D) H&E, ×200. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.2, p. 2103, with permission.
Table 22a.1
Sequence of Ocular Development
| Human (approximate postfertilization age) | Mouse (day postfertilization) | Dog (day postfertilization) | Developmental events | ||
| Month | Week | Day | |||
| 1 | 3 | 22 | 8 | 13 | Optic sulci present in forebrain |
| 4 | 24 | 9 | 15 | Optic sulci convert into optic vesicles | |
| 10 | 17 | Optic vesicle contacts surface ectoderm | |||
| 26 | Lens placode begins to thicken | ||||
| Optic vesicle surrounded by neural crest mesenchyme | |||||
| 2 | 5 | 28 | 10.5 | Optic vesicle begins to invaginate, forming optic cup | |
| Lens pit forms as lens placode invaginates | |||||
| Retinal primordium thickens, marginal zone present | |||||
| 32 | 11 | 19 | Optic vesicle invaginated to form optic cup | ||
| Optic fissure delineated | |||||
| Retinal primordium consists of external limiting membrane, proliferative zone, primitive zone, marginal zone, and internal limiting membrane | |||||
| Oculomotor nerve present | |||||
| 33 | 11.5 | 25 | Pigment in outer layer of optic cup | ||
| Hyaloid artery enters through the optic fissure | |||||
| Lens vesicle separated from surface ectoderm | |||||
| Lens surrounded by intact basement membrane (lens capsule) | |||||
| Retina: inner marginal and outer nuclear zones | |||||
| 11.5 | 29 | Basement membrane of surface ectoderm intact | |||
| Primary lens fibers form | |||||
| Trochlear and abducens nerves appear | |||||
| Lid folds present | |||||
| 6 | 37 | 12 | Edges of optic fissure in contact | ||
| 12 | 30 | Tunica vasculosa lentis present | |||
| Lens vesicle cavity obliterated | |||||
| Ciliary ganglion present | |||||
| 41 | 12 | 32 | Posterior retina consists of nerve fiber layer, inner neuroblastic layer, transient fiber layer of Chievitz, proliferative zone, outer neuroblastic layer, and external limiting membrane | ||
| 17 | 32 | Eyelids fuse (dog) | |||
| 7 | Anterior chamber beginning to form | ||||
| 12.5 | 40 | Secondary lens fibers present | |||
| 48 | 14 | 32 | Corneal endothelium differentiated | ||
| 8 | 51 | Optic nerve fibers reach the brain | |||
| Optic stalk cavity is obliterated | |||||
| Lens sutures appear | |||||
| Acellular corneal stroma present | |||||
| 54 | 30–35 | Scleral condensation present | |||
| 9 | 57 | 17 | 40 | First indication of ciliary processes and iris | |
| Extraocular muscles visible | |||||
| Eyelids fuse (occurs earlier in the dog) | |||||
| 10 | 45 | Pigment visible in iris stroma | |||
| Ciliary processes touch lens equator | |||||
| Rudimentary rods and cones appear | |||||
| 45- | Hyaloid artery begins to atrophy to the disc | ||||
| 3 | 12 | Branches of the central retinal artery form | |||
| 4 | 51 | Pupillary sphincter differentiates | |||
| Retinal vessels present | |||||
| 56 | Ciliary muscle appears | ||||
| Eye axis forward (human) | |||||
| 56 | Tapetum present (dog) | ||||
| Tunica vasculosa lentis atrophies | |||||
| Short eyelashes appear | |||||
| 5 | 40 | Layers of the choroid are complete with pigmentation | |||
| 6 | Eyelids begin to open, light perception | ||||
| Pupillary dilator muscle present | |||||
| 7 | Pupillary membrane atrophies | ||||
| Rod and cone inner and outer segments present in posterior retina | |||||
| Pars plana distinct | |||||
| 9 | Retinal layers developed | ||||
| Regression of pupillary membrane, TVL, and hyaloid artery nearly complete | |||||
| Lacrimal duct canalized | |||||

Times represent gestational days (numbers only) or postnatal days (numbers annotated with “P”).
From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Table 53.1, pp. 2099–2101, with permission.
The eye can be divided into three basic layers or tunics: (1) the fibrous tunic, the external part of the eye composed of the corneal and sclera; (2) the uveal tract, internal to the fibrous tunic and composed of the iris, ciliary body, and choroid, which houses the vascular supply to the eye; and (3) the internal neural layer, composed of the retina and optic nerve head. The eye can be further divided into anterior and posterior segments, the first consisting of the cornea, anterior and posterior chambers, iris, ciliary body, and lens, and the second composed of the vitreous and vitreal space, retina, choroid, sclera, and optic nerve.
Eyelids (Palpebra)
The eyelids’ primary function in mammals is protection of the ocular surface from exposure or from the risk of accidental trauma. The lids also have important functions in tear film formation and distribution (see later). In rodents, rabbits, and dogs, the upper and lower lids are fused at birth and separate only after retinal development is adequate for vision (between 12 and 15 days of age in the mouse and rat). In the species commonly used in toxicity studies, the eyelid presents a palpebral (inner) surface lined by conjunctiva, a specialized lid margin that contains openings for multiple meibomian (sebaceous) glands, and haired skin on the outer surface. Carnivores, rabbits, and humans present an almond-shaped eye fissure, while most rodents and nonhuman primates have a round one. Lid closure is moderated by the orbicularis oculi (Figure 22a.2) and levator palpebrae muscles.
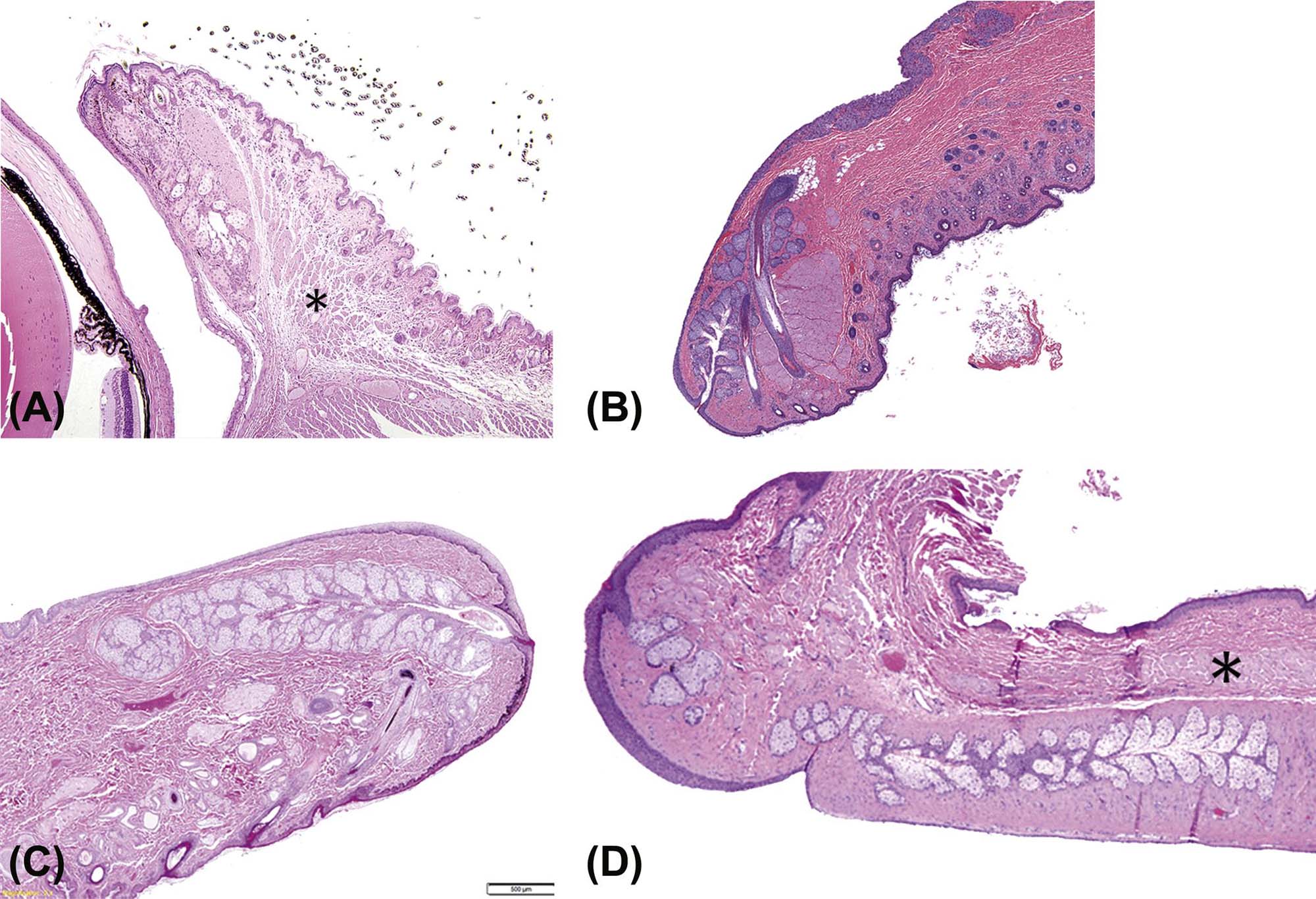
(A) Mouse, (B) rabbit, (C) dog, (D) nonhuman primate. Major features include haired skin on the external surface, nonhaired sebaceous (meibomian) glands with ducts reaching to the inner surface or mucocutaneous junction at the palpebral tip, and orbicularis oculi muscle (*). H&E, (A) ×12.5; (B–D) ×40. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.6, p. 2114, with permission.
Of the test species of interest the dog, cat, and rabbit possess a nictitating membrane (third eyelid), a large fold of conjunctiva that protrudes from the medial canthus over the corneal surface of the globe. The third eyelid is supported by a curved, T-shaped plate of hyaline cartilage, the base of which is surrounded by a mixed (seromucinous) gland, the gland of the third eyelid. The third eyelid mechanically protects the cornea, aids in tear distribution over the corneal surface, and contributes to 30%–50% of the aqueous portion of the tear film.
Lacrimal System
The lacrimal system is comprised of a series of extraocular apocrine glands whose secretions contribute important elements to the tears that lubricate the surface of the eye. Primates (both human and nonhuman) only have one lacrimal gland, which is located dorsal and lateral to the globe. Dogs and cats have both an extraocular lacrimal gland and a gland of the third eyelid. Rodents and rabbits have lacrimal gland systems consisting of extraorbital (i.e., the exorbital lacrimal gland) and intraorbital (i.e., Harderian gland) components (Figure 22a.3). The intraorbital component is located behind the globe, and generally in a medial (nasal) location. The lacrimal gland is a seromucinous gland that contributes mainly to the aqueous component of the tears. However, significant quantities of lipid are present in the secretion of Harderian glands, particularly in rabbits. The continual formation of tears that moistens the ocular surface is largely done by the small (minor) lacrimal glands of the conjunctival fornix, with the major lacrimal glands mainly producing the excess tear formation (crying) that results from ocular discomfort. In all species, the glands’ secretions contain enzymes and cytokines as well as antimicrobial substances (peptides and proteins) that protect the ocular surfaces.
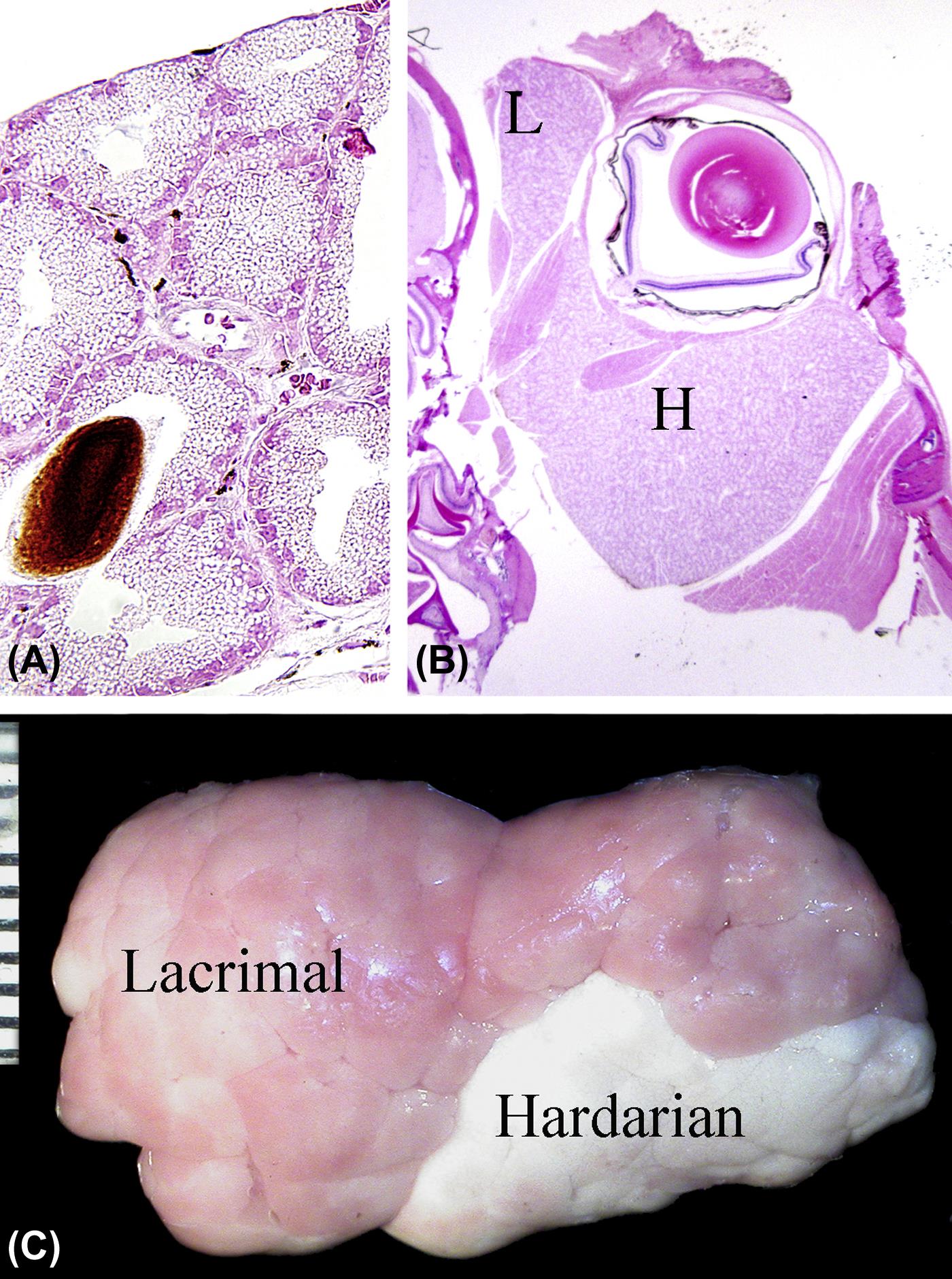
(A) High magnification image of the Harderian gland from an adult mouse showing the lipid vacuoles within the acinar epithelium and secreted porphyrin pigment within the lumen. (B) Low-magnification view of the mouse globe in situ showing the relative sizes of the Harderian (H) and lacrimal (L) glands. (C) The lacrimal and Harderian glands dissected from the orbit of a rabbit showing the difference in color. Scale, 1 interval= 1 mm. (A) H&E, ×200; (B) H&E, ×20. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.5, p. 2109, with permission.
The normal functions of the cornea are, in part, dependent on the existence of the precorneal tear film. This acellular fluid layer is adherent to the most superficial layer of the corneal epithelium and is composed of three main laminae: (1) the inner mucinous layer, secreted by the conjunctival goblet cells with contributions from the lacrimal glands, (2) the middle aqueous layer, secreted by the glands of the lacrimal system, and (3) the outer lipid layer, secreted by the sebaceous (meibomian) glands of the eyelid margins and the Harderian glands, particularly in rabbits. The tears are cleared from the ocular surface by a system of drainage ducts, in the conjunctiva near the medial canthus, called the nasolacrimal drainage system.
Cornea and Conjunctiva
Together the cornea and conjunctiva form the ocular surface. The cornea is the central translucent zone through which light reaches the intraocular structures, and the conjunctiva is the opaque mucosal tissue that lines the inner aspect of the eyelids (palpebral conjunctiva), the surface of the sclera (bulbar conjunctiva), and (when present) the inner and outer aspects of the third eyelid.
The conjunctiva has several main functions. These are maintenance of the tear film by secreting its mucinous component and aiding in its redistribution during blinking, to provide a loose flexible connection between the globe and the skin, to act as a mechanical barrier, and local immune surveillance through the conjunctiva-associated lymphoid tissue.
The conjunctival epithelium is a thin, multilayered, nonkeratinized epithelium that contains many unevenly distributed goblet cells. At the limbus (the border between the cornea and the sclera), the conjunctival epithelium forms a specialized, circumferential zone containing the stem cells of the cornea. The cells that migrate and repopulate the corneal epithelium during normal cell turnover and under pathological cell loss that result in corneal defects are derived from these stem cells. The substantia propria, a connective tissue layer beneath the epithelium, contains numerous glands, and is densely populated with both blood and lymphatic vessels. The lymphatic vessels are more common than blood vessels in the underlying loose fibrous connective tissue.
The cornea is a highly specialized, highly ordered tissue with many unique anatomical features that render it transparent to light in the visible spectrum. The mammalian cornea is covered on the surface by several layers of nonkeratinizing, stratified squamous epithelium organized into three layers: a deep layer of basal cells resting on a basal lamina, an intermediate layer of “wing” cells, and a surface layer composed of multiple layers of squamous cells, the number of which depends on the species (mice, rats, and rabbits, 3–5 layers, and dogs, cats, and primates, 5–7). This epithelium has a remarkably uniform thickness and structural consistency across the entire surface of the cornea. A modified acellular region of stroma (Bowman’s layer) composed of fine, randomly arranged collagen fibers is present posterior to the epithelial basal lamina in humans and nonhuman primates. Although Bowman’s layer is not visible by light microscopy in the mouse, a thin layer of randomly arranged collagen fibrils can be seen immediately underneath the corneal epithelium by electron microscopy. The function of Bowman’s membrane is not known. The bulk of the cornea is the stroma, which is located deep to Bowman’s membrane (if one is present) or to the corneal epithelium (Figure 22a.4). Optical transparency of the cornea stroma is dependent on a precise spatial relationship between stromal collagen fibers, noncollagenous matrix, and the functional stromal cells (keratocytes). A monolayer of low cuboidal epithelial cells (corneal endothelium) separates the cornea from the aqueous humor of the anterior chamber. The corneal endothelium secretes Descemet’s membrane, a thick basement membrane positioned between the endothelium and the inner surface of the corneal stroma. Descemet’s membrane is composed mainly of laminin, fibronectin, and type IV collagen, which gives it elastic properties. The morphology of corneal endothelium is similar across mammalian species, but cellular density can vary from 2211 cells/mm2 in the rat to 4450 cells/mm2 in nonhuman primates and humans. The endothelium actively moves water and electrolytes between the corneal stroma and anterior chamber, thus supplying the cornea with nutritional support from the aqueous while maintaining corneal transparency (Figure 22a.5).
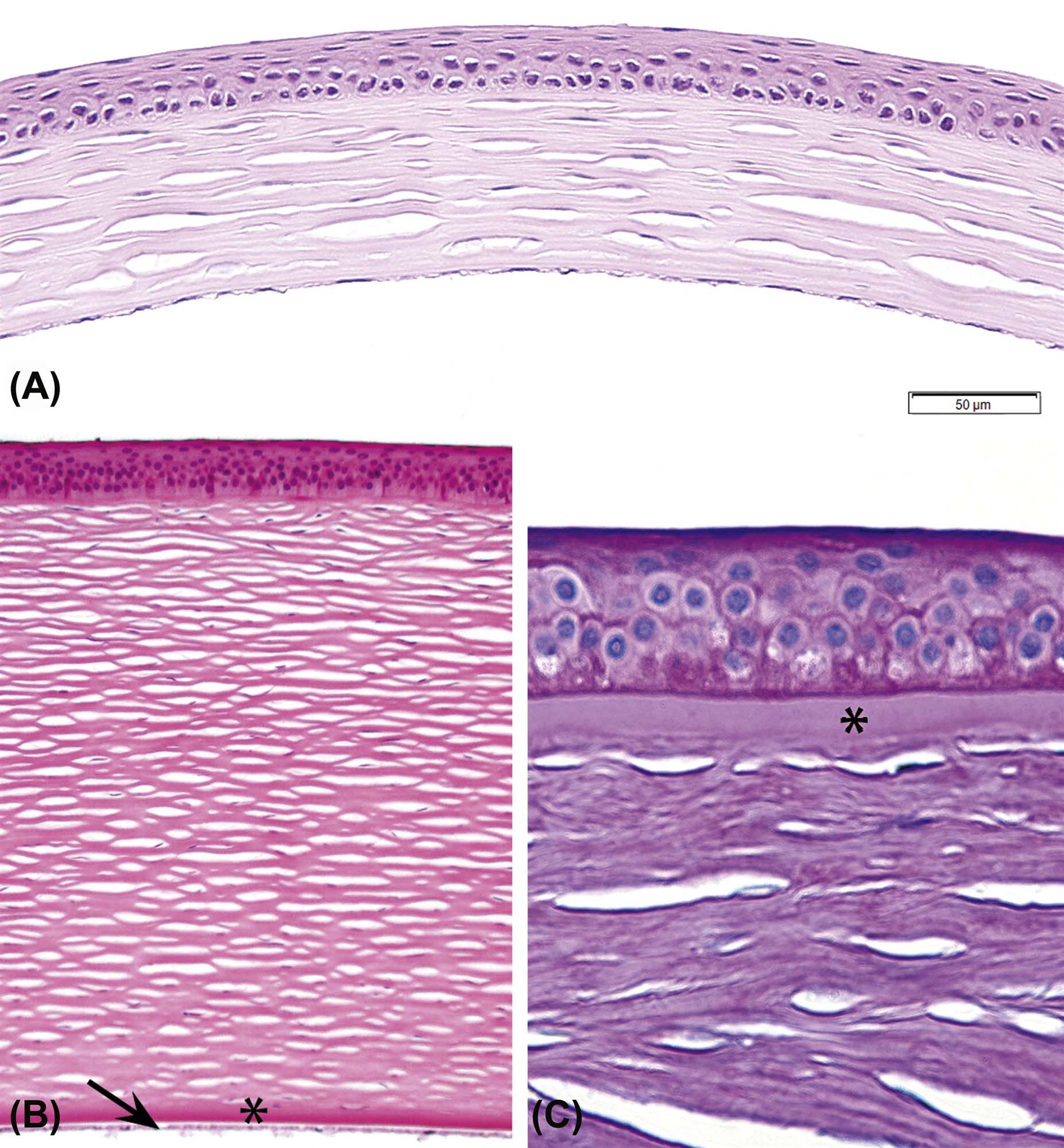
Axial (central) cornea from an adult mouse (A) and an adult dog (B) showing the relative thickness of the epithelium, stroma, Descemet’s membrane (*), and corneal endothelium (arrow) in these two species. (C) Epithelium and anterior stroma of the cornea from a nonhuman primate stained to show Bowman’s membrane (*). In all three images, the clear spaces within the stroma are processing artifacts and not vascular channels. (A) H&E, ×400; (B) H&E, ×400; (C) Periodic acid-Schiff (PAS), ×600. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.10, p. 2124, with permission.

(A) Epithelium and superficial stroma from a nonhuman primate cornea. (B) Posterior corneal stroma bordered by Descemet’s membrane (thick red band) and the corneal (posterior) endothelium from a nonhuman primate. (C) Scanning electron micrograph of the surface of the corneal endothelium from a dog. (A) H&E, ×400; (B) PAS, ×600; (C) ×2500. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.9, p. 2123, with permission.
Sclera
The sclera forms the main part of the fibrous tunic of the eye and functions to protect the ocular contents, maintain intraocular pressure (IOP), and preserve the shape of the globe, even during contraction of the extraocular muscles, which have tendons inserted on the globe’s surface. The sclera is relatively avascular and possesses great tensile strength, extensibility, and flexibility. It is composed of fibroblasts and dense irregular connective tissue containing mainly collagen type I. Anteriorly the sclera blends with the cornea at the limbus. The sclera surrounds the limbus and extends posteriorly where it is penetrated by the optic nerve exiting the eye at the lamina cribrosa. The sclera merges with dura mater that surrounds the optic nerve.
Uveal Tract
The uveal tract (or uvea) forms the continuous, heavily vascularized middle tunic of the globe. Its main functions are to provide the globe’s blood supply and, through its high melanin pigment content, absorb reflected light and prevent glare when light is focused on the retina. The uveal tract can be divided into iris, ciliary body (anterior uvea), and choroid (posterior uvea).
The iris is a thin contractile circular tissue analogous to a camera diaphragm. It is cantilevered across the front of the globe where it separates the anterior from the posterior chambers. The anterior chamber represents the space between the cornea and the anterior surface of the iris, while the posterior chamber is the space between the posterior surface of the iris, lens, and anterior face of the vitreous. The chambers are connected by the pupillary space, which is formed within the marginal rim of the iris. Both chambers contain aqueous humor. The anterior surface of the iris has no epithelial lining, so aqueous is free to diffuse from the iris stroma to the anterior chamber with no barrier. The iris stroma consists of a loose connective tissue containing fibroblasts, melanocytes, and collagen fibers. The posterior surface of the iris is lined by simple cuboidal pigmented epithelium (posterior pigmented epithelium), which is in direct contact with the posterior chamber. Tight junctions between these cells create a barrier between the posterior chamber and iris stroma. Contractile myoepithelial tissue forms the iris dilator muscle in the posterior iris stroma interior to the epithelium as well as the sphincter muscle that is positioned circumferentially at the leading edge of the iris (Figure 22a.6).
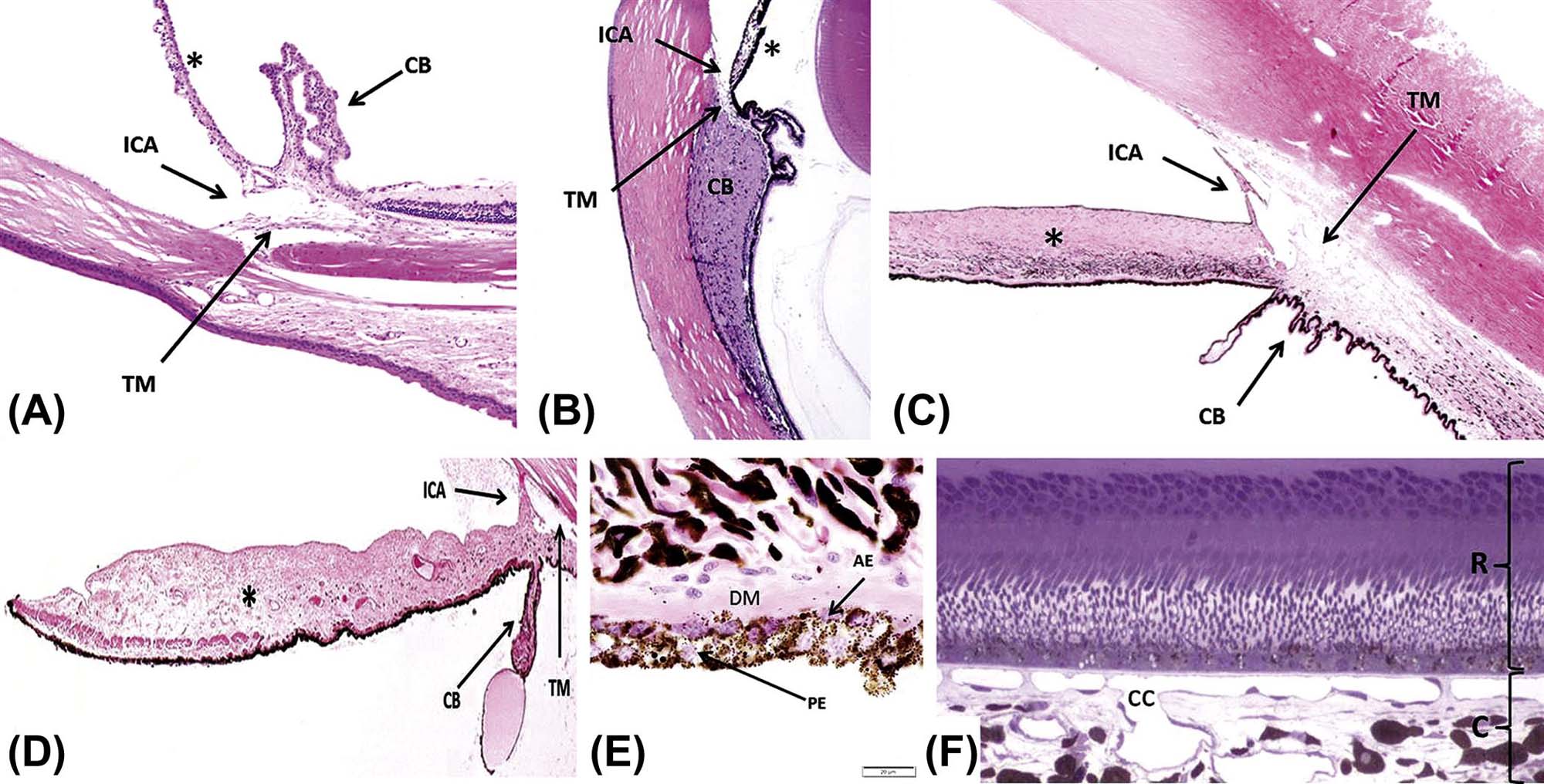
The iridocorneal angle (ICA), iris (*), trabecular meshwork (TM), and ciliary body (CB) from an albino mouse (A), nonhuman primate (B), dark-eyed dog (C), and blue-eyed dog (D). Note the marked lack of pigmentation of the iris stroma, characteristic of light-colored eyes, in the mouse (A) and blue-eyed dog (D). (E) Posterior iris epithelium showing the pigmented posterior epithelium (PE), the anterior epithelium (AE) with melanin surrounding its nuclei on the apical (near the posterior epithelium) aspect, and the smooth muscle appearance of its basal aspect, which contains large amounts of myofilaments and forms the dilator muscle of the iris (DM). (F) The outer retina (R) and inner choroid (C) from a nonhuman primate that was perfusion-fixed to allow better observation of the capillary network, the choriocapillaris (cc). (A–D) H&E, ×12.5; (E) H&E, ×1000; (E) Toluidine blue, ×400. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.13, p. 2136, with permission.
The ciliary body is subdivided into (anterior) pars plicata and (posterior) pars plana. In the pars plicata, there are multiple radially arranged folds, known as the ciliary processes. The inner aspect of the ciliary body is lined by a neural tube-derived double epithelium composed of an inner layer of nonpigmented epithelium and an outer layer of pigmented epithelium. The ciliary epithelium secretes aqueous humor and vitreous glycosaminoglycans and collagen. The zonular ligaments that suspend the lens are also produced by the ciliary epithelium and are anchored on the epithelial apical basement membrane. The ciliary body stroma is similar to the iris stroma and contains the ciliary muscle, a smooth muscle which functions in visual accommodation by controlling the suspension of the lens and thereby its curvature. The ciliary muscle also plays a role in aqueous filtration in primates by regulating the tension of the trabecular meshwork (TM) at the level of Schlemm’s canal. The relative tone of smooth muscle within the ciliary body controls the visual accommodation of the lens; thus the relative amount of smooth muscle present in the ciliary body is reflective of visual acuity. The ciliary muscle is robust in primates and accommodation is, by far, more effective in these species than in most other mammals. Accommodation is poor in rodents and dogs.
The filtration apparatus is made up of several structural features that function to reabsorb aqueous humor back into the blood. Aqueous is secreted into the posterior chamber, passes through the pupil into the anterior chamber, and is drained laterally though the TM situated within the iridocorneal angle (ICA) (Figure 22a.6). The balance between secretion and drainage of the aqueous humor determines intraocular pressure (IOP). The ICA is formed by the base of the iris and the corneal-scleral tunic. The ICA extends into the anterior ciliary body forming a recession, the ciliary cleft, or cilioscleral sinus, where the TM is located. The TM is composed of crisscrossing beams of collagen and elastin that are covered by a unique population of endothelial-like trabecular cells. The TM is subdivided into three distinct layers: corneoscleral meshwork (CSM), uveoscleral meshwork, and juxtacanalicular or cribriform meshwork (JCM). There are two major pathways of aqueous drainage: (1) the classic pathway and (2) the alternative pathway. For the classic pathway, aqueous flows through the JCM, collecting ducts [Schlemm’s canal, angular aqueous sinus, or angular aqueous plexus (AAP)], intrascleral channels, and exits the eye though the episcleral and conjunctival veins. In primates, rats, and mice, aqueous flows into Schlemm’s canal, a continuous circumferential duct lined by endothelium. Schlemm’s canal is absent in dogs, cats, and rabbits, which instead have intermittent drainage canals called the AAP that connects the CSM to the intrascleral venous plexus (Figure 22a.7). For the alternative pathway, aqueous flows into a potential space posterior to the TM (i.e., into the anterior face of the ciliary muscle and suprachoroidal space) just inside the sclera and is resorbed by vessels located within the choroid or sclera.
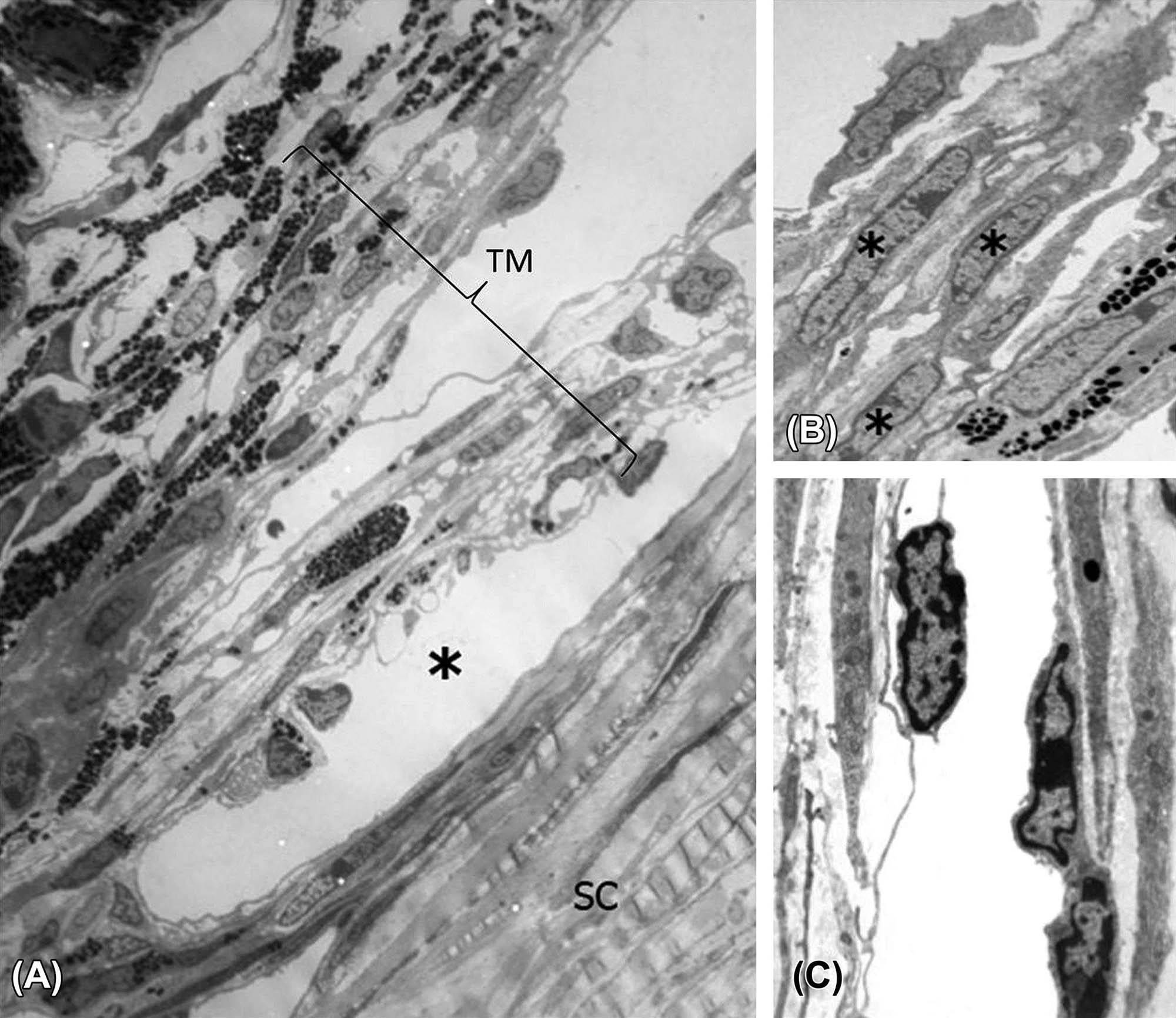
(A) In the mouse, the TM, Schlemm’s canal (*), and sclera (SC) may be shown to be closely related by transmission electron microscopy (TEM). (B) Trabecular beams composed of trabecular endothelial cells (*) surrounding a core of collagen fibers and small amounts of elastic fibers. (C) Detail of Schlemm’s canal with vascular endothelial cells lining the lumen (central clear space). (A) TEM, ×2250; (B) TEM, ×3300; (C) TEM, ×7100. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.15, p. 2139, with permission.
The choroid is the posterior portion of the uvea, a layer of blood vessels and connective tissue situated between the sclera and the retina. The main functions of the choroid are to deliver oxygen and nutrients to the high-metabolizing cells located in the outer retina, provide a conduit for vessels traveling to other parts of the eye, and absorb excessive light radiation, thereby reducing the amount of light reflection back to the retina. The choroid supplies oxygen and nutrients to the retina primarily through the choriocapillaris, an extensive interconnected capillary network located posterior to the retinal pigmented epithelial (RPE) cells (Figure 22a.6F). The choriocapillaris is separated from the RPE layer by Bruch’s membrane, a structure formed by five layers: the RPE basal lamina, an inner collagenous zone, a middle elastic layer, outer collagenous zone, and the basement membrane of the endothelial cells of the choriocapillaris (Figure 22a.8). Bruch’s membrane selectively filters the passage of macromolecules between the retina and choriocapillaris. Vessels of the choriocapillaris have a fenestrated endothelium through which macromolecules leak into the extracellular space of the choroid; this vascular network has the highest perfusion rate of any capillary bed in the body, which is reflective of the high metabolic demands of the ocular tissues. In humans, Bruch’s membrane is subject to a variety of senescent changes representing a significant cause of age-related visual defects, including age-related macular degeneration (AMD).
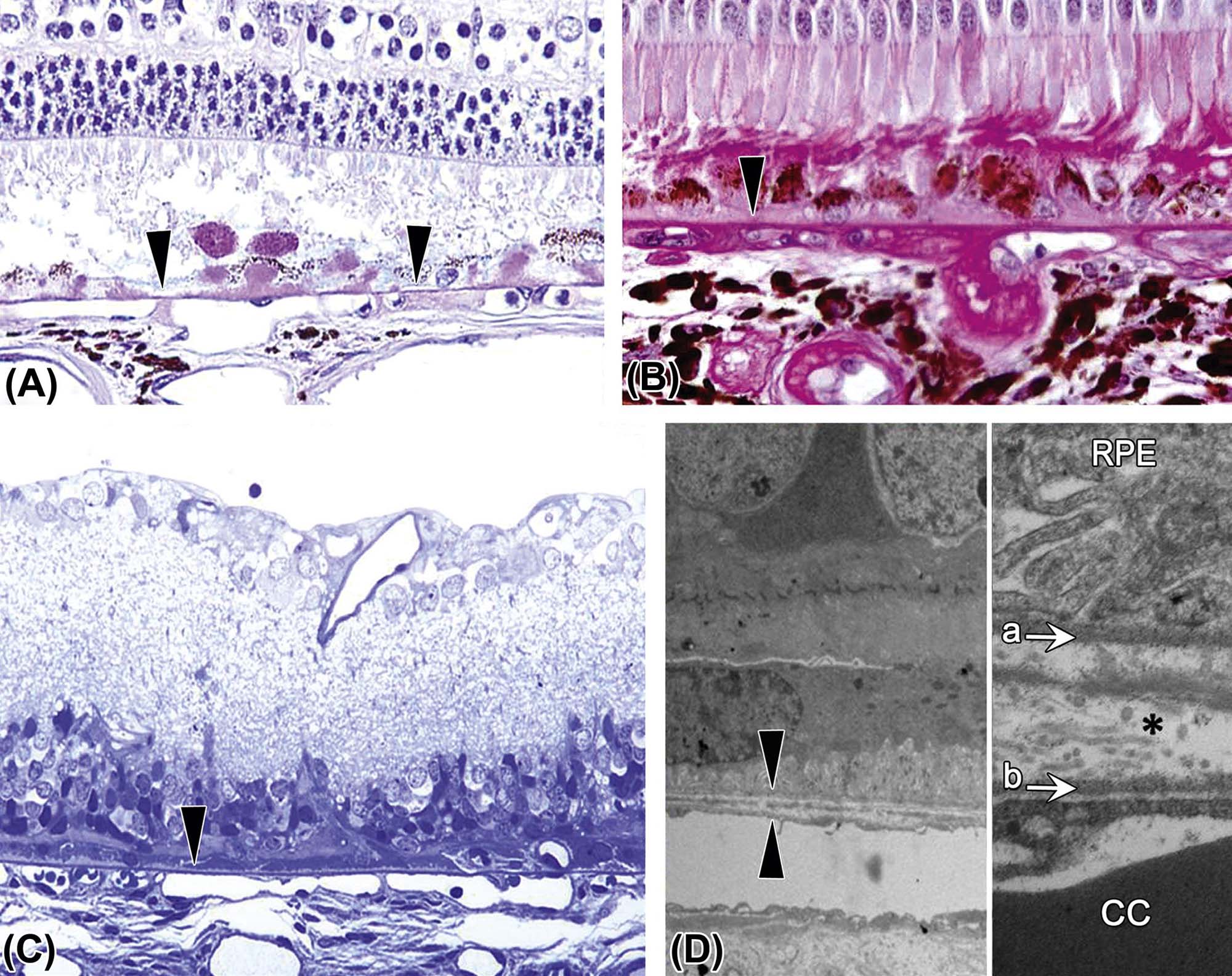
This structure lies between the retinal pigmented epithelium (RPE) and uveal capillary bed (choriocapillaris). (A) Image of the outer retina from a dog demonstrating the location and uniform thickness of the normal Bruch’s membrane (arrowheads). (B) Bruch’s membrane (arrowhead) from a nonhuman primate with hypertension and diabetes, demonstrating irregular thickening. (C) Bruch’s membrane from a rat with outer retinal atrophy, showing an intact Bruch’s membrane (arrowhead) in spite of the marked outer retinal atrophy. (D) Transmission electron micrograph (TEM) from a rat showing the normal ultrastructural appearance of Bruch’s membrane (between the two arrowheads). (E) At higher magnification, Bruch’s membrane consists of an inner layer (a), formed by the basal lamina of RPE cells, and an outer layer (b), formed by the basal lamina of the choriocapillaris (cc). Between these two basal laminae the membrane contains delicate layers of collagen and elastic tissue (*). (A) Periodic acid-Schiff (PAS), ×200; (B) PAS, ×400; (C) Toluidine blue plastic section, ×200; (D) TEM, ×1025; (E) TEM, ×3100. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.14, p. 2138, with permission.
Most domestic species, including the dog and cat, have a tapetum lucidum, a layer of reflective tissue interspersed between the choriocapillaris and the mid-sized vessels. The tapetum is composed of a multilayered complex of polyhedral cells (iridocytes) that contain cytosplasmic light-reflective crystals. In the dog, these are composed of zinc cysteine (Figure 22a.9). The tapetum is thought to enhance vision under scotopic (dim light) conditions by reflecting light that has passed through the retina back to the photoreceptors, increasing their activation. The organelles and crystal composition of these cells varies across species, and may have drug-binding characteristics that are also species variable.
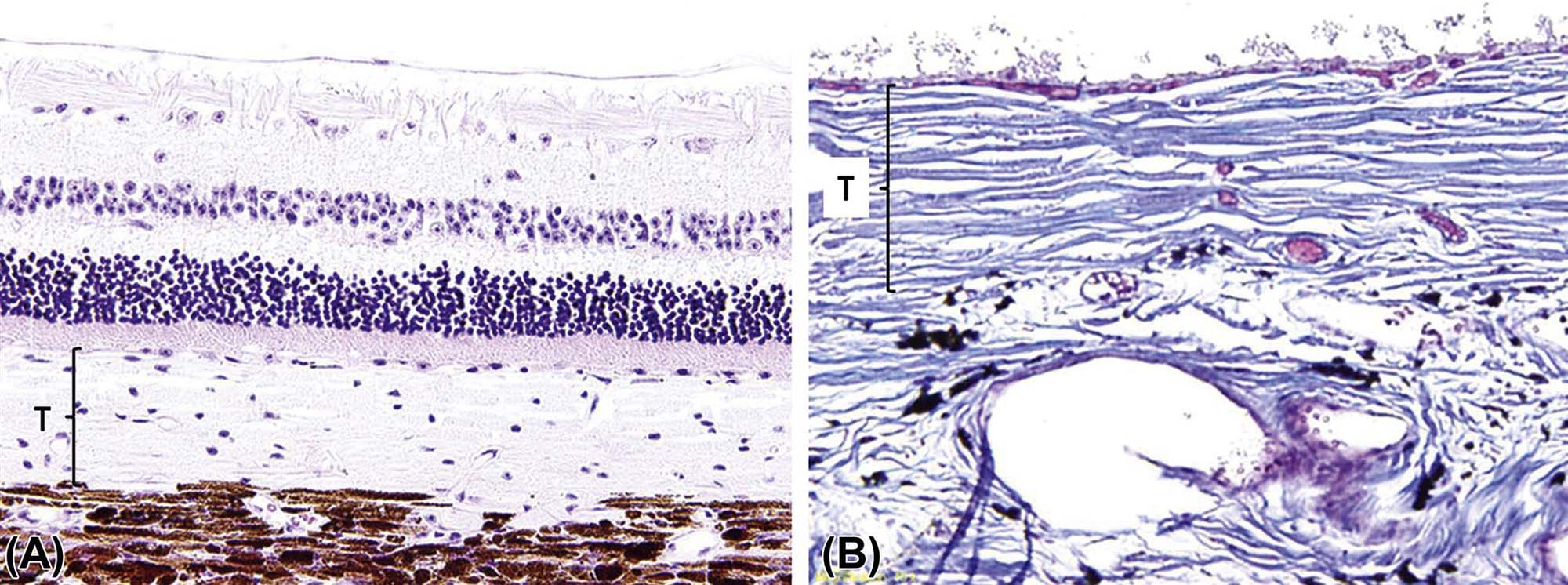
(A) Cellular tapetum (T) typical of a carnivore, such as a dog or cat. (B) Fibrous tapetum (T) from a goat, which is made up of collagen. (A) H&E, ×100; (B) Masson’s trichrome, ×200. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.27, p. 2127, with permission.
Lens
The lens is a transparent, exquisitely organized tissue composed of highly specialized cells (lens fibers) that plays an important role in the ocular optical system by refracting and focusing the light on the plane of the retina. It is suspended in the aqueous humor of the posterior chamber and held in place by the zonular fibers (zonules) and the anterior face of the vitreous body. Structurally the lens is composed of three parts: the lens capsule, the lens epithelium, and the lens fibers. The lens capsule is a thick and elastic basement membrane composed of collagen type IV and sulfated glycosaminoglycans. It is produced by the lens epithelium and completely envelops the lens. The anterior aspect of the capsule is characteristically thicker than the posterior portion. The lens epithelium is composed of simple cuboidal cells that are only present under the anterior and equatorial lens capsule. These cells become columnar and migrate toward the lens equator where they elongate, lose their nuclei, and transform to the relatively homogenous lens fibers that comprise the majority of the lens (Figure 22a.10). As the lens fibers elongate, they expand to become hexagonal structures up to 4×7 µm in cross-section and up to 12 mm in length, extending from the anterior to the posterior pole of the lens.
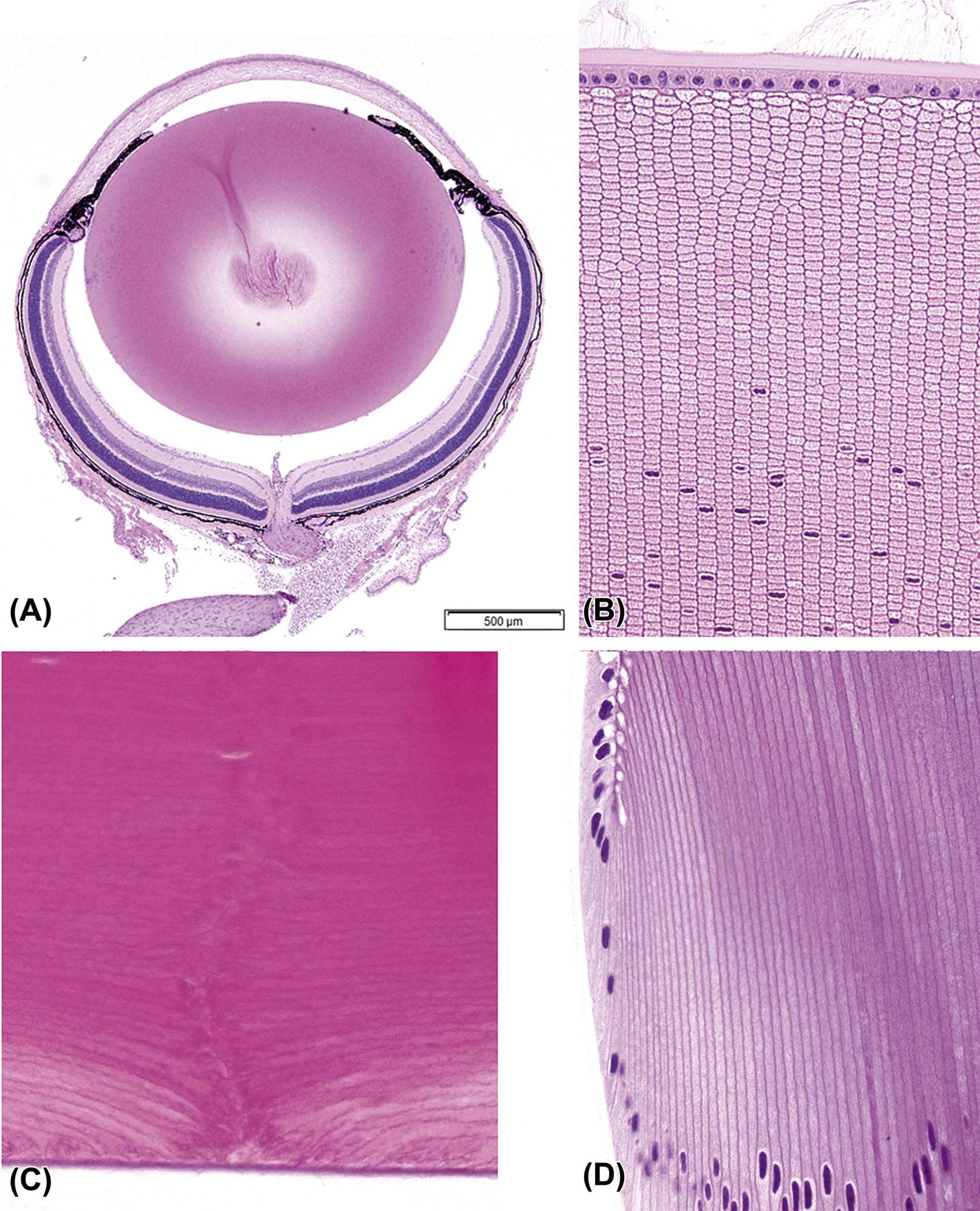
(A) Low-magnification image of an adult mouse eye showing the large relative size of the lens compared to other components of the globe. (B) The anterior pole of a mouse lens fixed with Davidson’s fixative showing individual profiles of well-differentiated lens fibers. (C) The posterior pole of the lens from an adult dog showing the posterior suture (curved seam in the middle where fibers meet). (D) The equator of a rabbit lens showing the nuclear bow (bottom). (A) H&E, ×40; (B) H&E, ×400; (C) H&E, ×600; (D) H&E, ×600. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.16, p. 2144, with permission.
Vitreous
The vitreous body is an optically clear, viscoelastic, gel-like extracellular matrix that fills the vitreous cavity, the space that spans the distance from the posterior pole of the lens to the inner aspect of the retina. More than 95% of the vitreous gel weight is water, while the remaining balance is comprised of the structural components hyaluronon, heterotypic fibrils of collagens type II, V/XI, and IX; fibronectin; fibrillin; and opticin as well as a small population of resident macrophages (hyalocytes). The anterior face of the vitreous closest to the lens has a more robust collagen structure. The vitreous harbors matrix attachment points to the retinal inner limiting membrane, which help maintain the retina in place. Average vitreous volumes vary by species: human, 4.5 mL; dog, 2.9 mL; cynomolgus macaque, 2.2 mL; rabbit, 1.6 mL; rat, 0.03 mL (30 μL); and mouse, 0.01 mL (10 μL).
Retina
The retina is a highly differentiated and complex multilayered neural tissue derived from an outward extension of the rostral neural tube. Vertebrate vision is initiated through the transduction of light energy to neural impulses by the retinal photoreceptors. The retina can be roughly divided into an inner and outer neural retina. The outer neural layer is supported by the retinal pigmented epithelium (RPE). The retina and RPE are not physically attached to one another, but are separated by a potential space. The outer neural retina, particularly the photoreceptor cells, consumes oxygen and nutrients at a higher rate than any other tissue in the body. For this reason, the choroidal blood supply to the RPE is designed to deliver oxygenated blood and remove metabolic wastes for the retina through high-volume, fast-flow rates. The metabolic needs of the inner retina are less than that of the outer retina, and are supplied by an endogenous retinal vasculature, which has a more conventional blood flow rate. Mice, rats, dogs, and nonhuman primates all have an extensive intrinsic retinal vascular bed (i.e., a holangiotic pattern) in addition to the choroidal system (Figure 22a.11). In contrast, rabbits have a limited retinal vascular system (i.e., a merangiotic pattern), and most of the retina lacks an endogenous blood supply. Vessels in the rabbit are located internal to the surface of the medullary rays, and are attached to the retinal surface by glial cell processes. The metabolic needs of the rabbit retina are largely met through diffusion from the medullary ray vasculature.
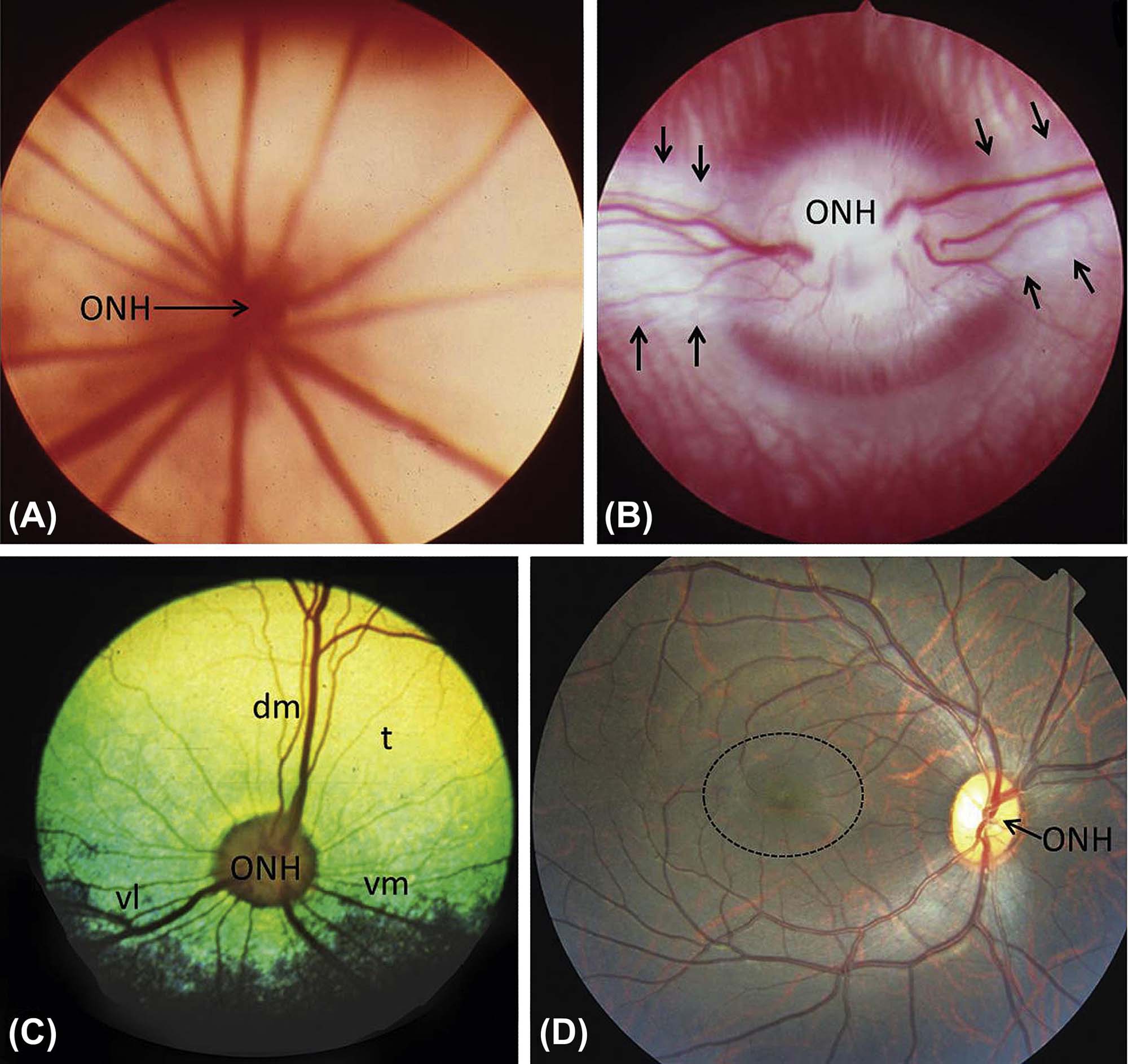
(A) Albino mouse, holangiotic fundus with blood vessels radiating outward widely from the optic nerve head (ONH). (B) Albino rabbit, merangiotic retina, with retinal vessels emanating from the ONH only in discrete zones along the horizontal plane; these zones, or medullary rays (between arrows), represent a thick white band of myelinated nerve fibers that are characteristic of the rabbit. (C) Cat. Holangiotic retina, with retinal vessels departing the ONH in all directions but especially in the dorsomedial (dm), ventrolateral (vl), and ventromedial (vm) directions. The cat has a prominent, highly reflective yellow-green tapetal area (t) dorsally. (D) Nonhuman primate. Holangiotic fundus, with retinal vessels exiting the ONH in all directions but arcing around the relatively avascular—and thus vulnerable—macula (dotted circle). This fundus image is almost indistinguishable from a human fundus. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.22, p. 2160, with permission.
The neural retina is a nine-layered neural tissue responsible for phototransduction, and the signal processing across a three-neuron network that ultimately ends in the transmission of nerve impulses to the brain by the retinal ganglion cells. The layers of the neural retina are listed in the following order, from the inner to the outer layer of the retina (Figure 22a.12).
1. Inner limiting lamina (ILL). A thin, transparent basement membrane, synthesized by the basal foot processes of the Müller cells (retinal glia). The ILL separates the retina and the vitreous body.
2. Nerve fiber layer. Axons from the retinal ganglion cells, interspersed with astrocytes and other glial cells, form the nerve fiber layer that course across the surface of the retina to converge at the optic disc, forming the optic nerve.
3. Retinal ganglion cell layer. Ganglion cells are “third order” neurons that collect integrated signals from interneurons of the inner nuclear layer (INL) and transmit nerve impulses via the optic nerve to the brain. Astrocytes are also present in this layer.
4. Inner plexiform layer (IPL). Synapses between retinal ganglion cells and the interneurons of the INL (bipolar, amacrine, and interplexiform cells) are formed in the IPL.
5. Inner nuclear layer (INL). Nuclei of interneurons (“second order” neurons: bipolar, amacrine, horizontal, and interplexiform cells) and Müller cells (retinal glia) are housed in the INL.
6. Outer plexiform layer (OPL). Houses the synapses between the photoreceptor cells and the interneurons of the INL. Horizontal cells form connections between groups of rods and cones through lateral synapses. Rod spherules and cone pedicles also reside in the OPL.
7. Outer nuclear layer (ONL). Houses the cell bodies and nuclei of the rod and cone photoreceptors. Rod cell nuclei are distributed throughout the layer, while cone nuclei form a row inner to the external limiting membrane. Photoreceptor axons extend into the OPL, while dendrites form the photoreceptor segments.
8. Outer limiting membrane (OLM). Visualized as a line on H&E, the OLM reflects the presence of an anastomosing network of tight junctions formed between photoreceptor cells and Müller cells.
9. Photoreceptor Segments. Photoreceptor dendrites form the inner and outer segments of the retina. The portion of the dendrite residing in the inner segment has a high content of mitochondria, Golgi apparatuses, and rough endoplasmic reticulum, all of which are necessary for high-efficiency protein synthesis. Phototransduction occurs in the outer segment, which houses the discs of the rods and cones.
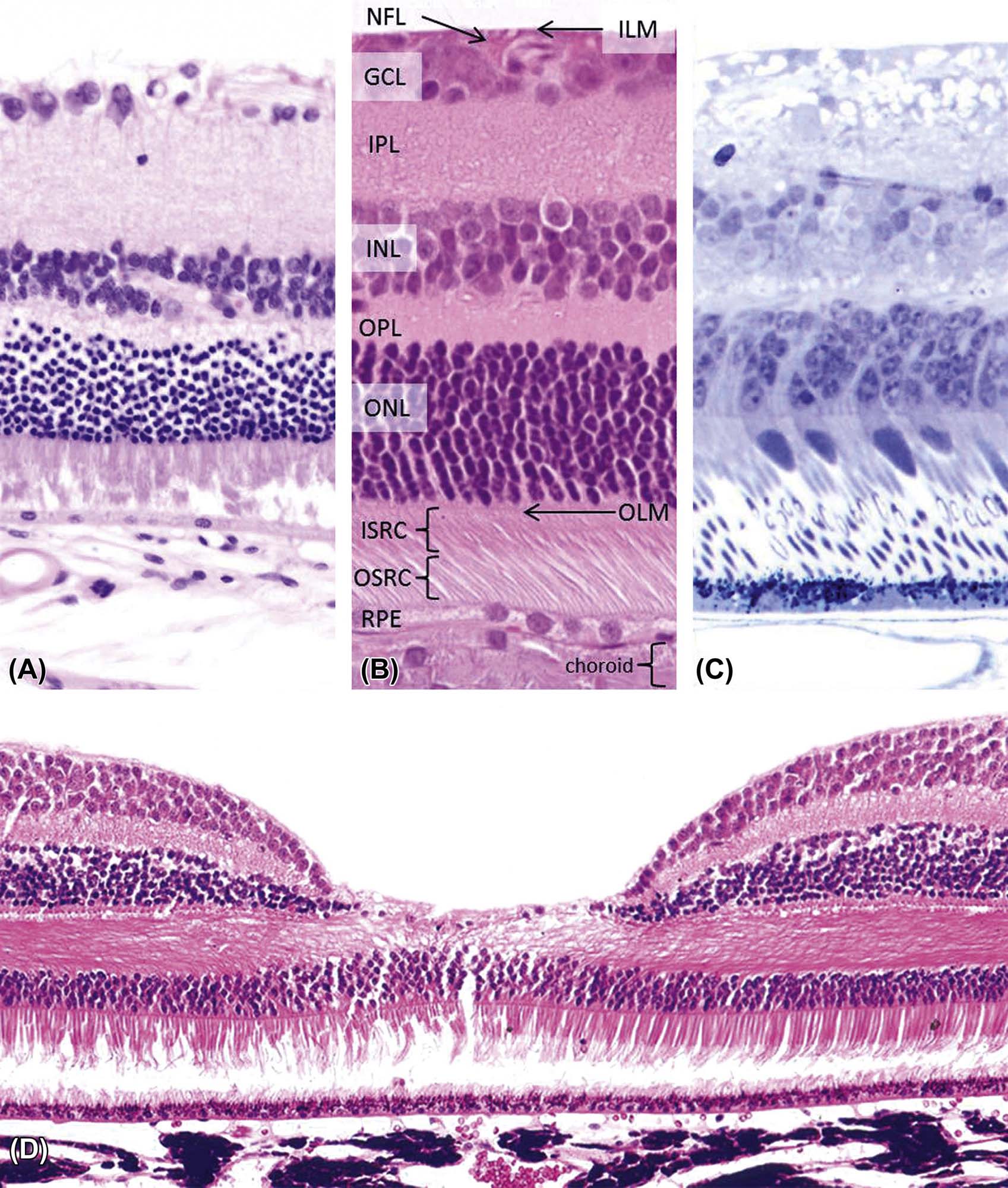
(A) Normal retina from an albino mouse. (B) Area centralis from a normal cat retina, with the retinal layers labeled from outside (bottom) to inside (upper). (C) Plastic section of the retina from a nonhuman primate. (D) Image of the fovea from a nonhuman primate retina. This site contains a heightened number of cone photoreceptors, and thus is critical for proper perception of color and visual acuity in bright light. (A,B) H&E, ×400; (C) Toluidine blue, ×400; (D) H&E, ×200. Abbreviations (listed from top): ILM, inner limiting membrane; NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; OLM, outer limiting membrane; ISRC, inner segments of rods and cones; OSRC, outer segments of rods and cones. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.20, p. 2154, with permission.
While the circuitry and function of the retina is remarkably conserved in vertebrates, marked morphological variation in the retina does exist for among species. Perhaps the most important differences to be considered in toxicologic studies are the vascular pattern (described earlier) and distinctive distribution of photoreceptors (rods and cones). As a rule, the cones (which are specialized in the discrimination of color and detail) are concentrated in the central retina, while the rods [adapted for performance in dim light (i.e., for low resolution)] are more common at the peripheral retina. Most primates, including humans, possess a fovea (also termed the fovea centralis), which is a small circular region characterized by a higher concentration of cones. The fovea is located in the temporal retina near the optic nerve and is responsible for sharp vision. The fovea in primates in turn is surrounded by the macula, a larger retinal region that exhibits an increased ganglion cell density. In contrast, other laboratory animal species have an area centralis, a specialized region that has both more cones and greater retinal ganglion cell density. With the exception of rabbits, the area centralis often is located in the central retina temporal to the optic nerves. Rabbits possess a visual streak, which is a linear, horizontal band (2–3 mm wide) of increased cone and ganglion cell density. This visual streak is located ~3 mm ventral to the optic disc, and runs parallel and below the ventral margin of the medullary ray.
Retinal Pigmented Epithelium
The RPE is derived from neural tube ectoderm and is analogous to ependymal cells in form and function. As the optic vesicle folds on itself to form the optic cup, the RPE remains as a single cell layer in direct contact with the outer cells of the neural retina, which eventually become the photoreceptor cells. The photoreceptor cells and the adjacent RPE function as a unit, and together are responsible for phototransduction.
The RPE cells are smooth and hexagonal in shape when viewed from the inner surface. In histologic sections, the RPE cells consist of an outer nonpigmented basal region with an oval nucleus and an inner, pigmented portion, which extends as a series of straight villous processes between the photoreceptor outer segments. The basal surface of the RPE cells is characterized by extensive infolding. The basal lamina of the RPE cell forms the innermost layer of the five-layered Bruch’s membrane, which separates the retina from its rich vascular supply in the choriocapillaris. There is an anastomosing network of tight junctions near the apex of the RPE cell layer that forms the outer blood–retinal barrier, which is similar in structure and function to the blood–brain barrier (Figure 22a.8). The melanin pigment in the RPE protects the outer retina from excessive light reflection and glare, and thus from unrestrained oxidative damage. The RPE also secretes the interphotoreceptor matrix (IPM), a specialized extracellular matrix containing hyaluronan, proteoglycans, glycosaminoglycans, matrix metalloproteases, and growth and immunosuppressive factors that mediates key interactions between the photoreceptors and RPE including adhesion, phagocytosis, outer segment stability, nutrient exchange, development, and vitamin A trafficking in the visual cycle. The IPM also helps prevent vascular proliferation in the surrounding tissues.
Optic Nerve
The term “optic nerve” is a misnomer since this structure actually is a central nervous system (CNS) tract. The optic nerve is composed primarily of the axons from retina ganglion cells (RGC), which extend from the inner retina, coursing posterior, and centripetally within the inner nerve fiber layer to converge into bundles at the optic nerve head (also known as the optic disc) and form the nerve. The position of the optic disc demonstrates some species variation. In dogs, primates, and rodents, it tends to be positioned in the equatorial region, in the rabbit, and in the dorsal posterior pole. The central area of the optic disc is depressed and is supported by a thickening of the retinal inner limiting membrane (called the supporting meniscus of Kuhnt). Before exiting the eye, the RGC axons are arranged into bundles surrounded by retinal astrocytes. As the axon bundles exit the eye posterior to the choroid, they transverse an open meshwork of horizontally oriented collagenous beams or plates continuous with the sclera (lamina cribrosa). The lamina cribrosa (LC) contains elastin and provides structural support for the nerve. In glaucoma, physical distortion of the globe resulting from elevated IOP causes outward bowing of the LC; the physical distortion and misalignment of the laminar plates results in axon compression, atrophy, and subsequent ganglion cell death. The composition of the LC is similar across species, but there are structural differences. Nonhuman primates, pigs, dogs, cats, and rabbits have a robust LC comparable to humans; in rats, single bundles of collagen form the trabeculae, resulting in a delicate LC (Figure 22a.13). Mice do not have a distinct LC. The axons of the optic nerve are myelinated by oligodendrocytes; the extent of myelination varies across species. In dogs, myelination occurs within the optic disc, with variable extension within the peripapillary nerve fiber layer. The rabbit presents a unique arrangement in that it has a medullary ray, a myelinated and vascularized region on either side of the optic nerve. In other species (nonhuman primates, cats, pigs, and rodents), myelination commences at the outer margins of the LC similar to humans. The optic nerve proper is surrounded by extensions of the three meningeal sheaths of the CNS.

(A) Mouse optic nerve, demonstrating the absence of the lamina cribrosa. The presence of pigmented cells within the optic nerve head is a normal variation. (B) Dog optic nerve, highlighting (in blue) the many parallel collagenous beams of the lamina cribrosa. (C) Rabbit optic nerve, showing the thick optic nerve head is composed of a large concentration of myelinated ganglion cell axons that form the retinal medullary ray and the vessels in the retinal surface (merangiotic vasculature). (D) Nonhuman primate optic nerve, resembling that of humans, has a prominent lamina cribrosa (arrows) and a central retinal artery that can be observed as a circular profile on the inner aspect of the optic nerve head. (A,D) H&E, ×12.5; (B) Masson’s trichrome, ×100; (C) H&E, ×200. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.31, p. 2173, with permission.
The neural signals initially processed by the retina are transmitted to the optical centers of the brain for further integration. Impulses arising in the retina travel via the axons of the ganglion cells through the optic nerves, where they undergo a variable degree of crossing (decussation) before entering the optic tract. Axon crossing is an adaptive feature that allows the brain optical centers to view the same hemispheric visual field from both eyes. Generally, fibers from the temporal retina project to the ipsilateral hemisphere while fibers from the nasal retina cross to the contralateral side. The amount of decussation, however, varies among species. The percentage of axonal crossover is approximately 50% in humans and most primates, 25% in dogs, 33% in cats, and up to 99% in rodents and rabbits. After leaving the optic chiasm and entering the optic tract, signals from the retina reach the lateral geniculate nucleus (LGN), a relay center in the thalamus that exerts dynamic control over the amount and nature of information that is transmitted to the visual processing centers of the brain. Primates and carnivores have a laminated dorsal LGN due to the large number of axons arriving from the contralateral retina, while rodents and rabbits (which have almost no decussation) do not exhibit LGN lamination. Axons from the LGN neurons form the optic radiations that relay visual signals to their final destination in the visual (occipital) cortex, where impulses will be integrated to produce images. In most mammals, the optic radiations also contain reciprocal fiber tracts of axons from neurons in the visual cortex that descend to the LGN and rostral (superior) colliculus (a midbrain structure, often termed the “optic tectum,” that helps direct certain visual-related behaviors), thus enabling feedback control of the visual signal processing.
Evaluation of Toxicity
Use of Animal Models
In preclinical drug development, animal test systems are the backbone of hazard identification and risk assessment. While in vitro test systems are being used increasingly to probe relevant questions, none of the current methods for culturing ocular tissues can mimic the whole animal setting in performing these safety assessment functions. When choosing the most appropriate animal test system in an ocular toxicity experiment, many factors need to be considered. The most important of these factors are described here.
The model should be well characterized. The animal species that are commonly used as models in ocular toxicologic pathology are rodents (mouse, rat); rabbits; and larger animals (dog, nonhuman primate). Although there may be specific reasons to choose some other species, the bias for using one of these animal models is founded in the large mass of historic data (with well characterized background changes and expected variations), desirable husbandry characteristics, including knowledge regarding their potential effects on ocular health, and proven and familiar standard operating procedures and handling protocols.
The model should be appropriate for the experimental question. Although small rodents have incalculable value as test models in toxicologic pathology, the small size of the rodent eye and the relatively large size of the lens often preclude the use of such models when certain in-life procedures are a part of the experimental design. Some examples of procedures that are either unachievable or achievable only with great effort and/or risk in rodents are intraocular injections, sampling of aqueous or vitreous, tonometry (measures of IOP), fundoscopy (visual evaluation of the retina), and conventional surgery (e.g., glaucoma filtration procedures). On the other hand, the small eye size in rodents makes it possible to sample the entire eye by making serial or short-interval step sections through the globe.
The model should be anatomically appropriate. The usual species of interest when evaluating the impact of ocular toxicants to the visual system is the human. While eyes of vertebrates share many more similarities than differences regarding structure and function, many experimental hypotheses are most reliably answered using an animal model that best recapitulates the anatomic and functional properties of the human situation. Largely for this reason, nonhuman primates are almost a default favorite as the animal model of choice.
The model should be useful for both acute and chronic studies. It is not efficient to use animal models where aging is an important part of the testing strategy if the animal in question takes 10 years or longer to age. Laboratory rodents achieve advanced age in 2 years, making them useful for chronic toxicity studies.
The model should be cost effective. Small rodents provide obvious cost efficiency, although careful consideration should be given to the likelihood of success if certain protocols and procedures are to be employed.
The model should be appropriate for answering questions about efficacy. Many animal models of disease have been developed to mimic aspects of particular human ophthalmic diseases. These models may be useful in drug testing, particularly when evaluating molecule effectiveness. Some examples are genetically engineered mice models of the distinctive human retinal disease (e.g., Leber’s congenital amaurosis) and spontaneous canine models of dry eye.
Ocular Diagnostic and Functional Tests
Multiple experimental endpoints in toxicity and drug development studies are measured by clinicians or an ophthalmologist rather than a pathologist. For example, nonhuman primate models with induced high IOP treated with IOP-lowering drugs can be monitored rapidly and quantitatively by measuring the IOP antemortem using a tonometer without the need to necropsy valuable animals. Many analytical procedures are available for the clinical examination of eyes in live animals (Figure 22a.14). The ophthalmic diagnostic and functional tests most commonly used in toxicology and drug development are described in Table 22a.2.
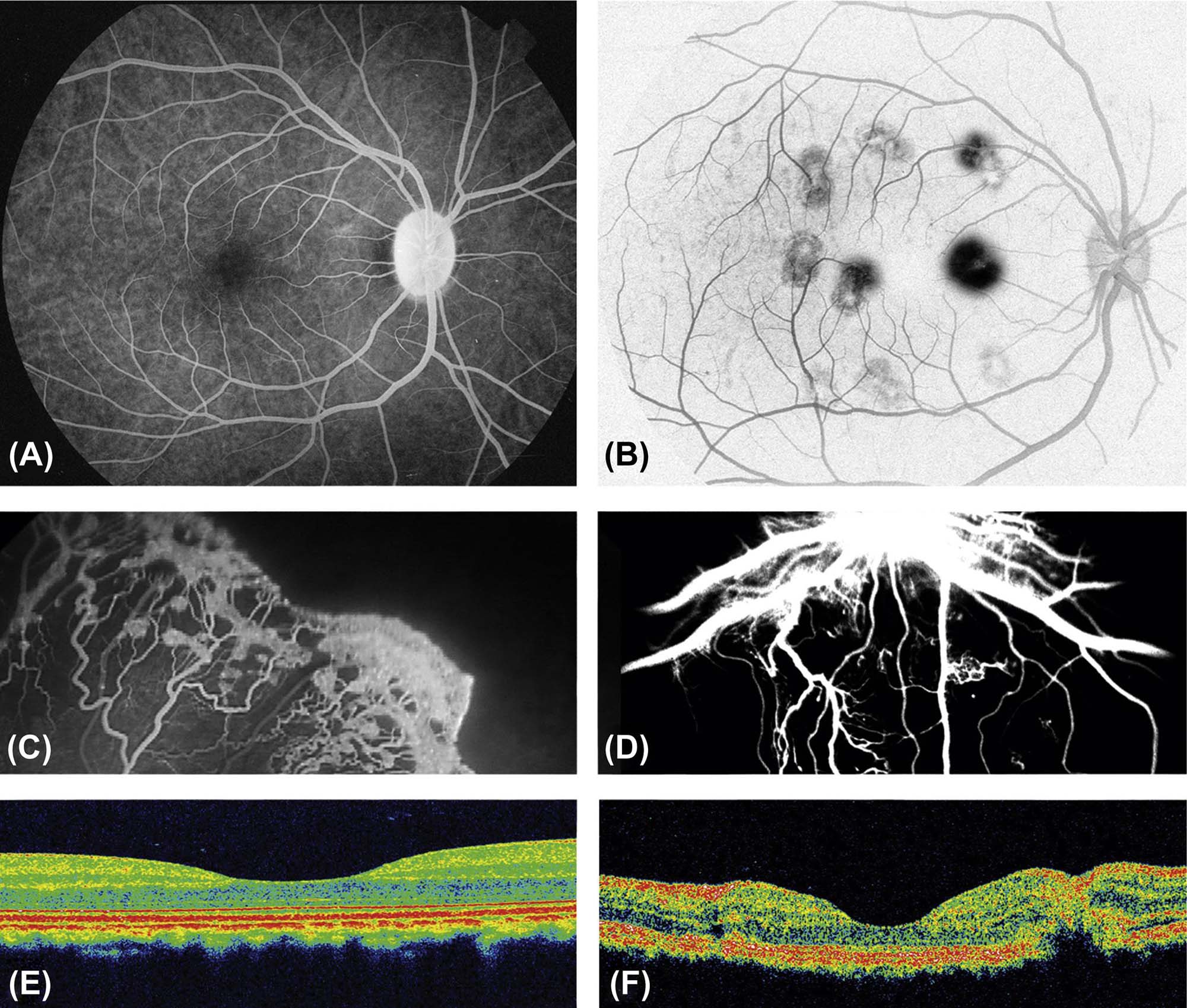
(A–D) Fluorescein angiography of retinal vasculature. (A) Positive image of fluorescein angiography from a normal (control) nonhuman primate. (B) Negative image of fluorescein angiography from a nonhuman primate in which laser burns (dark spots) were used to destroy Bruch’s membrane, causing vascular proliferation and leakage, as a model of age-related macular degeneration (AMD). (C) Fluorescein angiography of the retina of a human infant with vascular proliferation associated with retinopathy of prematurity, demonstrating proliferation of tortuous, newly formed vessels. (D) Fluorescein angiography of retina in a dog model of diabetic retinopathy, showing markedly thickened central vessels and proliferation of tortuous neovascular profiles. (E,F) Optical coherence tomography (OCT), which uses light waves to take high-resolution cross-sectional images of retinal layering. (E) Spectral domain OCT image of the macula from a normal nonhuman primate. The inner surface of the retina is located at the top of the image. The depressed central region represents the fovea centralis. (F) Spectral OCT showing an experimentally induced laser burn leading to retinal distortion (disruption of the normal retinal layering) in a nonhuman primate; again, the depressed central region is the fovea centralis. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figures 53.23 and 53.24, pp. 2161, 2162, with permission.
Table 22a.2
Ophthalmic Diagnostic Tests Commonly Used in Ocular Toxicity Testing
| Test | Measurement | Description |
| Schirmer’s tear test | Tear film aqueous production | Absorbent paper placed in the conjunctival fornix measures the amount of tear produced in a defined period of time |
| Tear film breakup time | Function of mucin and lipid layers of the tear film | Measurement of the time interval following a blink to the occurrence of a break in the tear film |
| Fluorescein staining | Detection of corneal ulcers | Sodium fluorescein dye applied to the corneal surface bonds to exposed stromal allowing detection of epithelial defects |
| Tonometry | Intraocular pressure (IOP) | Indirect measurement of the IOP through a tonometer |
| Pachymetry | Corneal thickness | Corneal thickness measured by a contact ultrasound machine |
| Confocal microscopy | Morphology of the cornea | In vivo, noninvasive microscopic imaging of the corneal epithelium, stroma, Descemet’s membrane, and endothelium |
| Specular microscopy | Morphology of corneal endothelium | In vivo noninvasive imaging of the corneal endothelial cells |
| Gonioscopy | Morphology of the iridocorneal angle (ICA) | Clinical examination of the anterior chamber angle through a goniolens (a special type of contact lens) |
| Ultrasound biomicroscopy | Morphology of the anterior segment of the eye | High-frequency ultrasound is used to image the cornea, ICAs, iris, ciliary body, and lens. Can be used to determine corneal thickness and monitor changes in the ICA. |
| Indirect/direct ophthalmoscopy | Morphology of the posterior segment of the eye | Indirect ophthalmoscope provides binocular, inverted, and reversed wide field aerial image of the posterior segment of the eye. Technique of choice for routine screening. |
| Fluorescein angiography | Vascular integrity of intraocular structures | Intravascular fluorescein dye injection followed by multiple timed images of the iris, choroid, and retina |
| Electroretinography (ERG) | Retinal function | Measurement of the electrical potential generated by the retina when stimulated by light |
| Visual evoked potential | Retinal and optic nerve function | Measurement of the brain’s electrical response to a light stimulus in the eye |
| Optical coherence tomography (OCT) | Morphology of the anterior segment, retina, and optic nerve | High-resolution, noninvasive imaging technique that provides real-time cross-sectional imaging of ocular structure (more commonly retina and optic nerve) at an axial resolution of 2–10 μm |
Morphologic Examination of the Ocular Tissues
Light microscopic examination of the eye requires several factors including: knowledge of the clinical ophthalmic findings, an understanding of comparative ocular anatomy and histology, an awareness of iatrogenic ocular findings and artifacts, an awareness of toxicant-induced changes that may occur in ocular tissues, knowledge of appropriate terminology, and good histologic sections of globes. Of that list, one of the most basic factors, and conversely the one that generates the most enquiries, is the histologic processing of ocular tissues. The basic concepts and procedures for proper ocular tissue fixation and trimming vary from those of other tissues, and so deserve reiteration here.
Tissue Fixation and Handling
Intact globes usually will be fixed by immersion of the entire organ, typically with a portion of the optic nerve. When fixing larger eyes, an intravitreal (IVT) injection of the fixative (0.1–0.3 mL by a 25–27 ga needle) or initially submerging the eye in the fixative for a brief (5–30 minutes) period of time before making a small (5 mm long axis) window in the equatorial sclera are possible (but not necessary) additional steps to insure proper fixation. The excess soft tissues including eyelids, muscles, orbital tissues, and (in rodents) the Harderian glands typically should be removed before fixation. However, there are special circumstances where tissues adjacent to the globe should be kept intact and sampled along with the globe. These might include cases where a surgical field is important (e.g., to define where a glaucoma device was oriented, when analyzing the effects of subconjunctival injections, or to evaluate the invasiveness of a tumors adjacent to the eye). Many options exist for suitable fixation of ocular tissue, each with its own advantages and disadvantages. Regardless of the fixative used, the volume of tissue to be fixed should be kept to a minimum, without sacrificing the integrity of the tissue’s anatomic orientation. In most cases, not less than 10 volumes of fixative should be used for one volume of tissue (e.g., 10 mL of solution per 1 gram of tissue). The commonly used fixatives for ocular tissues destined for microscopic evaluation are described in Table 22a.3.
Table 22a.3
| Fixative solution | Composition | Fixation | Use | Positive characteristics | Negative characteristics | |
| 10% Formalin, neutral buffered (NBF) | 24–96ha | LM, IHC | Readily available, excellent tissue penetration, reasonable histologic detail preservation, preservation of tissue color, gold standard for IHC | Less than adequate tissue rigidity. Can lead to folding and artifactual retinal detachments | ||
| Davidson’s fixative | LM | Good histologic preservation of the retina and lens, excellent tissue penetration (better with modified Davidson’s), good tissue rigidity | Opaque white discoloration of tissues, (impairs gross identification of landmarks and lesions). Over-fixation can cause artifacts: vacuolation of corneal epithelium and endothelium, oblong spaces in the corneal stroma, shattering of lens fibers and capsule, indistinct photoreceptor segments | |||
| Modified Davidson’s fixatives | ||||||
| Bouin’s fixative | LM | Good histologic preservation of the retina, decalcifies tissues, excellent tissue rigidity | Opaque yellow discoloration of tissue, corrosive to metal, explosive potential | |||
| Glutaraldehyde | EM | Good histologic preservation, preservation of tissue color | Very poor tissue penetration, tissues fixed should be <1 mm3 | |||
| Karnovsky’s fixative | 12–24 h (sg)b24–48 h (lg)b | EM | Good histologic preservation, preservation of tissue color, better tissue penetration than glutaraldehyde | Still relatively low tissue penetration, tissues fixed should be <1 mm3 | ||
| Zenker’s | 12–24 h | LM | Excellent fixation of nuclear chromatin, connective tissue fibers and some cytoplasmic features | Toxic, contains mercury |

Abbreviations: LM, light microscopy; IHC, immunohistochemistry; EM, electron microscopy; sg, small globes; lg, large globes.
aIf IHC is anticipated, fixation time should be minimized to 24–48 hours.
bAfter initial fixation, transfer to 10% NBF for 24 hours to increase ocular tissue rigidity.
Tissue preparation by frozen sectioning on a cryostat is preferred for processing the eye when immunohistochemistry (IHC) using fixed tissue is not possible due to inadequate preservation of labile antigens or when preservation of enzymatic activity is need for histochemistry. Tissues may be frozen without fixation, or more commonly they may be fixed for a brief period (typically 30 minutes or less) in ice-cold neutral buffered 10% formalin to improve cell morphology. Specimens then should be transferred to 20% sucrose in Dulbecco’s phosphate-buffered saline for 15 minutes and then dipped three times into a 1:1 mixture of 20% sucrose:tissue freezing medium. Next, the sample should be oriented in a cryomold surrounded by 100% freezing medium, frozen by immersion in liquid nitrogen-cooled isopentane, and stored at −80°C until sectioning.
Trimming the Eye
The default orientation for evaluating eyes of animal species other than nonhuman primates is the vertical median (midsagittal) plane, which samples both the tapetal [typically dorsal (superior)] and nontapetal [typically ventral (inferior)] portions of the retina. Exceptions are made in order to sample more effectively known or suspected lesions in the temporal or nasal aspects of the globe. The horizontal plane is the preferred default orientation in viewing eyes of nonhuman primates because the fovea is lateral (temporal) to the optic nerve head. In the extremely small eyes of rodents, trimming is to be avoided. Instead, the whole globe is submitted and oriented at the time of paraffin embedding. Rodent eyes are positioned in the vertical plane, and a central section through the pupil and the optic nerve is obtained by serial sectioning. For best results over time, select a standard technique for trimming globes of each species. This strategy will reliably provide sections with a single orientation and similar morphological features; such standardization will speed the pathologist’s histopathological analysis. Generally, the goal should be to obtain an ocular section that includes the optic nerve and passes through the pupil. Below are several techniques that are helpful in obtaining reproducible sections among animals and across studies.
For large globes, make the initial trimming cut near the optic nerve. The lens is sectioned with a quick forceful cut while supporting the globe on the cutting board. A major advantage to this approach is that suitable sections for analysis may be obtained after facing only a few sections from the embedded tissue (just sufficient to reach the optic nerve). Another advantage is that the globe is cut close to the anatomic center, which is appealing for the purpose of macroscopic photography. The disadvantage is that the lens may be misplaced or damaged during trimming. To maximize the chances of preserving good lens morphology when trimming, make an initial off-center cut that avoids the lens. Subsequent step-sectioning once the tissue has been embedded in paraffin can be used to achieve a central cut through the lens and optic nerve. An advantage of this latter approach is that the lens is untouched during trimming and thus is less likely to be artifactually dislodged.
Ensure the inclusion of lesions in the plane of section. The ideal strategy is to have the clinician indicate the location of a focal lesion in the globe with a careful clinical description and/or drawings or photographs, as well as some indication directly on the tissue with a suture or another tissue-marking technique (e.g., insoluble ink applied to the outer surface). Gentle palpation of the globe often is valuable in detecting large localized lesions before sectioning. The orientation of the initial section can be changed in such a way as to sample the palpable lesion. Transillumination (candling) the globe by holding it in front of a bright light in a darkened room can help to localize an opaque focal lesion. Careful examination of the sectioned globe using a dissecting microscope also facilitates the identification of focal lesions. Additional steps may be indicated to sample a focal lesion. The primary calotte (i.e., the term used for an isolated portion of the globe) might be retrimmed so that only the segment of the globe with the lesion is embedded. The histology technician is then instructed to step-section to a specified depth to sample focal lesions. This added step is sometimes essential to fully characterize discrete lesions.
In Vivo Ocular Irritation Test
The Draize test is an acute ocular toxicity test devised in 1944 to provide a method for assessing the irritation potential of materials that might accidentally come in contact with human eyes, such as household and office products, agricultural or environmental chemicals, and volatile organic compounds. Because of the widespread acceptance of this method, it was later adopted for testing eye care products and drugs designed specifically for topical ophthalmic use prior to marketing. The current Draize method involves the instillation of 10 µL of a test liquid (or 10 mg of a test solid) into the lower conjunctival cul-de-sac, followed by a saline rinse. Observations of various criteria (i.e., corneal opacity and area of corneal involvement, conjunctival hyperemia, chemosis, ocular discharges, and iris abnormalities) are taken at predefined intervals: 1 hour, 24 hours, 48 hours, 72 hours, 7 days, and 21 days after administration. The test is usually performed in rabbits due to their large eyes, well-described anatomy, ease of handling, relatively low cost, and ready availability. A slit-lamp examination has been added to allow better assessment of corneal lesions, and topical fluorescein is applied routinely to reveal any cornel ulceration. Optical or ultrasonic pachymetry now is implemented to measure the extent of corneal thickening. Although the Draize test is still the official model for eye irritation and toxicology studies worldwide, it has suffered major criticism in recent years due to the lack of objective quantification within its grading system, its unreliability in predicting chronic toxic reactions, and marked opposition by animal welfare groups.
In Vitro Ocular Toxicity Tests
Several in vitro assays have been developed in order to circumvent the aforementioned limitations of the Draize test and to minimize the exposure of animals to potentially irritating materials. No single in vitro assay has been validated as a full regulatory replacement for the Draize test.
Key ocular-based platforms may be used for in vitro testing. Isolated rabbit and chicken eyes (removed immediately after death) are placed in a temperature-controlled chamber and perfused with physiological saline. Test substances are applied topically to the cornea and conjunctiva, and the effects are observed over several days with a slit-lamp biomicroscope. Isolated corneal preparations are typically limited to assessments of corneal irritation, and are done using isolated bovine corneas collected from slaughter houses. The corneas are mounted in specially designed holders, incubated in Eagle’s minimum essential medium at 32°C, and exposed on the external surface to the test article for 1 hour. Subsequently, changes in the opacity and permeability of the corneal tissue are measured by an opacitometer and by spectrophotometry, respectively. The corneal cup method uses isolated fresh bovine corneas to form a “cup” that can be used to assess the local production of leukocyte chemotactic factors. The corneal fluorescence probe method measures the degree of epithelium permeability using penetrance of a fluorescent probe after application to fresh rabbit corneas. Cultured conjunctival, corneal, and lens epithelial cells are readily available without the need for specifically terminating test animals, and in some instances may be of human origin. Methods for assessing cytotoxicity in cell cultures include measurements of plasminogen activation and the 51Cr-release method.
Alternatives exist to the use of eyes and ocular derivatives for in vitro ocular irritancy testing. The Eytex system is a corneal opacification assay that uses a vegetable protein gel extracted from jack beans (Canavalia ensiformis). It predicts ocular irritation of materials based on alterations induced in its vegetable protein matrix. This method is quantitative and reproducible and presents a high degree of correlation to the Draize test.
Substances that irritate ocular tissues also have the potential to cause irritation in other sensitive nonocular tissues. The chicken egg test (Huhner-Embryonen test) uses embryonated hens' eggs to demonstrate chemical toxicity through embryo lethality, teratogenicity, and systemic toxicity. It is considered a borderline assay falling between an in vivo test and in vitro test. Chicken eggs also provide chorioallantoic membrane (CAM) for the chorioallantoic membrane test. In this method the vascular CAM is exposed to the test article in multiple dilutions, and the tissue is scored for irritation effects such as hyperemia, hemorrhages, and coagulation activity. Mucous membranes from other tissues may be used to assess chemical irritancy. Examples include the rat vaginal mucosa (using prostenoid production as an indicator) and also mouse and rabbit ileum mucosa (using altered contractility as an index).
Response to Injury by Specific Ocular Tissues
Lacrimal System
All of the glands comprising the lacrimal system are apocrine glands, and they respond to injury much like other apocrine glands throughout the body. They are prone to ductular obstruction secondary to inflammation. This often leads to ascending inflammation affecting the gland acini and can result eventually in atrophy. Lymphocytic clusters among acini are a common background lesion in safety studies, especially in rodents. Viruses such as sialodacryoadenitis virus (SDAV), a coronavirus of rats, can directly infect the acinar epithelium in lacrimal glands as well as salivary glands, leading to necrosis, inflammation (intra- and periacinar), and periacinar fibrosis, which ultimately may lead to atrophy of the remaining parenchyma. Chronic infection with SDAV can cause corneal desiccation [keratoconjunctivitis sicca (KCS)], corneal ulceration and uveitis leading to hyphema (blood accumulation in the anterior chamber), intraocular inflammation, formation of fibrovascular membranes over the iris (preiridal fibrovascular membrane), and eventually to obstruction of the aqueous outflow pathways with secondary (unilateral or bilateral) glaucoma.
Squamous metaplasia of the epithelium lining lacrimal gland ducts is occasionally seen. This change is usually idiopathic, but metaplasia associated with vitamin A deficiency is a possible cause. Ionizing irradiation used for cancer therapy can affect the lacrimal glands, leading to acinar atrophy. The exact mechanism for this atrophy is unclear, but the likely explanation is that cumulative gene damage in irradiated stem cells leads to their apoptosis and a reduced capacity to regenerate gland acinar epithelium.
Like other apocrine glands, lacrimal and Harderian glands are capable of neoplastic transformation. These tumors occur in aged rodents (more common in mice than rats or hamsters), dogs, and cats (the last two presenting only with lacrimal tumors since they do not have Harderian glands). The spectrum of neoplasms seen in lacrimal glands is similar to those that develop in other apocrine glands: acinar, ductular, or solid adenomas (benign) or adenocarcinomas (malignant). When the myoepithelial component that surrounds acini is involved, the architecture can form complex tumors that includes both epithelial and spindle cell components (with the spindle cell element ranging from minimal to major); if there is osseous or cartilaginous metaplasia of the myoepithelial component, truly mixed tumors can arise in lacrimal glands. Rarely, the mesenchymal component will be the predominant malignant cell population so that osteosarcoma or chondrosarcoma may arise as a primary tumor in a lacrimal gland.
Eyelids
The response of eyelid tissue to injury is not complicated. The most common reaction is inflammation secondary to infection or trauma. Subsequent fibrosis (scarring) can have a profound effect on the function of the lid by interfering with its motion or conformation. An inwardly turned lid (entropion), usually affecting the lower lid, can result in hairs contacting the cornea. This abnormal interaction causes pain and irritation and may lead to erosion or ulceration of the cornea. In contrast, an outwardly turned lower lid (ectropion) can invite spillage of tears, which can result in "dry eye" (KCS) and secondary infection. Weakening of the dorsal (superior) tarsal muscle associated with a defect in sympathetic innervation, as in Horner’s syndrome, causes ptosis (drooping of the upper eyelid). Blepharospasm, the abnormal contraction or twitching of an eyelid, is a nonspecific response to painful or irritating stimuli. This clinical abnormality can be triggered by many factors, including conjunctival or corneal irritation or even injury or inflammation in the uvea. The identification of blepharospasm is important when clinically accessing the immediate response to topically administered drugs and ocular discomfort. Neoplasms may develop from any of the structures in the eyelid, including the skin. The most commonly reported tumors include papilloma, squamous cell carcinoma, peripheral nerve sheath tumors, melanocytomas/melanomas, and sebaceous and meibomian gland adenomas.
Conjunctiva
The conjunctiva responds to injury like most mucous membranes of the body. The most common diseases of the conjunctiva are inflammatory (conjunctivitis). Most species have viral diseases that affect the conjunctival epithelium, causing epithelial necrosis and ulceration that often lead to secondary bacterial conjunctivitis with suppurative inflammation and tissue destruction. The conjunctiva also is a common site to manifest allergic diseases, presenting as acute eosinophilic inflammation with edema driven by local mast cell infiltration. A varied pattern of inflammatory reactions can be found within the conjunctiva, ranging from dispersed lymphoplasmacytic to granulomatous to perivascular lymphocytic lesions with neutrophilic vasculitis. Infectious agents are hardly ever associated with these inflammatory infiltrates, suggesting that such infiltration results from an immune-mediated reaction of unknown etiology. With chronicity, squamous metaplasia of the conjunctival epithelium can occur. If generalized, this metaplastic change can reduce the numbers of mucus-producing goblet cells leading to a vicious cycle of tear film dysfunction and dry eye that can eventually progress to permanent KCS. The conjunctival surface is exposed to ultraviolet (UV) radiation and thus also can react with dysplastic changes and neoplastic transformation. Tumors typically exhibit squamous cell differentiation, but dogs also may develop vascular endothelial neoplasia. In general, these conjunctival changes are not reported to reflect prior exposure to toxicants.
Cornea
Atrophy of the corneal epithelium is characterized by a decrease in the numbers of corneal epithelial cells and/or a thinning of the epithelial layers. Corneal epithelial atrophy can be secondary to stromal changes, especially edema or a deficiency of the limbal stem cells that replenish the corneal epithelium. The corneal epithelium does not have a native stem cell population, so there is a limited capacity of the epithelium to proliferate in response to injury. After ulceration, the existing epithelial cells disconnect from each other and migrate to cover the wound (a process termed “restitution”). However, newly differentiated corneal epithelial cells are recruited from a stem cell population that exists only in the conjunctiva at the limbus. If this population of cells is destroyed (e.g., by herpes virus, chemical burns, necrotizing inflammation, or some other disease process), the corneal epithelium is at grave risk of permanent deficiency. Conjunctival epithelium will regenerate over the corneal surface, but this scar will not achieve the same optical clarity as normal corneal epithelium since it lacks the differentiation state needed to maintain the proper tissue organization to permit light passage at the proper angle. In addition, the scar will remain thin and at risk of renewed ulceration or even a full-thickness corneal rupture. Limbal stem cell transplantation from the healthy eye to the diseased eye has made it possible for affected humans to regain the function of unilateral eye damage.
Corneal epithelial hyperplasia, keratinization, and melanin pigmentation are nonspecific responses of the corneal epithelium to chronic injury. These lesions are seen in the late stages of chronic keratitis, corneal surface exposure (exposure keratopathy), and dry eye, and are characterized by thickening and keratinization of the epithelium, along with rete ridge formation and migration of melanocytes leading to pigmentation of the basal layers (Figure 22a.15).
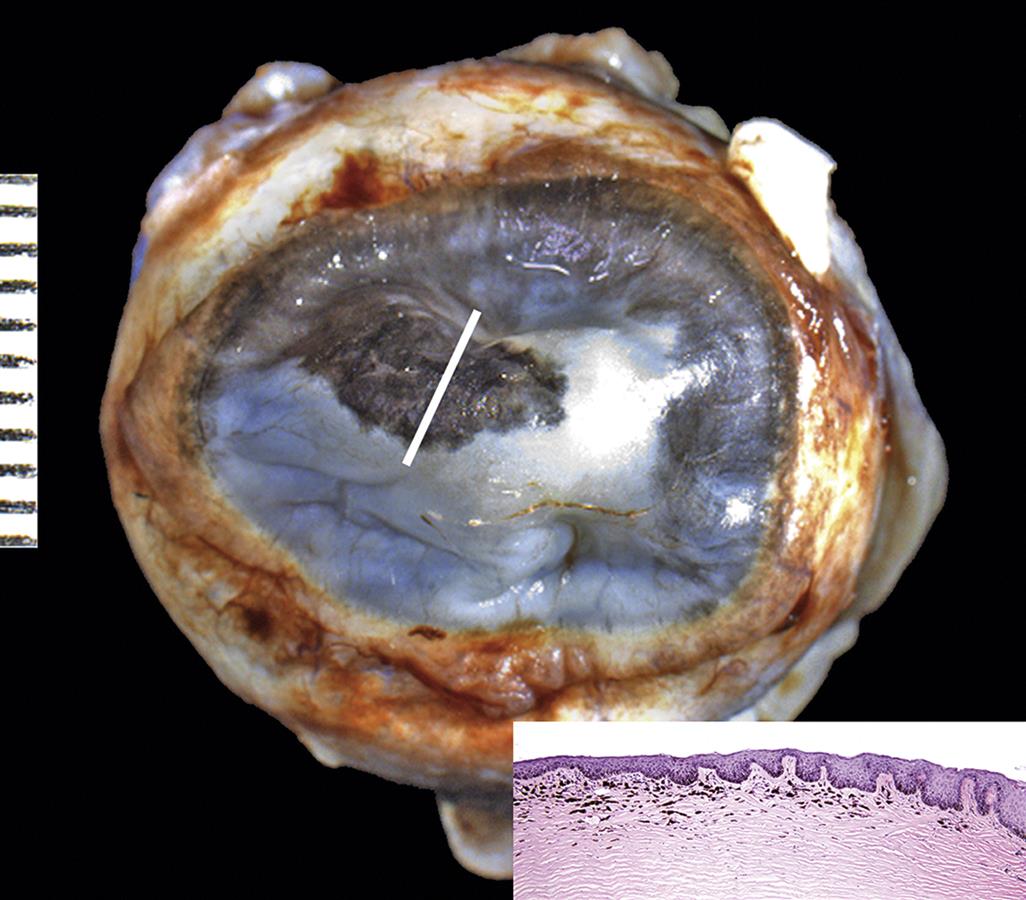
Gross image of a formalin-fixed dog cornea exhibiting marked opacity and dark pigmentation as reactive changes to a chronic inflammatory process. Inset: Histological section corresponding to the region of the cornea marked with a white line. Note the marked corneal hyperplasia and hyperkeratosis and pigmentation of the basal layers of the corneal epithelium and superficial corneal stroma. Scale, 1 interval=1 mm. Inset: H&E, ×100. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.8, p. 2120, with permission.
Corneal epithelial ulceration and erosions can be associated with trauma, reduced tear production resulting in dry eyes, exposure to topical or gaseous caustic agents, and corneal mineralization. Abrasions may be incidental in rodents, often related to caging conditions or environmental irritants. Direct trauma with epithelial ulceration can provide a portal for bacterial colonization, and an edematous cornea secondary to damage at the corneal endothelium makes the risk of infection and progressive stromal damage all the more serious. Ulcerations are usually associated with corneal stromal vascularization and inflammation (keratitis).
Corneal stromal lipidosis. Metabolic disorders, tumors, or hemorrhage can lead to deposition of lipid or the formation of lipid granulomas (often with high cholesterol content) in the corneal stroma. Similar deposits of iron (usually after hemorrhage) and calcium (mineralization) in the superficial stroma may develop if the tear film is allowed to dry (Figure 22a.16).
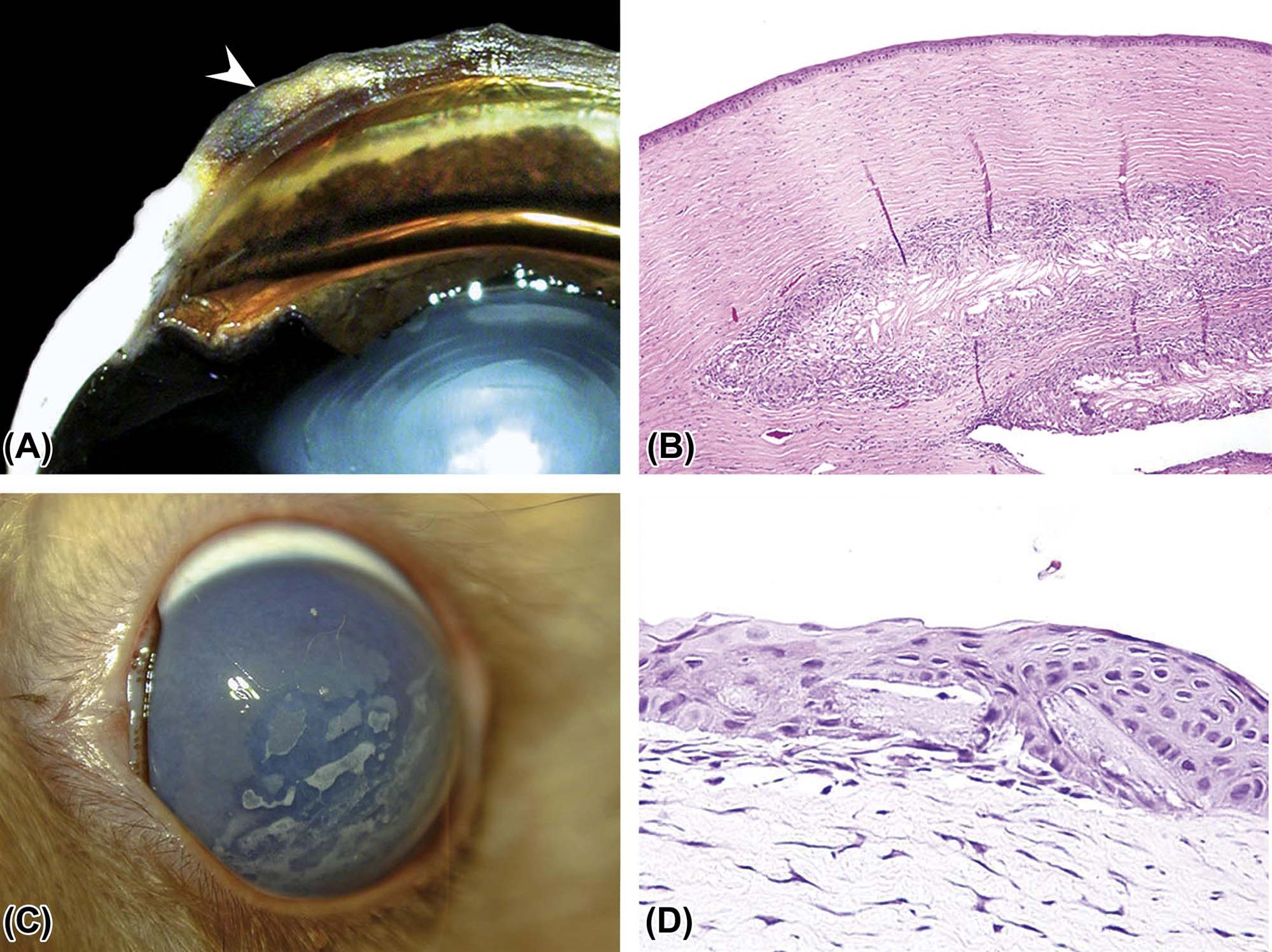
(A) Gross image of a dorso-ventral section of a dog globe showing yellow material, consistent with lipid, deposited in the corneal stroma (arrow). (B) Histologic section presenting a large cholesterol granuloma, comprised of numerous cholesterol clefts surrounded by a marked granulomatous reaction, embedded in the deep corneal stroma. (C) Gross image of a rabbit cornea showing white irregular plaques, composed of mineral and lipids deposited in the corneal stroma. (D) Multiple brick-shaped cholesterol crystals embedded in the corneal epithelium of the rabbit in C. (B) H&E, ×100; (D) H&E, ×400. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.12, p. 2131, with permission.
Corneal subepithelial and stromal mineralization (corneal dystrophy, calcific band keratopathies, dystrophic calcification) is a relatively common lesion that can be associated with hypercalcemia and metastatic mineralization, or more commonly as a secondary response to chronic corneal irritation and/or ulceration. Mineralization has also been associated with high ammonia levels due to urease-positive bacteria in the bedding. It is a prominent feature of spontaneous corneal pathology in some mouse strains. For instance, BALB/c, C3H, and DBA/2J, mice frequently possess Von Kossa-positive, extracellular, basophilic corneal deposits of mineral that are age-related in incidence and severity and may be associated with focal corneal thinning and ulceration. Interestingly, these mouse strains are also prone to spontaneous cardiac calcinosis.
Corneal edema results from water accumulation in the stroma. The optical clarity of the cornea is dependent on the precise special distribution of keratocytes (i.e., stromal fibroblasts), collagen and proteoglycans in the stroma. Even slight fluid accumulation in the corneal stroma leads to a shift from transparent and colorless to translucent blue (Figure 22a.17). The normal corneal stroma is maintained in a slightly dehydrated state by the intact epithelium, the exclusion of blood vessels from the stroma and, importantly, by the energy-dependent removal of fluid into the anterior chamber across a pressure gradient created by the corneal endothelial cells. Due to the importance of maintaining normal levels of transparency and light refractivity, the cornea presents a very low threshold of tolerance for damage.
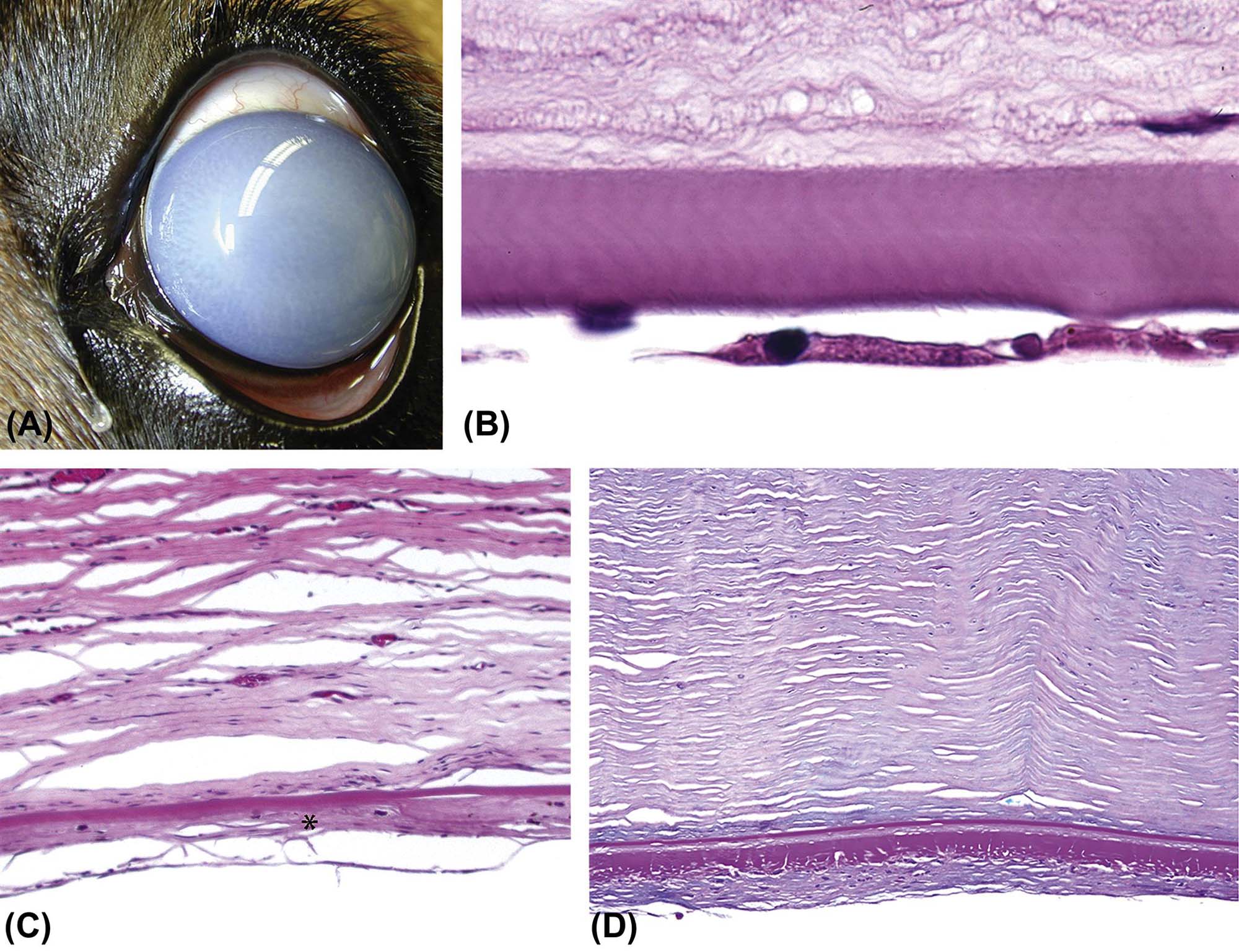
(A) Clinical image of a dog eye with a diffusely opaque and blue cornea secondary to edema caused by corneal endothelial loss. (B) Attenuated corneal endothelium in a dog with a hereditary endothelial dysplasia. Note the discontinuity and nuclear condensation in the endothelial cell layer beneath the thick eosinophilic band of Descemet’s membrane. The stromal connective tissue exhibits an abnormal granular appearance, likely a reflection of fluid accumulation due to disruption of the endothelial barrier. (C) Replacement of the endothelium with a fibrous layer (retrocorneal membrane, *) in a dog secondary to longstanding mechanical disruption of the endothelium. Using Descemet’s membrane as a reference, note the increased thickness of the retrocorneal membrane relative to the normal endothelial cell monolayer. (D) Retrocorneal membrane in a dog associated with disorganized deposits of basement membrane proteins. (B) H&E, ×600; (C) H&E, ×200; (D) Periodic acid-Schiff (PAS), ×200. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.11, p. 2126, with permission.
Several toxic effects might be associated with corneal edema. For example, permeability of the surface epithelium may be altered. This can occur as a result of physical or chemical damage to the epithelium and is important when irritants come in contact with the ocular surface. Second, the structural integrity of the stroma may be disrupted. This can be caused by damaging the profile of cytokines, which regulate the balance in the arrangement and composition of the stromal elements. Deposition of lipids, mineral, and/or molecules, which might enhance the colloidal pressure in the stroma, can also cause the stroma to become opaque. Third, vascularization of the stroma and/or enhanced permeability of the limbal blood vessels may impact the ability of the cornea to bar the entry of excess fluid. Toxicant-induced changes in vascular permeability can lead to corneal edema even if new blood vessels do not proliferate into the corneal stroma. Finally, malfunction of the corneal endothelium will predispose to fluid accumulation within the corneal stroma due to the cessation of its active transfer from the corneal stroma of excess fluid. Microscopically, corneal stromal edema is characterized by paleness an irregular expansion of the stromal lamellae sometimes associated with edema and spongiosis of the adjacent epithelium and subepithelial cleft formation. It is important, and sometimes challenging, to differentiate stromal edema from artifactual splitting of the corneal lamellae, a very common and almost predictable artifact following conventional histological processing.
Corneal stromal collagenolysis (keratomalacia). Many factors that affect the health of the corneal stroma induce the release of matrix metalloproteinases, which can degrade the stromal collagen. In the worst cases, the collagenolysis is so fast and complete that the corneal stroma can be completely dissolved in a day or less. An example is the “melting” corneal ulcer, a devastating disease that is rare in humans but occurs frequently as a spontaneous condition in dogs, particularly the Shih Tzu breed. Affected dogs present with an acute episode of corneal opacity and edema that is cause by abrupt qualitative changes in the corneal stroma: loss of stromal lamellar architecture and severe liquefaction (collagenolysis) of the tissue, usually associated with marked neutrophilic infiltration. If left unchecked, extensive areas of the corneal stroma are affected, and the lesion evolves to a descemetocele (prolapse of Descemet’s membrane through an eroded corneal epithelium and stroma) and eventually corneal perforation.
Corneal stroma fibrosis and neovascularization are nonspecific responses of the corneal stroma to injury. Neovascularization is usually seen in response to corneal ulceration and inflammation, but it can also be stimulated by intraocular lesions such as endophthalmitis. Vessels start proliferating from the limbus into the peripheral corneal stroma approximately 3 days after the initial stimulus and progress centrally at a rate of approximately one-corneal-thickness (i.e., approximately 100–600 μm) per day. Corneal stromal fibrosis is seen in the late stages of chronic keratitis, corneal surface exposure (exposure keratopathy), and dry eye or as a reparative response after corneal wounds. Histologically, fibrosis is characterized by areas of randomly deposited dense collagen with increase numbers of fusiform cells and loss of the normal parallel arrangement of the corneal stroma—all of which lead to permanent opacity in the affected portion of the cornea.
Corneal inflammation (keratitis) can be present as a response to a variety of possible causes, such as corneal ulceration with or without secondary infections, chronic corneal exposure and dry eye, mechanical irritation, toxic and chemical reactions, photosensitization, immune-mediated processes, and others. The type of leukocyte response varies with the inciting cause, but usually neutrophils predominate in acute and subacute processes while the number of lymphocytes and macrophages increases with chronicity.
The corneal endothelium in most mammalian species presents a very limited proliferative capacity. That said, although rare, the corneal endothelium can respond to injury by proliferating. In such instances, the corneal endothelium migrates over the ICA and anterior aspect of the iris and sometimes produces a Descemet’s membrane-like material. This phenomenon is known as endothelialization and is associated with iridocorneal endothelial (ICE) syndrome and some forms of glaucoma in humans. In mice, ICE syndrome occurs in the DBA/2J and AKXD-28/Ty strains and can lead to progressive angle closure glaucoma.
Corneal endothelial attenuation may significantly impact corneal function because of its limited proliferative capacity. When endothelial cells are lost to injury or aging, the tissue attempts to cover the resulting defect by enlargement and stretching of the adjacent cells. Despite this adaptation, with time there is a critical point at which endothelial cells are unable to adequately sustain their function simply because there are too few of them. Microscopically, corneal endothelium attenuation presents as flattened and enlarged cells with an overall decrease in cell numbers and possibly associated corneal stromal edema (Figure 22a.17).
Doubling and breaks in Descemet’s membrane are usually seen secondary to traumatic events. These lesions can be accompanied by a fibrovascular formation on Descemet’s surface (retrocorneal membranes) and/or fibroplasia of the deep corneal stroma. Multiple small breaks in Descemet’s membranes can be seen in conjunction with globe enlargement secondary to glaucoma (buphthalmos). In this instance, these breaks are called Haab’s stria, and they represent linear (usually horizontal) fissures in Descemet’s membrane.
Uvea
Fibrovascular membranes result from proliferation of stromal and vascular tissue in the uvea. Because of the need for transparency in the light-transmitting media of the globe, vascular proliferation is tightly regulated. There are many disease states where the release of angiogenic molecules leads to neovascular proliferation associated with the uveal tissue. These membranes are seldom actually within the uveal tissue but rather extend out along the surfaces of the iris, ciliary body, and/or choroid, sometimes extending into the anterior and posterior chambers, vitreous and subretinal spaces. Such fibrovascular membranes can lead to synechiae [i.e., adherence of the iris to either the cornea (anterior synechia) or lens (posterior synechia)], ICA closure, and/or hemorrhage. When severe, the membranes block the aqueous outflow, increase IOP, and initiate glaucoma. Retinal hypoxia as a result of restricted perfusion or retinal detachment and subsequent release of pro-angiogenic factors [e.g., vascular endothelial growth factor (VEGF)] in the ocular chambers is a common cause of fibrovascular membrane formation; two diseases resulting from production of such factors are neovascular glaucoma and choroidal neovascularization in the “wet” form of AMD. Intraocular inflammation, deep ocular trauma, and intraocular tumors are other possible causes. Microscopically, these membranes are characterized by fusiform cells and small vascular sprouts surrounded by collagen fibers. The amount of collagen production and degree of vascular proliferation in these membranes vary markedly.
Uveal inflammation (uveitis). Anterior uveitis refers to inflammation of the iris and ciliary body. Posterior uveitis refers to inflammation of the choroid, which is more commonly denominated choroiditis. When the inflammatory process affects both the anterior and posterior uvea, it is termed panuveitis. Ocular trauma, irritation secondary to intraocular devices, or intracameral injections (i.e., into either the anterior or posterior chamber) may cause uveal inflammation.
Inflammation secondary to a toxic event must be differentiated from leukocyte accumulation as a spontaneous background change. Cynomolgus monkeys exhibit foci of uveal lymphocytic infiltration as an idiopathic background change. A review of hundreds of archival samples shows that these lymphocytic foci are seen in the ciliary body and choroid of approximately 27% of naïve control animals. Such infiltrates usually present as unilateral, focal, minimal to mild, lymphocytic aggregates with few plasma cells, most often in the pars plana of the ciliary body. Mononuclear cell infiltration in the iris, ciliary, and ICA is also observed as relatively frequent nonspecific change in rabbits. Rats and aged mice also develop spontaneous leukocyte infiltration of the anterior uvea. This control finding is properly considered an infiltration (i.e., cell accumulation of no pathologic significance) rather than an inflammation (i.e., where the accumulating cells actually damage the affected tissue).
Hyperpigmentation and depigmentation of the uvea are nonspecific alterations that can be associated with multiple causes. Hyperpigmentation can occur following systemic administration of compounds such as urethane in neonatal hooded rats or topical application of prostanoid compounds such as latanoprost. Increased pigment deposition occurs in DBA/2J mice homozygous for iris pigment dispersion or iris stromal atrophy. Decreased pigmentation may be associated with uveal inflammation or edema, and can also present as an aging change.
Lens
When reporting lenticular changes, it is important to fully characterize the pathologic changes. Features to describe include the lesion distribution (unilateral or bilateral, focal, multifocal or diffuse), tissues affected (capsule, epithelium, or fibers); and anatomical location in the lens (anterior, equatorial, or posterior; subcapsular, cortical, or nuclear). It is important to recognize that some lenticular opacifications observed clinically are reversible and usually do not present corresponding microscopic lesions. Reversible lens opacification can be induced by cold temperature, dehydration, asphyxia, anoxia, stress, and certain chemicals and drugs (see mechanism of toxicity section).
Cataract (degeneration). Transparency and refraction of light are the main functions of the lens, and toxicity may be manifested by changes in these features. The most readily apparent is a change in transparency, which is defined as a cataract. Not all cataractous changes can be detected microscopically. A good example is changes in the lens nucleus (or core) diagnosed clinically as nuclear cataract. In addition, cataracts may manifest histologically by changes indistinguishable from certain tissue artifacts related to sectioning and fixation. It is important to identify genuine lenticular changes to avoid misinterpreting lens artifacts as lesions. The most reliable cataract-related lesions that can be demonstrated using a microscope include swelling of lens fibers, formation of Morgagnian globules (large degenerate anuclear fibers) and bladder cells (large degenerate fibers with nuclei or nuclear fragments), liquefaction and mineralization of the cortical lens fibers, migration of the lens epithelial cells (normally only present in the inner aspect of the anterior lens capsule) along the inner surface of the posterior lens capsule, spindle cell (fibrous) metaplasia, and hyperplasia of the lens epithelial cells and wrinkling of the lens capsule (Figure 22a.18).
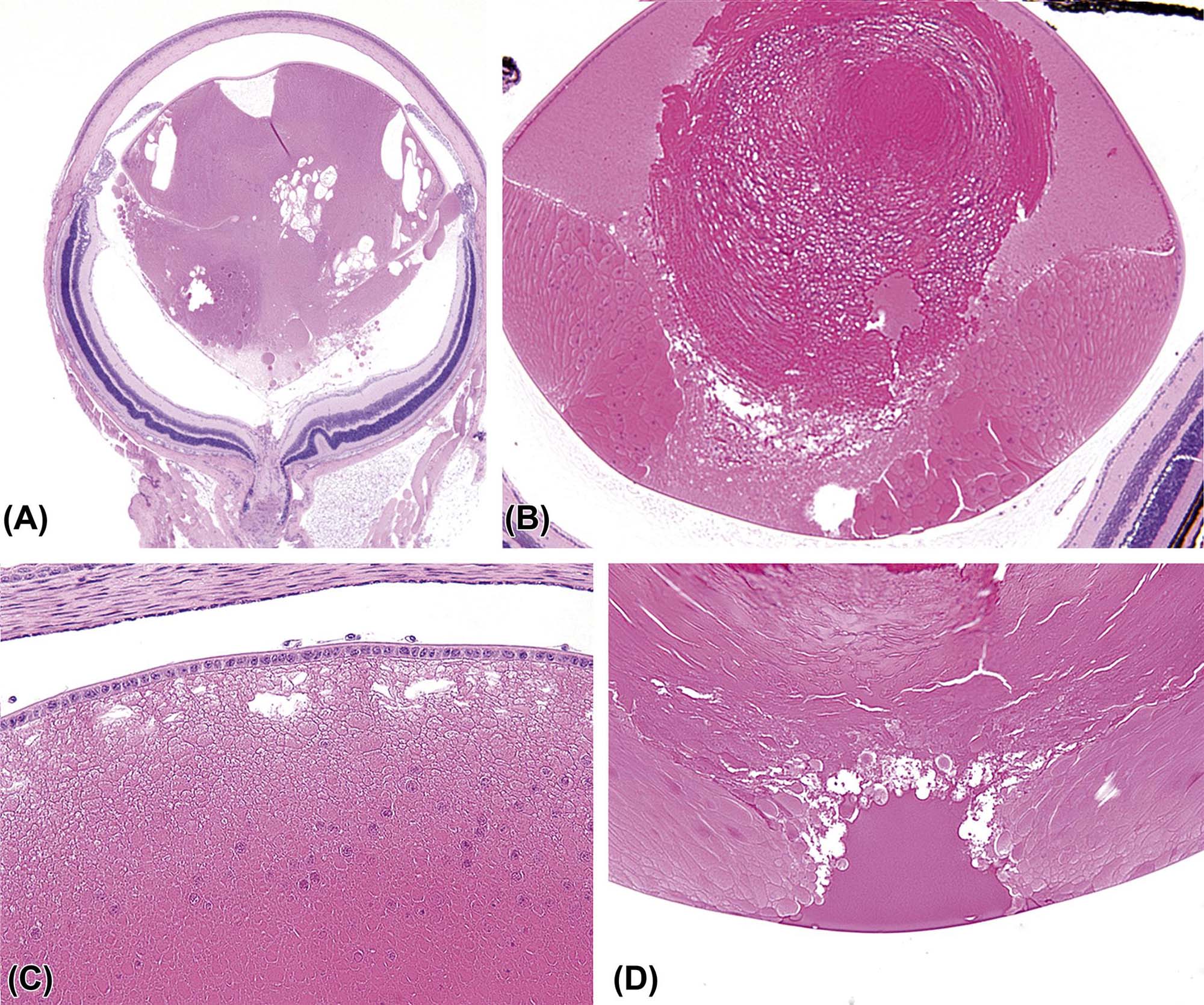
(A) Subgross view of a mouse globe with a cataract distorting the posterior pole of the lens. (B) Lens from a mouse with fairly normal equatorial regions but abnormal nucleus that protrudes caudally to disrupt the posterior pole of the lens. (C) Posterior migration of the lens epithelium (posterior cortical cataract) in a young mouse with persistence of the tunica vascularis lentis on the surface of the lens. (D) Cortical cataract at the posterior suture. (A) H&E, ×12.5; (B) H&E, ×100; (C,D) H&E, ×200. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.17, p. 2146, with permission.
Posterior migration of the lens epithelium is usually one of the earlier morphologic findings in cataracts. Another early morphologic change that appears to be more specific to mice is internal migration of nucleated lens epithelial cells beyond the nuclear bowl, especially at the lens equator. These initial changes are particularly important to be recognized when screening for morphological phenotypes, especially in developmental cataracts. Morgagnian globules and bladder cells are formed when abnormal cellular metabolism in lens epithelium causes cytoplasmic accumulation of coarse granular material that causes the cells to swell. Mineralization and liquefaction of the lens fibers are often present in advanced (chronic) cataracts. The contents of the liquefied lens fibers have the potential of leaking though the intact lens capsule, where they may incite an intraocular immune-mediated response culminating in uveitis. Lens epithelial cells can undergo spindle cell metaplasia and deposit collagen within the subcapsular space, creating a pattern of opacification clinically referred to as subcapsular cataracts. The metaplastic cells atrophy over time, and often only the collagen remains (Figure 22a.19).
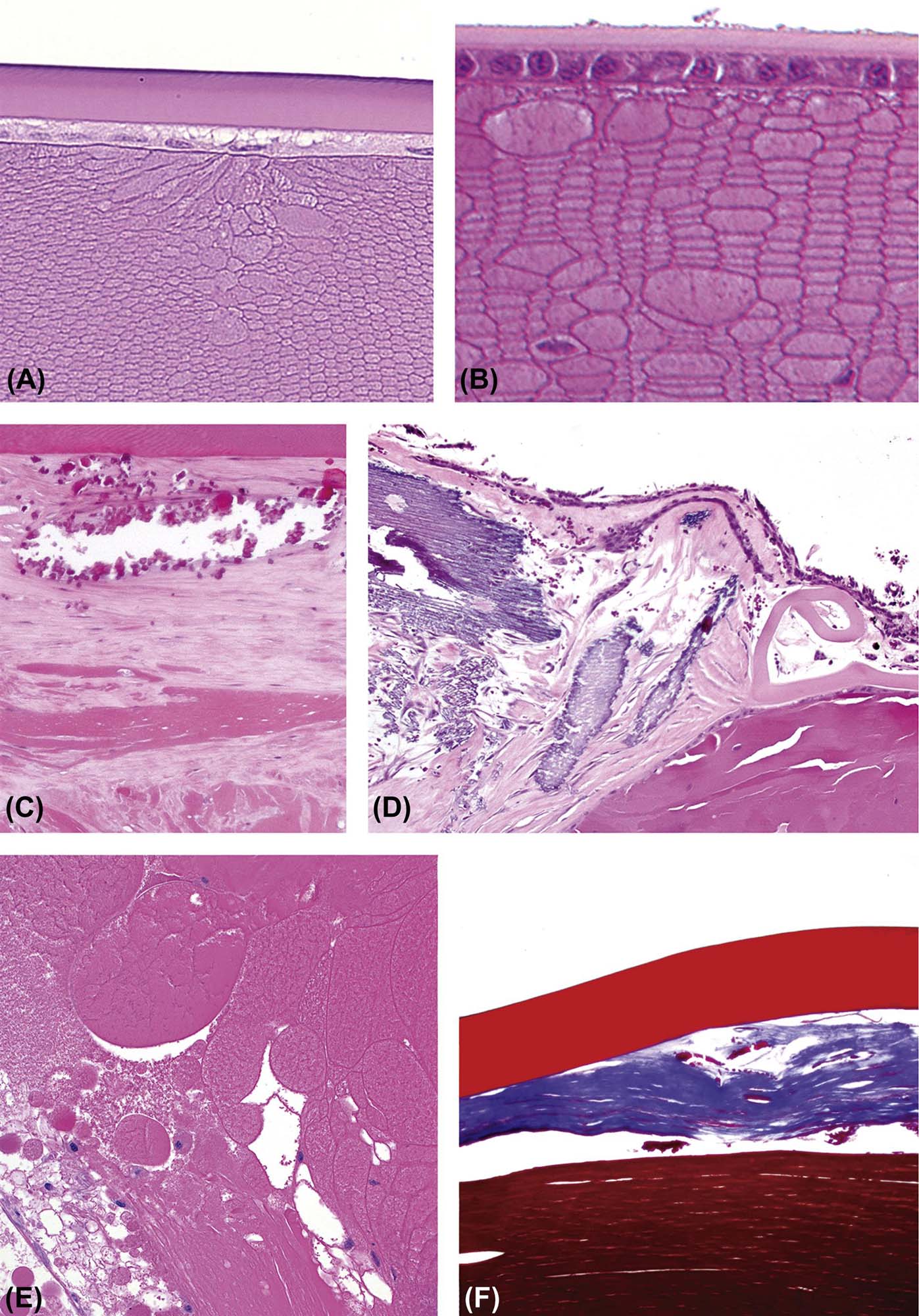
(A,B) Lens fiber swelling in the anterior cortex of a mouse eye, indicative of cortical cataract; Davidson’s fixative. (C,D) Collagen deposition, spindle cell metaplasia of lens epithelial cells, and lens fiber mineralization as part of a subcapsular cataract. The lens capsule is ruptured and coiling in D. (E) Morgagnian globules (round and swollen degenerate lens fibers without nuclei) and bladder cells (swollen degenerate lens fibers with nuclei or nuclear fragments) with extensive disorganization of the lens fibers in a mature cortical cataract. (F) Collagen (blue) subtending the lens capsule (thick upper red band) secondary to spindle cell metaplasia of lens epithelial cells in the formation of a subcapsular cataract. (A) H&E, ×200; (B) H&E, ×400; (C) H&E, ×600; (D) H&E, ×400; (E) H&E, ×100; (F) Masson’s trichrome, ×600. Source: From Haschek, W.M., Rousseaux, C.G., Wallig, M.A. (Eds.), 2013. The Handbook of Toxicologic Pathology, third ed. Academic Press, San Diego, CA, Figure 53.18, p. 2150, with permission.
Lens capsule rupture usually affects the posterior lens capsule, which is thinner than the anterior capsule. Lens capsule rupture usually occurs as a posttraumatic event (blunt or perforating trauma, including iatrogenic perforation after intraocular injections) but it can also occur secondary to fast developing (intumescent) cataracts, as seen in diabetic cataracts in dogs, or secondary to granulomatous lenticular inflammation in rabbits infected with Encephalitozoon cuniculi. Regardless of the cause, lens protein and fibers are exposed and extruded into the intraocular chambers after capsule rupture usually causing a secondary endophthalmitis and/or uveitis (phacoclastic inflammation). The inflammation associated with release of lens proteins is an autoimmune reaction produced by exposure to “foreign antigens” from the lens interior (which is an immunologically privileged site). Some mutant mouse models develop spontaneous rupture of the lens capsule. These include lens rupture (lr), lens opacity 10 (Lop10), and lens opacity 12 (Lop 12) animals. Unlike traumatic lens capsule rupture in most species, where a granulomatous inflammatory reaction occurs, these genetically mediated ruptures seldom mount an inflammatory response; the reason for this phenomenon is unknown.
It is very important to differentiate real lens capsule rupture from artifactual rupture produced during sectioning of the ocular tissues. The most reliable histologic changes associated with genuine lens capsule rupture are coiling of the free edges of the ruptured capsule away from the site of the break and infiltration of inflammatory cells among the exposed lens fibers. Because of the three-dimensional structure of the lens and the focal nature of some lesions, the exact point of capsule rupture might not be sampled in a given section. In these situations, the presence of inflammatory cells in the intralenticular space interacting with the lens fibers is evidence of lens capsule rupture at another location in the lens.
Lens luxation may present as dislocation either anteriorly or posteriorly. Primary lens luxation occurs in dogs (typically terrier breeds) that carry mutations affecting ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) 10, leading to dysplastic zonular fibers and zonular instability (weakness). This ADAMTS 10 mutation also has been described in a colony of beagle dogs that exhibit a primary glaucoma phenotype. These beagles traditionally have been used as an animal model of primary open angle glaucoma in humans.
Lens luxation is more commonly seen in association with trauma, secondary to glaucoma with buphthalmos (enlargement of the globe leading to rupture of the zonular fibers), and hypermature cataracts (where wrinkling of the lens capsule leads to rupture of the zonular fibers). Since the lens can be easily artifactually dislocated while trimming the eye, the adequate diagnosis of lens luxation relies heavily on the clinical and gross identification of a dislocated lens. If the lens is firmly entrapped in the anterior chamber or vitreous, the microscopic diagnosis of luxation is simple. Otherwise, the clinical and macroscopic diagnosis of lens luxation can be supported by the histologic findings of attenuation of the corneal endothelium (suggesting the lens was in contact with the corneal endothelial cells), posterior curving of the iris leaflets, and atrophy of the ciliary body processes.
Vitreous Body
It is tempting to think of the vitreous body as a structural lumen, but it is actually a cell-poor, transparent, extracellular space filled with a gel-like matrix. As such, it responds to injury in the same way other connective tissues do. If there is a stimulus for fibrovascular proliferation, then new blood vessels and spindle cells will move into the vitreous, and deposition of collagen fibrils in the originally rarefied matrix will lead to opacification. Proliferative vitreoretinopathy after retinal detachment, retinopathy, or premature birth are examples of diseases in which fibrovascular proliferation in the vitreous may be profound.
Persistent fetal vasculature (PFV) results from impaired regression if the fetal vitreous vasculature, which normally recedes shortly after birth. In such cases, retained remnants of embryonic vessels accompanied by fibrous connective tissue can be seen in the vitreous and posterior aspect of the lens for extended periods after birth. This condition is referred to as PFV, which is an umbrella term proposed to encompass multiple presentations that can range from persistent hyaloid vessels to persistent hyperplastic primary vitreous and persistent tunica vasculosa lentis (TVL) (Figure 22a.18C). PFV is a relatively common congenital abnormality in human eyes and has been reported as a congenital finding in Sprague–Dawley rats, Swiss mice, Göttingen minipigs, and Yucatan micropigs. PFV is also associated with several different mutant mouse models including the p53-null and Norie’s disease homologue (Ndph) strains, and it can be induced in animals with double null mutations for members of the retinoic acid receptor family. Microscopically, remnants of small vascular profiles admixed with variable amounts of collagen and hemorrhage are present in the central vitreal canal anywhere from the optic nerve head surface to the posterior aspect of the lens.
Degeneration of the vitreous body can be hard to detect microscopically, but it can be seen macroscopically as liquefaction of the gel-like vitreous matrix. Degradation of the vitreous also may be recognized indirectly as the cause of retinal detachment. Liquefaction of the gel-like vitreous causes the retina to be buffeted by energy waves in the fluid media; in the absence of structural protection, the resulting trauma to the neural retina leads to retinal detachment and ultimately retinal degeneration. The formation of fibrovascular membranes (vitreal membranes) in the vitreous is a less common cause of vitreal and retinal traction bands that might promote physical pulling of the retina away from the RPE, and sometimes lead to retinal tearing. With age, changes in the rheologic (i.e., gel-like) features of the vitreous can cause it to separate from the retina spontaneously.
Since substances injected into the vitreous freely diffuse into the retinal tissue, the IVT route has become a popular method of drug delivery to the posterior segment of the eye. The injections are usually made at the level of the equatorial sclera and ciliary body pars plana, and are directed into the vitreous body rather than the retina proper. These injection sites usually present histologically as small defects in the ciliary body epithelium, stroma, and sclera, and can be associated with mild fibrosis and minimal infiltration of macrophages and lymphocytes.
Asteroid hyalosis is a degenerative condition characterized by small white opacities in the vitreous. It occurs in humans, dogs, and chinchillas. Clinically, these opacities are quite refractile, giving the appearance of stars (or asteroids) shining in the night sky. Histologically, they present as round, amphophilic, laminated to radiating structures of variable size within the vitreous. Asteroid hyalosis has been associated with aging, diabetes mellitus, hypertension, hypercholesterolemia, and (in dogs) intraocular tumors.
Vitreal inflammation is usually a secondary change rather than a primary response to vitreal disease. Vitritis is more likely to develop as a sequel to corneal ulceration, endophthalmitis, scleral perforations, or systemic infections rather than toxicant exposure.
Retina
Retinal toxicity primarily affects the retinal ganglion cells, photoreceptors, vessels, and the RPE. The RPE is closely integrated with the photoreceptors such that a toxic effect on the RPE will often affect the photoreceptors, and vice versa. It is important to notice that not all functional impairments of the neural retina or RPE will result in morphological alterations. This discrepancy underscores the importance of combined histological and electrophysiological endpoints when evaluating the impact of xenobiotic exposures on the eye, especially when seeking to detect very early stages of toxicity.
Inner retinal atrophy refers to a thinning of the nerve fiber layer and loss of retinal ganglion cells and neuronal nuclei in the inner nuclear layer (INL). The most common causes for loss of retinal ganglion cells and their axons in the nerve fiber layer are increased IOP leading to optic nerve atrophy and compressive lesions on the extraocular portion of the optic nerve, leading to axonal degeneration and ganglion cell death. The retinal ganglion cells can also be targeted by several known retinal toxicants, including carbon disulfide and doxorubicin. A few toxicants, like ethylcholine mustard aziridinium ion (AF64A), preferentially target the interneurons in the INL. Ganglion cell loss can also be spontaneous. Rhesus and cynomolgus monkeys with a condition called idiopathic optic neuropathy present with a loss of ganglion cells in the macular region, which is correlated to atrophy of axons in the temporal portion of the optic nerve.
Outer retinal atrophy is characterized by shortening of the photoreceptor outer segments along with loss of nuclei from the outer nuclear layer (ONL). These features are often seen following exposure to ocular toxicants. Other causes of outer retinal atrophy include inherited genetic defects, aging, light-induced damage, nutritional deficiencies, retinal detachment, and inflammation (Figure 22a.20). A common mistake in designing ocular toxicity studies is to automatically choose rodents as the test species. Hereditary outer retinal atrophy occurs in several strains of mice and rats, such as Royal College of Surgeons (RCS) rats or retinal degeneration (rd) mice. These animals are docile and make good experimental animals, but they lose essentially all of their photoreceptors by adulthood as a normal background condition.