30.1 Introduction
In criminal and civil trials, DNA is one of the most potential and prime evidence that plays a vital role in the framing of judgement. Personal identification, determination of maternity and paternity, and knowing the involvement of personnel, among others are possible with the invention DNA typing technique. Compared to various others, the DNA technique in some ways is more authentic. It is also considered as ‘new gold standard’ of scientific evidence and at times even circumvent other established evidence such as fingerprints. During the middle 1980s, the DNA technique was introduced by Sir Alec Jeffreys and his team of the University of Leicester, UK (Lynch 2003; Ansell 2013).
Forensic DNA analysis may be defined as the process of identification and individualisation of biological evidence for legal proceedings using DNA technology. It is used in both criminal and civil cases. Criminal cases mostly involve aggravated assault, murder, rape, arson, grievous hurt, robbery, etc. In contrast, civil cases may include paternity disputes, proof of kinship, custodial disputes, etc. which may involve DNA typing. It helps establish a link between the suspect, victim and the crime scene (National Research Council (US) Committee 1992). In every case, evidence should be handled on a priority basis. It is imperative for every individual coming in contact with the exhibits to maintain the integrity and authenticity of the evidence. Maintaining the high and uniform standards of quality is imperious.
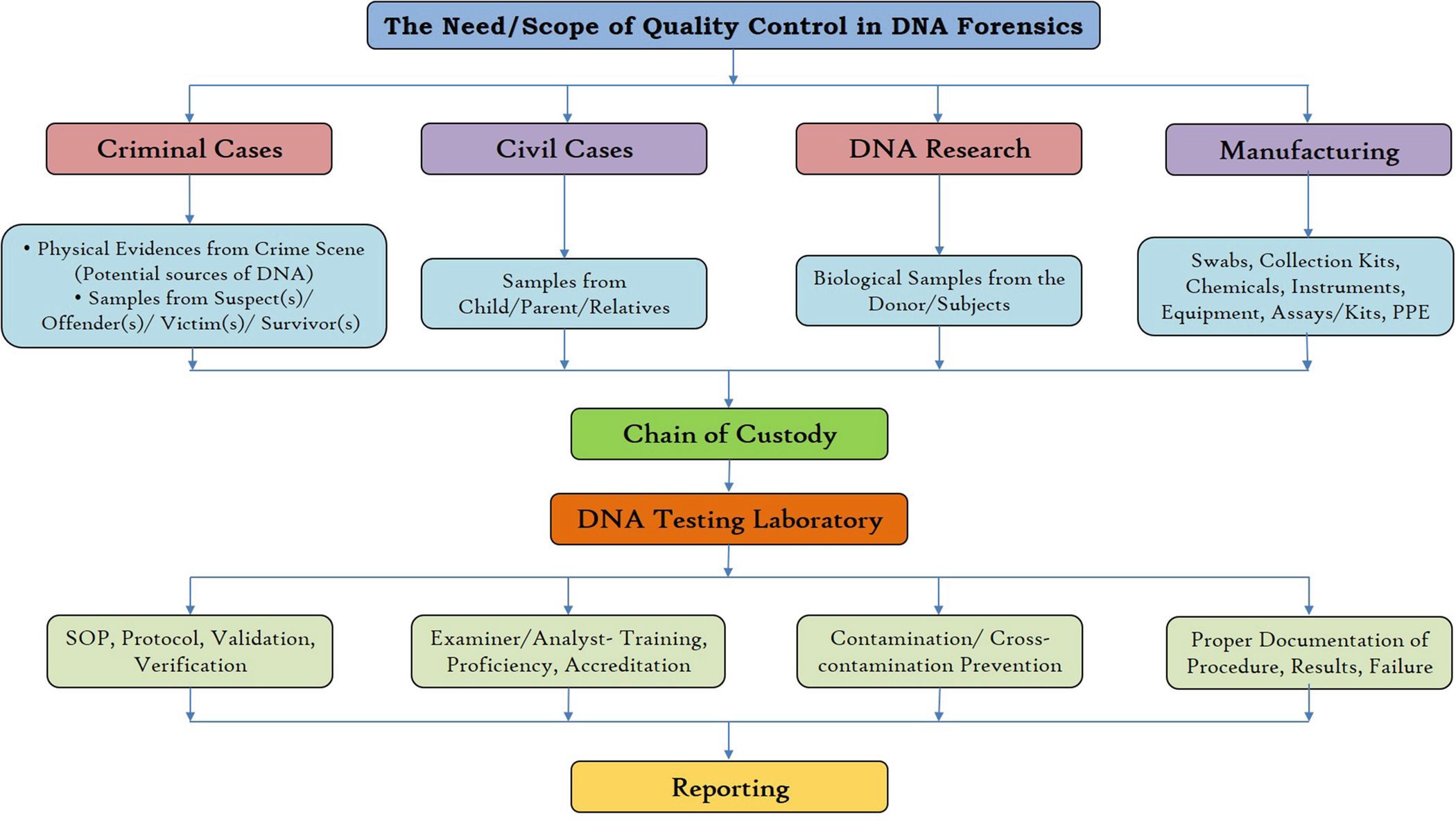
The need and scope of ‘quality control’ in DNA forensics
‘Quality control’ is the term associated with maintaining the quality of every small entity that proves its adherence to the said set of programs. The ISO 9000 defines quality control as ‘a part of quality management focused on fulfilling quality requirements (requirements related to quality)’ (ASQ n.d.). So, it applies to every section of testing where quality is the prime motto. Whereas, ‘quality assurance’ is referred to as ‘the systematic and premeditated approach to build confidence among the service and product, which is required for giving quality result’.
In DNA testing laboratories, quality control is critical because of the vulnerability to produce false (positive/negative) results. Forensic DNA analysis begins with the collection of biological evidences and continues until the report is finally admitted in the court proceedings and has withstood the scrutiny of the defence and the court. When considering the legal purview, the evidence is never treated directly or sent for DNA testing. It has to go through the pipeline before reaching the DNA testing laboratory. In many cases, it first passes through preliminary examination and then after several procedures, at last, is sent for DNA testing. Hence, the quality control in DNA testing is not limited to the quality of the testing laboratory but has to be taken into consideration during every step of the investigation. Lacking quality control can lead to the conviction of an innocent and exoneration of the guilty (Balažic and Zupanič 1999). The defence counsel raises not only the question on the DNA typing technique but also the procedure and methods used for DNA collection, preservation, documentation, chain of custody, reporting and even the proficiency of the analyst/expert (to name a few).
The ‘quality control’ (QC) is the check on routine operational technique followed by an expert for conducting the DNA analysis. While ‘quality assurance’ (QA) is referred to as the systematic and premeditated approach to build confidence in the testing to provide a consistent quality result. Each DNA testing laboratory has a set of pretested protocols that needs time to time progression/updating and regular validation of methods and instruments for reliable results. For consistency and transparencies in result, every DNA lab should undergo a regular audit, proficiency test, expert and routine training programs for DNA experts, and along with the accreditation of DNA laboratories (Butler 2005). ‘Validation’ is described as the procedure followed by the personnel in a laboratory is capable of producing a vigorous, trustworthy and reproducible results (Butler 2005).
The ‘proficiency test’ is an assessment of a laboratory’s performance in conducting DNA analysis procedures. These tests may be conducted periodically, or on a semi-annual basis, for each DNA examiner in the testing laboratory. The ‘ISO/IEC 17043:2010 Conformity Assessment—General Requirements for Proficiency Testing’ standard is used by internationally recognised accreditation bodies to accredit proficiency test providers. This standard also provides the purpose of proficiency testing. As per the guidelines of DNA Advisory Board Standard 13.1, this proficiency test can be performed by two ways, i.e., internal proficiency test and external proficiency test. In internal proficiency test, the DNA analyst has to undergo a performance test conducted by some other DNA expert within the lab/organisation/company. In external proficiency test, the proficiency of the analyst examined by some other experts belonging to a different organisation. The most effective way of external proficiency test is when it is performed without the knowledge of DNA analyst being examined. This type of proficiency test is termed as a ‘blind external proficiency test’. According to the guidelines, every examiner/analyst handling DNA examination should undergo a proficiency test within 180 days of service. The German DNA profiling group (GEDNAP) has built up a productive and successful blind proficiency-testing program (DNA Box 16.2) (Butler 2005). Further, the analyst engaged in DNA analysis must endure a proficiency test based on new Laboratory Validation Error 393 in typing. This Laboratory Validation Error 393 in typing was proposed by DNA Advisory Board Standards, which was then recognised as the National Standard in the United States (Butler 2005). In India, NABL provides the guidelines for the ‘Proficiency Testing Providers’ in accordance with ISO/IEC standards.
The ‘laboratory audit’ is one of the crucial aspects. It is a systematic examination/audit that may be performed by the laboratory management (internally) or may be conducted by calling experts from another independent organisation/laboratory. It is a substantial and obligatory requirement for the DNA laboratory to keep the audit record and the course of action undertaken to resolve the points/problems raised by the audit committee.
The ‘laboratory accreditation’ is the third-party verification related to the predetermined protocols, instrument performance, personal proficiency and regular audits of a DNA laboratory. Accreditation of a laboratory is the resulting outcome from the successful inspection by a recognised body of a country. It is essential to maintain good lab practices. The ‘accreditation process’ generally involves several steps such as a ‘laboratory self-evaluation, filing the application and supporting documents to initiate the accreditation process, on-site inspection by a team of trained auditors, an inspection report, and an annual accreditation review report’. The inspection evaluates the facilities and equipment, the training of the technical staff, the written operating and technical procedures and the casework reports and supporting documentation of the applicant laboratory (Butler 2005).
30.2 Quality Control at the Crime Scene
Quality control of the samples starts from the crime scene itself. The samples for DNA testing may be collected from crime scenes, victims, suspects and hospitals. When collecting the samples, one should follow the proper method, as making mistakes at this point will cause the failure of the entire procedure.
DNA evidence is collected as blood, hair, bone, teeth, seminal stains, other body fluids, etc. [7]. The type of material used for collecting this evidence also plays a role in quality control. The material used for the collection process should be completely free from any foreign or extraneous substance. The material should be completely sterile, and its quality should be checked before using it to avoid contamination of the sample. This can be achieved by manufacturing the material, especially for collecting DNA evidence under proper guidance. The cotton swab, filter paper, FTA paper modified cellulose, foam, nylon, polyester and rayon-tipped swabs used for collecting the evidence should be kept separately and labelled thoroughly (Eugene 2011). Every sample should be handled carefully to avoid contamination of original evidence with biological and non-biological samples. Contamination of evidence with non-biological origin sample may cause failure in results, whereas contamination with biological origin sample will change the result completely. It will result in a mixed genotype which will further cause problems in the interpretation of the result. This can be avoided only by handling the evidence with precautions, wearing gloves and avoiding mixing of the evidence by keeping them separate from the time of collection itself (Balažic and Zupanič 1999). Care should be taken to avoid contamination as well as cross-contamination.
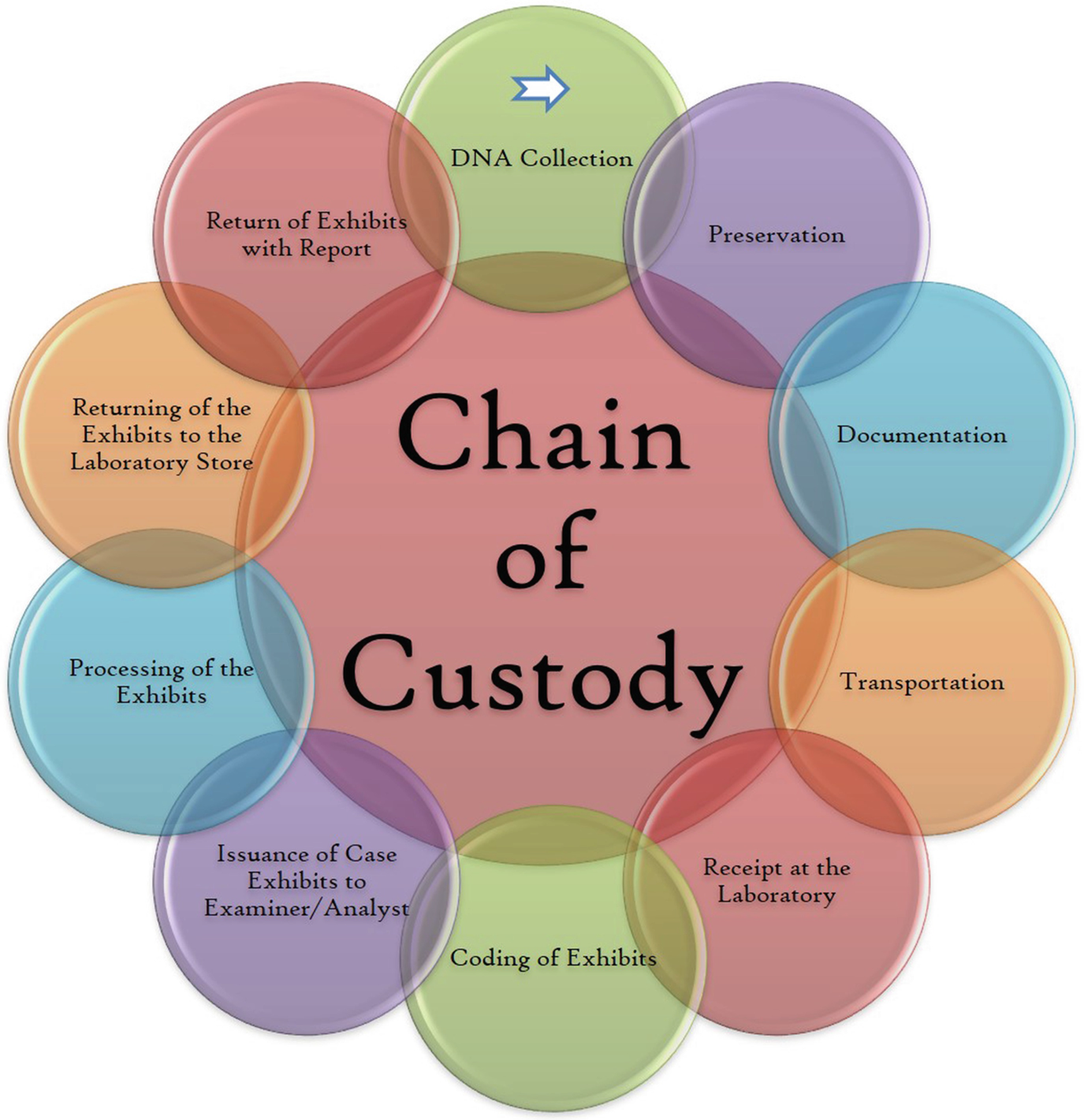
‘Chain of custody’ of DNA exhibits
30.3 Quality Control in the Laboratory
Every DNA testing laboratory should follow Quality Assurance Standards for Forensic DNA Testing Laboratories. The laboratories that perform DNA testing should always work according to the established standards while considering all the ways to maintain the quality of the results produced.
30.3.1 Personnel
The laboratory must have qualified DNA analysts who have experience in examination and testimony. It should have personnel with technical skills and other managerial staff with all required qualifications to complete the assigned work. At the time of hiring laboratory personnel, they must pass the competency test (to confirm his/her knowledge about the field). They must then be trained irrespective of their experience(s), with the updated testing procedures of the laboratory. Based on the expertise and the accuracy required, individuals may be assigned separate tasks.
30.3.2 Laboratory Manuals
The laboratory must have the manuals or SOP (standard operating procedures booklets) soliciting all the analytical procedures performed during testing for maintaining the consistency of testing procedures, and the result produced through it. The laboratory should always follow well established, tested and validated methodologies for DNA testing. The manual should be regularly maintained and updated from time to time, considering the accurate testing and innovations in the field. Along with manual methods, automated or robotic methods should also be validated. Care should always be taken to evaluate the updated procedure for maintaining consistency in the result. Every single modification or update to the software will require a routine check to maintain the integrity of results. Validation of the procedures adopted should be performed by the laboratory (Butler 2001). There should be safety manuals for the laboratory personnel. When dealing with ‘unique’ samples, the method may be modified as per the existing literature and/or experience of the senior analyst. However, the process should be documented and must be with appropriate justification.
30.3.3 Requirements
Fully equipped lab is the prime requirement for each testing laboratory, including reagents, apparatus, equipment, personal safety wears, etc. The laboratory should always use calibrated equipment. All equipment should be sterilised after every use. The use of reagents should always be according to the written protocol given in the laboratory manual/SOPs. The reagents that are prepared in the laboratory should also be labelled, including the composition, date of preparation, expiration, the identity of individual preparing reagents, and other relevant details. Depending upon the class of reagent, they should be kept in favourable/suitable storage conditions (dark bottle, away from sunlight, etc.). Logs of the reagents and chemicals used by the laboratory must be maintained as a routine practice. No one should be allowed to enter the laboratory without prior permission and appropriate authorisation. All the entries in and out of the laboratory should be documented and controlled.
30.3.4 Evidence Maintenance
Each laboratory should have a secure and governed area for evidence storage and testing materials (FBI n.d.). The samples, when received at the laboratory, should be appropriately marked using the common standards. The laboratory should also maintain the chain of custody of every piece of evidence, so that when the report is presented in the court, it should stand admissible. The evidence at the laboratory should be accessed by the dedicated personnel only.
Every time only a single sample should be analysed to avoid sample switching. It should be a common practice to try and avoid analysing the reference and suspected sample parallelly.
The result obtained should be uniform throughout the testing procedure. Regular inspection and professional assessments are also the critical factors in maintaining the testing laboratories. Professional assessments will involve internal and external testing. Internal testing will comprise of testing within the laboratory, i.e., the same sample will be tested by multiple personnel to check the consistency of the results. Whereas in external testing, the samples will be tested by authorised external bodies to check the consistency and/or variability of the results (Balažic and Zupanič 1999).
The laboratory should always be cleaned before and after the testing procedures. Care should be taken for not exposing the testing area to direct environment. Primary as well as secondary transfers must be prevented. The appropriate environmental and health safety guidelines and norms must be followed while disposing of the chemicals and waste.
30.3.5 Accreditation
Accreditation boards and agencies function to provide accreditation to the laboratories by assessing their technical competency. The laboratory accreditation services to testing and calibration laboratories are provided following ISO/IEC 17025: 2005 and ISO/IEC 17025: 2017 or other established standards that describes the general and/or specific requirements for the competence of testing and calibration laboratories. The guidelines, standards, and parameters followed vary from country to country. The results of accredited laboratories have the upper hand over their non-accredited counterparts. Regular auditing of the laboratories is the essential procedure of quality control. Table 30.1 shows the list of organisations providing accreditation to the Forensic DNA Testing Laboratories.
30.3.6 Proficiency Testing
This should be carried out regularly by participating in various proficiency testing schemes. It indicates the ability of the laboratory to produce excellent and valid results independently (Juniper 1999). Some accreditation body requires proficiency testing as their prerequisite. It can be performed in two ways by open testing and blind testing. The open testing states the purpose of testing, whereas blind testing will be done without prior information of sample analyses. Also, this can be done by sending the samples for testing to all participants and then comparing the results produced by them. This is also known as external quality assessment. These schemes focus on increasing the quality control standards of the laboratory (Dequeker et al. 2001). Table 30.2 shows the list of global institutions/organisations conducting proficiency tests for the DNA examiners.
30.3.7 Reporting of Non-conformance and Near-Failures
According to ISO/IEC 17025, the reporting of non-conformance and near-failures plays a vital role in the quality control of the laboratory. The non-conformance can be an instantaneous indication of errors or failure of the test or procedure, or it is possible that it may occur after a while.
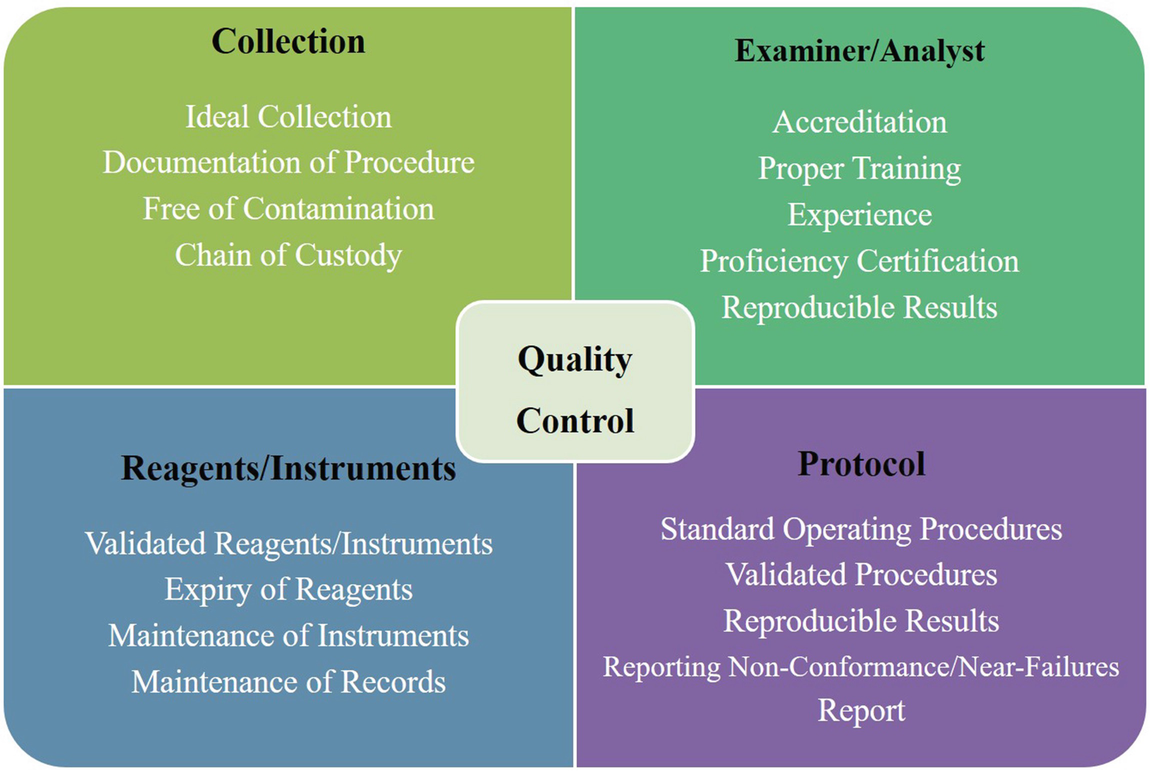
Quality control highlights
30.4 Quality Control in Reporting
The reports generated by the testing laboratories are admissible in the court unless the questions are raised on its authenticity. The laboratory should maintain everything in writing to support the conclusions drawn through it. The data should be maintained in hardcopy as well as in softcopy. It should be maintained in a defined format so that everyone could get it when going through it. It should contain all the details of the case and the process. The report will contain information including case identifier/serial number, detailed description of the evidence analysed, description of the procedures and techniques performed, result or conclusion, interpretation of the statements made, date issued, disposition of the evidence and lastly the signature of the expert/authorised person who performed the primary operations in the case. The laboratory should follow the rules for maintaining the privacy of the evidence and information related to it. The final report should be verified by more than one person before dispatch. The reports should never be biased. The laboratory should always hand over the reports to the concerned authorised person. There should not be any leakage of the information from the laboratory. The chain of custody should be maintained throughout the procedure (FBI n.d.).
30.5 Conclusion
In forensic DNA typing setups, ‘Quality control’ is one of the critical components in ensuring high quality and standards. The path of the evidence from the crime scene to the courtroom is quite lengthy and intricate. The characteristic unpredictability of every crime and the biological evidence thus recovered and analysed adds to the complexity. Different QCs along the whole process shall assist in providing justice by eliminating the chances of errors and thus being admissible in the court of law. Maintenance of the chain of custody as well as ensuring quality control measures at each and every step during the whole investigation shall be given utmost priority and importance.