The Use of Human Acellular Dermal Matrices in Irradiated Breast Reconstruction
Abstract
This article examines the effects of radiation on prosthetic breast reconstruction when human dermal allograft is used in the reconstruction. A brief review of radiation terminology and techniques as applied to the breast is given, followed by a review of the effects of radiation on wound healing in human tissue. The effects of radiation on prosthetic breast reconstruction before the advent of dermal allografting are reviewed. The addition of dermal allograft in reconstruction has led to a reduced number of complications. An algorithm for surgical treatment of irradiated prosthetic breast reconstructions is presented, with a discussion of the authors technique.
Keywords
• Breast reconstruction • Radiation • Acellular dermal matrix • Tissue expander • Fat graft • Implant capsule
Roentgen discovered the x-ray in 1895.1 Within 1 year, Gocht in Germany treated the first 2 patients with x-ray radiation for painful locally advanced disease and obtained relief,2 demonstrating the potential benefits of radiation. Shortly thereafter, in 1901, Becquerel noted the deleterious effects of radiation when he reported that he developed a wound on his chest skin after leaving a vial of radium in his breast pocket for 6 hours. He noted the acute erythema and subsequent ulceration that took a prolonged time to heal.1 Thus, the double-edged sword that is radiation became known to physicians, and this therapeutic/wounding effect has remained a challenge to this day.
Key Points: ACELLULAR DERMAL MATRICES RADIATION BREAST RECONSTRUCTION
Ionizing radiation
Energy from radiation can be absorbed by biological material and can result in excitation of electrons in the tissue or ionization of the atoms within this tissue. Release of enough ionizing energy within the tissue can cause localized damage to that tissue. This damage, known to medicine for longer than a century, can be therapeutic or harmful depending on several factors.
Radiation can be classified as electromagnetic or particulate. Electromagnetic radiation such as the short-wavelength x-ray can cause damage to human tissue, as can the particulate radiation produced by discrete particles such as electrons and protons (among others). This particulate radiation can be delivered by a variety of techniques, by intracavitary means or via external beam. This article discusses the effects of external beam radiation therapy in common use today, but recognizes that newer techniques of intracavitary and partial breast irradiation are currently being evaluated.3
External beam radiation
As would be expected, the higher the beam energy, the deeper the penetration into human tissue. Earlier beam energy devices were relatively lower-voltage x-ray machines that delivered low energy and were only able to penetrate superficially into tissue. These devices are termed orthovoltage, and are still useful for skin irradiation. Most breast tumors are now treated with high-energy megavoltage machines, commonly a linear accelerator, which can generate beams of sufficient energy to penetrate deeper into tissue.
The unit of measure of the biological effect in tissue caused by radiation is termed the radiation absorbed dose or rad. At present the gray (Gy) is used to represent 100 rad. Thus the more commonly used 50 Gy is equivalent to 5000 rad.
The degree of tissue penetration for a given radiation dose varies depending on the energy delivered. Fortunately, depth of penetration can be calculated very precisely for the different energy sources, and this depth of maximum dose (Dmax) follows a predictable curve. Initially there is a zone of superficial penetration with a rapid buildup of energy, then a loss of energy as the beam penetrates deeper in the case of low-energy orthovoltage radiation. This process would yield the greatest effect in the skin, for example. By contrast, the high-energy megavoltage beam would have a slow buildup of energy superficially and reach its maximal energy deeper in the tissue, thus sparing the skin from the maximal radiation effect and damage while delivering the maximal effect and damage to tumors deeper in the tissue, such as the breast.
Dosing schedule for radiation
The fact that certain cells are more sensitive to the effects of radiation than others was first noted in 1906.1 Since then it has become clear that cells are most sensitive to the effects of radiation during the G2-M phase of the cell cycle and are most resistant during the S phase.1 Thus, a single dose of radiation will not affect all the cells that are irradiated, because of differences in cell-cycle status. This situation has led to the concept of fractionation or delivery of radiation in installments over time, which is the current practice.
Radiation effects on skin and wound healing
As noted earlier, the effect of radiation on skin has been known for more than a century. Radiation has both acute and chronic effects that, of course, vary with the type, dose, and delivery schedule used.1,4
The acute effects include erythema, dry desquamation at lower doses, and moist desquamation at higher doses. The erythema is the direct result of an inflammatory process caused by increased capillary permeability. Dry desquamation is the result of a dose of radiation strong enough to kill some epidermal cells, but allowing enough of the remaining epidermal cells to survive and proliferate. Moist desquamation occurs when an inadequate number of epidermal cells survive and the exposed dermis oozes serous fluid.
The chronic effects include either an increase or a decrease in pigmentation, thickening and fibrosis of the skin, telangiectasia, and alteration of hair, sweat, and sebaceous gland function. Pigmentation is altered by death of melanocytes or by deposition of pigment into the dermis. Dermal thickening can occur, as the collagen within the involved dermis will swell. If the dose is strong enough, fibroblasts are impaired and unable to synthesize collagen, and the dermis will atrophy and could ulcerate. Telangiectasia is the result of thrombosis of deeper vessels. Active hair follicles and sebaceous glands are killed by the radiation. This aspect is particularly important, because these structures provide migrating epidermal cells that are necessary for reepithelialization and wound repair.
As a direct result of these effects, a skin wound that is radiated is prone to prolonged healing, with a resultant scar that is thin in the dermis as well as the epidermis, has poor vascularity and strength, is susceptible to opening, and is unable to defend adequately against infection.
Effects of radiation on expanded skin in the animal model
Understanding the effects of radiation on skin, soft tissue, and wound healing, the question occurs as to what effects radiation may have on expander/implant (silicone or saline) breast reconstruction. A few studies have examined the effects of radiation on previously expanded skin in an animal model. Goodman and colleagues5 used New Zealand white rabbits and evaluated the effects of radiation on expanded skin 6 weeks after a single radiation dose of 25 to 35 Gy. A thickening of the epidermis but no change to the dermis was found, although the histology was performed only 6 weeks postradiation. Dvali and colleagues6 studied effects of radiation on Yorkshire pigs. Skin was irradiated in fractions up to a total dose of 48.6 Gy and expanders placed under this skin 3 months later, and the skin flaps were then expanded. The skin flaps were evaluated almost 6 months after completion of the radiation. At this time the irradiated skin flaps were reduced in overall area by 23% compared with nonirradiated controls. It was also found that radiation reduced expanded skin flap viability by almost one-third compared with controls. Performing a capsulectomy did not significantly worsen this decreased viability.
Effects of radiation on prosthetic breast reconstruction before the use of acellular dermal matrices
Much has been written on the effects of radiation on silicone and saline implant breast reconstruction. It is helpful to review the literature published before the introduction of acellular dermal matrices (ADM) in these reconstructions, to better assess the effects of the radiation with the subsequent use of these materials and to determine if there are any differences.
Given the proven negative effects of radiation on wound healing, it is not surprising that external beam radiation has been shown to exert a negative effect on the outcome of breast reconstruction using tissue expanders and saline or silicone implants.
Krueger and colleagues7 showed a 68% complication rate for tissue expander/implant reconstructions that were irradiated postoperatively compared with a 31% complication rate without radiation, resulting in a reconstructive failure rate of 37% compared with an 8% failure rate without radiation. Other reports show very high complication rates, high Baker III-IV capsule rates, and higher rates of reconstructive failure resulting in expander or implant loss when radiation follows tissue expander/implant reconstructions.8-11
Similarly, Spear and Onyewu12 reported on a variety of tissue expander/implant reconstructions treated with and without radiation, and included reconstructions that had been irradiated before reconstruction, during expansion, and after reconstruction. A capsular contracture rate of 32.5% was found, regardless of when the patient received the radiation, compared with a rate of zero in the nonirradiated group. There was also a 37.5% incidence of adding a transverse rectus abdominis muscle (TRAM) or latissimus flap in the radiated group versus a 10% incidence in the nonirradiated patients to either salvage or improve the cosmesis of the reconstruction. Indeed, to combat these very high rates of complications, advanced capsule formation, and/or implant loss, Spear and Onyewu advise their patients that addition of a TRAM or latissimus flap at the time of expander removal/implant exchange will be needed in 50% of cases involving radiation.
Parsa and colleagues13 advise delaying reconstruction by at least 6 months after radiation to assess skin damage, and then to proceed with expander/implant reconstruction if there is no induration and moderate or better skin changes.
Kronowitz and colleagues14 propose a delayed-immediate reconstruction whereby an expander is placed immediately at the time of the mastectomy and, if radiation is needed postoperatively, the expander is left in place until completion of the radiation, before being replaced by an autologous flap.
Fine and Hirsch15 at Northwestern suggest a similar plan, but do not routinely place an autologous flap after radiation, and use an implant depending on desires of the patient.
Cordeiro and colleagues16 reported a totally different timetable whereby the tissue expander is placed immediately and inflated during subsequent chemotherapy, then replaced with an implant before initiation of radiation therapy. These investigators report very similar results between the irradiated and nonirradiated groups overall in terms of complications, with only a higher Baker III capsule rate among the irradiated reconstructions.
Despite these various protocols, tissue expansion followed by implant/exchange breast reconstruction continues to challenge us all as plastic surgeons, especially when the reconstruction has been or will be irradiated. The question is what effect, if any, has ADM had on these problems? To answer this question one needs to briefly examine the relatively short history of ADM and implant breast reconstruction, which is examined in greater detail in the article by Baxter elsewhere in this issue.
Brief review of ADM and implant reconstruction
Although the use of ADM in immediate breast reconstruction is relatively new, first reported in 2001,17 its use has dramatically increased in popularity since then. Many publications support its use, safety, and efficacy in immediate single-stage implant (silicone, saline, adjustable) reconstruction17-21 as well as in a 2-stage tissue expander/implant exchange technique.22,23 The use and history of these materials is well covered elsewhere in this issue and is not the focus of this article. Previous work has shown histologically that human dermal allograft promotes less fibrosis and inflammation in capsules formed around implants.24 The history and the expanding use of these materials is, however, important in evaluating these materials in the context of breast irradiation. As experience with these materials has increased, so have the applications that are generating more reports and evaluations of ADM materials subjected to radiation during the course of breast reconstruction.
Experimental evidence for protective effect of ADM in irradiated prosthetic reconstruction
In an important animal study, Komorowska-Timek and colleagues25 reported on the protective effect of AlloDerm® (LifeCell Corp, Branchburg, NJ, USA), a popular human acellular dermal matrix, on irradiated implant capsules formed in Sprague-Dawley rats using small saline implants. The implants were inflated and irradiated 2 days later with a single dose of 21.5 Gy. The resultant capsules were examined at 3 weeks postradiation and a second group at 12 weeks postradiation, and compared with irradiated non-AlloDerm® controls and nonirradiated controls. The AlloDerm® group was seen to have a protective effect on the radiation changes that were seen. There was less radiation-related inflammation and, importantly, only 1 of the 20 rats that were radiated developed a pseudoepithelium on the AlloDerm®, whereas all of the non-AlloDerm® radiated control capsules developed this pseudoepithelium. These findings, which have been confirmed elsewhere,26 are very important because it is believed that formation of this pseudoepithelium is a precursor to the formation of a fibrotic capsule in humans.27
Armed with the knowledge of the protective effects of ADM on irradiated implant capsules in the animal model and knowing that ADM has been shown to diminish inflammation and fibrosis in capsules formed around implants in humans, the question is whether ADM offers any protective benefit to an implant breast reconstruction that has been or will be radiated. An increasing body of literature has evaluated the results of implant breast reconstruction using ADM that have been radiated. Less has been written on the effects of radiation if the radiation has preceded the reconstruction.
Effects of radiation on ADM/tissue expander or implant breast reconstructions
In an elegant review of 12 published studies on complication rates in prosthetic breast reconstruction using ADM, Newman and colleagues28 found an overall complication rate of 12%.
More recently, Salzberg and colleagues17 reported a complication rate of 3.9% following an 8-year experience using ADM and immediate implant reconstruction. Their review reported a 14.3% complication rate if the reconstruction was irradiated (this included either prereconstruction or postreconstruction radiation) which, though greater than their complication rate without radiation, is still consistent with the 12% reported by Newman in the absence of radiation.
Further evaluation by Nahabedian29 reviewed the rate of infection, seroma, and skin necrosis in prosthetic reconstructions using AlloDerm® both with and without radiation. Nahabedian found that the timing of the radiation (prereconstruction or postreconstruction) was not a factor. He also found an infection rate of 3.9% without radiation and 9.4% with either preoperative or postoperative radiation. Seromas occurred in 2.6% of nonirradiated breasts and in 13% of irradiated breasts. No irradiated breast showed skin necrosis. Wound dehiscence occurred in 1.3% of nonirradiated breasts and in 13% of irradiated breasts. Statistical significance of these differences was not evaluated; the number of all these complications was small.
In a review of 231 patients undergoing a 2-stage prosthetic reconstruction, Seruya and colleagues30 found a 15.4% capsule rate in irradiated reconstructions versus 2% in nonirradiated reconstructions, but no difference in the revision rate or the rate of explantation caused by infection after stage 2.
Again, it is important that even though the effects of radiation increased the number of complications even with ADM, this increased rate is still consistent with the overall rate noted by Newman and colleagues28 in their meta-analysis of complications in nonirradiated reconstructions. Perhaps most importantly, the complication rates in prosthetic reconstructions using ADM discussed here are markedly less than the greater than the 40% complication rates described by Christante and colleagues8 and also by Ascherman and colleagues11 when ADM was not used with the prosthetic reconstruction that was irradiated.
The use of fat graft in radiated tissue
The newest addition to prosthetic breast reconstruction involves the use of fat grafting. After pioneering work by Coleman,31 the use of staged, serial fat grafts as an adjunct to fill and soften implant reconstructions has been shown to be very effective even in the face of radiation.32,33 The fat is injected after completion of the radiation either at the time of delayed insertion of a tissue expander, at the time of implant exchange, or subsequent to implant exchange and the demarcation of deformity around the implant.
The author’s approach to irradiated breast reconstruction
Like many approaches to problems in plastic surgery, a staged approach is appropriate when dealing with the prosthetic breast reconstruction that has been or will be irradiated. My approach varies depending on the quality of the tissue at the time of the reconstruction. I find it is the quality of the skin/soft tissue that is most important and that the occurrence of the radiation (prereconstruction or postreconstruction) carries less of an impact. Working with a hard reconstruction under skin that is heavily pigmented and telangiectatic from radiation 10 years previously can be less satisfying than with a breast reconstruction that was irradiated after tissue expander placement 3 or 4 months previously that is relatively soft and minimally pigmented.
To begin with, I believe it is better to perform implant reconstructions in 2 stages, for 3 reasons:
I also prefer to do opposite breast symmetry work at the time of the second stage when I have a much clearer idea of what the unilateral reconstruction looks like.
The vast majority of my reconstructions are immediate. I do not always know if radiation will or will not be used when I am in the operating room. I follow the same protocol regardless. I have used many different types of ADM, all with similar good results. I currently use AlloDerm® (LifeCell Corp, Branchburg, NJ, USA). I rarely see the “red breast” on the overlying skin flap but when I do, I worry and treat with oral antibiotics, although it is likely due to inflammation rather than infection. I will gladly switch to a different product when one becomes less expensive than what is currently available, provided continued reliability is consistent with what I use now.
The author’s operative technique for placing the ADM
When the mastectomy incision is in the mid-breast at the nipple areola (either excising or sparing it):
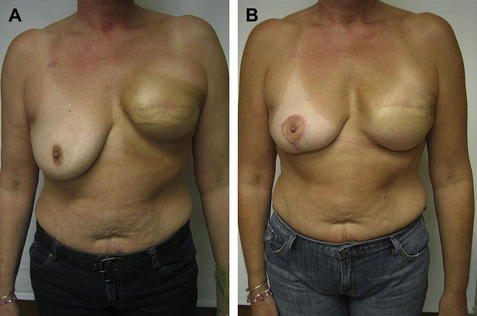
Fig. 1 (A) Typical elevation of implant and inframammary fold (IMF) after radiation. (B) Postoperative appearance after lowering the left IMF only, and a vertical mastopexy on the right.
The author’s algorithm for the expander/implant exchange
The decision as to what to do, and when, after radiation depends on the appearance and quality of the skin/soft tissue over the tissue expander. Where I work, the radiation oncologists prefer that I do not fully inflate the expander before radiation.
Once the radiation course has been completed, I begin to consider the implant exchange no sooner than 3 months after radiation. I believe the skin is too acutely damaged before this time. When the skin is not heavily pigmented and not indurated, I plan the exchange procedure. This procedure may be at the 3-month point or could be delayed for 9 months or so if the radiation was given postoperatively. If the radiation was given more than 1 year ago (as would be the case with a completion mastectomy following a recurrence after lumpectomy/radiation done in the past), the second stage can commence as early as 6 weeks after placing the expander, depending on skin quality. When I determine that the skin/soft tissue is optimal for that patient, I use an algorithm for the second-stage procedure(s). I use the same algorithm if I am working on a reconstruction that was done in one stage with an implant at the time of the reconstruction (Fig. 2).
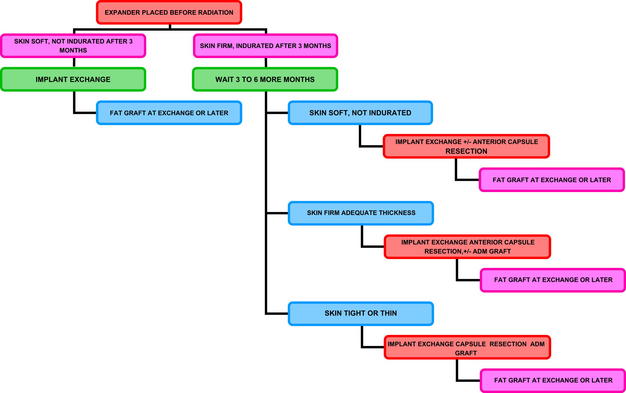
Fig. 2 Algorithm for adjunct procedures done with the implant exchange when the expander is placed before radiation. A similar algorithm is used if the radiation preceded placement of the tissue expander.
The second-stage procedure is straightforward expander removal and implant exchange. I advise smooth round silicone gel implants (I do not have access to the shaped, highly cohesive gel implants). Implant size is based on patient preference for size (often larger, rarely smaller), skin envelope quality, base diameter of the breast pocket, and the size and shape of the opposite breast in unilateral cases. It is important to fill the base diameter to minimize lateral shift and cleavage widening. I place an implant that is larger in volume than the expander because I believe the volume of the expander shell is 50 to 100 mL greater than the shell of the implant, and this must be accounted for.
This straightforward exchange is easiest but, in my practice, not a common situation.
Non-Straightforward Implant Exchange Procedure
Frequently the capsule is firm and implant exchange must be accompanied by anterior capsulectomy. Hydrodissection with a dilute epinephrine solution such as tumescent solution is helpful in removing the capsule.
Patients are cautioned about the trade-off of more postoperative pain with the addition of lateral fold sutures into the serratus.
Complex Implant Exchange Procedure
If the skin quality is thick or tight after 9 months postradiation (or 3–6 months after placing an expander in a patient with a previous history of radiation, such as after a completion mastectomy for a recurrence after segmental resection/radiation), the second stage is more complex.
Patients are advised that often 2 or 3 sessions of fat grafting done 3 months apart may be needed (Fig. 3).
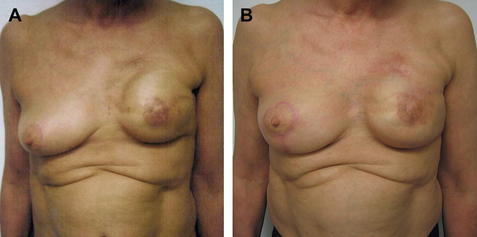
Fig. 3 (A) Preoperative appearance of irradiated implant. (B) Postoperative appearance after 2-stage procedure: first, placement of acellular dermal matrices (ADM) second, 2 sessions of fat grafting.
This same concept of capsulectomy, additional ADM, and fat grafting is used in secondary reconstructions of radiated implants in cases where the implant has been already placed (Fig. 4). If the capsule is thin or if there is significant rippling, I do not remove it but instead release it as needed and then reinforce with additional ADM and fat graft if needed.
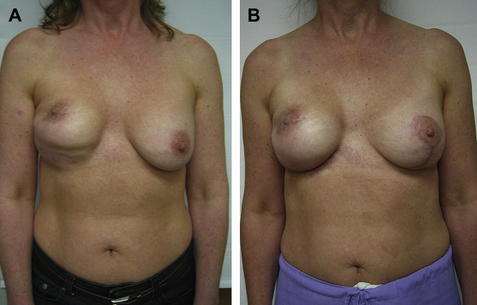
Fig. 4 (A) Preoperative appearance of patient referred after having completion mastectomy after previous lumpectomy/radiation and placement of implant. (B) Appearance status post total capsulectomy, anterior coverage with ADM, adjustment of IMF, implant replacement, 2 sessions of fat grafting, and left mastopexy. Note the improvement but also the persistence of retracted nipple areola.
Summary
Radiation to the breast continues to present a difficult problem to the reconstructing plastic surgeon. The effects of radiation on skin and soft tissue can be classified as acute and long term, but there is always permanent impairment to the tissue to some extent. Wound healing in radiated tissue is slower and more prone to dehiscence and/or infection. Prosthetic breast reconstruction that has been irradiated can be compromised aesthetically compared with nonirradiated reconstructions. The addition of ADM to prosthetic breast reconstructions has improved the results and reduced complications compared with those reconstructions without this material that have been irradiated. The author’s technique for using ADM is presented in detail. An algorithm is also presented, which assists in thinking about and planning the reconstruction.
References
1. E. Bernstein, F. Sullivan, J. Mitchell, et al. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg. 1993;20(3):435-453.
2. P. DeMoulin. A short history of breast cancer. Boston: Martinus Nijhoff; 1983.
3. M. Lehman, B. Hickey. The less than whole breast radiotherapy approach. Breast. 2010;19(3):180-187.
4. J. Burns, J. Mancoll, L. Phillips. Impairments to wound healing. Clin Plast Surg. 2003;30(1):47-56.
5. C. Goodman, R. Miller, C. PatrickJr., et al. Radiotherapy effects on expanded skin. Plast Reconstr Surg. 2002;110(4):1080-1083.
6. L. Dvali, A. Dagum, C. Pang, et al. Effect of radiation on skin expansion and skin flap viability in pigs. Plast Reconstr Surg. 2000;106(3):624-629.
7. E. Krueger, E. Wilkins, M. Strawderman, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):713-721.
8. D. Christante, S.E. Pommier, B. Diggs, et al. Using complications associated with postmastectomy radiation and immediate breast reconstruction to improve surgical decision making. Arch Surg. 2010;145(9):873-878.
9. E. Vandeweyer, R. Deraemaecker. Radiation therapy after immediate breast reconstruction with implants. Plast Reconstr Surg. 2000;106(1):56-58.
10. C. McCarthy, A. Pusic, J. Disa, et al. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116(6):1642-1647.
11. J. Ascherman, M. Hanasono, M. Newman, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117(2):359-365.
12. S. Spear, C. Onyewu. Staged breast reconstruction with saline filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105(3):930-942.
13. A. Parsa, D. Jackowe, W. Johnson, et al. Selection criteria for expander/implant breast reconstruction following radiation therapy. Hawaii Med J. 2009;68:66-68.
14. S. Kronowitz, K. Hunt, H. Kuerer, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113(6):1617-1628.
15. N. Fine, E. Hirsch. Keeping options open for patients with anticipated postmastectomy chest wall irradiation: immediate tissue expansion followed by reconstruction of choice. Plast Reconstr Surg. 2009;123(1):25-29.
16. P. Cordeiro, A. Pusic, J. Disa, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113(3):877-881.
17. C. Salzberg, A. Ashikari, R. Koch, et al. An 8-year experience of direct to implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127(2):514-524.
18. K. Breuning, S. Warren. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55(3):232-239.
19. A. Salzberg. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57(1):1-5.
20. R. Zienowicz, E. Karacaoglu. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120(2):373-381.
21. B. Topol, E. Dalton, T. Ponn, et al. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann Plast Surg. 2008;61(5):494-499.
22. S. Spear, P. Parikh, E. Reisin, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418-425.
23. A. Losken. Early results using sterilized acellular human dermis (Neoform) in postmastectomy tissue expander breast reconstruction. Plast Reconstr Surg. 2009;123(6):1654-1658.
24. C. Basu, M. Leong, M. Hicks. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg. 2010;126(6):1842-1847.
25. E. Komorowska-Timek, K. Oberg, T. Timek, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg. 2009;123(3):807-816.
26. A. Uzunismail, A. Duman, C. Perk, et al. The effects of acellular dermal allograft (AlloDerm) interface on silicone-related capsule formation—experimental study. Eur J Plast Surg. 2008;31:179-185.
27. W. Siggelkow, A. Faridi, K. Spiritus, et al. Histologic analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials. 2003;24:1101-1109.
28. M. Newman, K. Swartz, M. Samson, et al. The true incidence of near-term postoperative complications in prosthetic breast reconstruction utilizing human acellular dermal matrices: a meta-analysis. Aesthetic Plast Surg. 2011;35:100-106.
29. M. Nahebedian. AlloDerm performance in the setting of prosthetic breast surgery, infection and irradiation. Plast Reconstr Surg. 2009;124(6):1743-1753.
30. M. Seruya, M. Cohen, S. Rao, et al. Two-stage prosthetic breast reconstruction using AlloDerm: a 7-year experience in irradiated and nonirradiated breasts. [abstract]. Plast Reconstr Surg. 2010;126(Suppl 4):22-23.
31. S. Coleman. Structural fat grafting. St Louis (MO): Quality Medical Publishing; 2004.
32. G. Rigotti, A. Marchi, M. Galie’, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409-1422.
33. J. Serra-Renom, J. Munoz-Olmo, J. Serra-Mestre. Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: formation of new subcutaneous tissue. Plast Reconstr Surg. 2010;125(1):12-18.