Patients commonly ask about the use of marijuana for treatment of glaucoma, especially in the setting of increasing legalization across the United States and Canada. Currently in the United States, there are 33 states in addition to the District of Columbia, Guam, and Puerto Rico that have legalized either medical, recreational, or both uses of marijuana, while Canada has federally legalized the use of this substance.
The ocular endocannabinoid system has become a point of active research for its involvement in three separate functions within the ocular system: intraocular pressure (IOP) control, visual processing, and inflammatory regulation. Marijuana clinical trials in the United States remain limited in large part because of current Schedule I classification, defined as “no medical indication but high abuse potential,” but also because of its relatively short duration of action, high cost, ocular irritation with topical (eye drop) routes of administration, and general health risks and side effects [1]. Scientific references to the effect of marijuana on the ocular system have stemmed from 1971 and extend to present day.

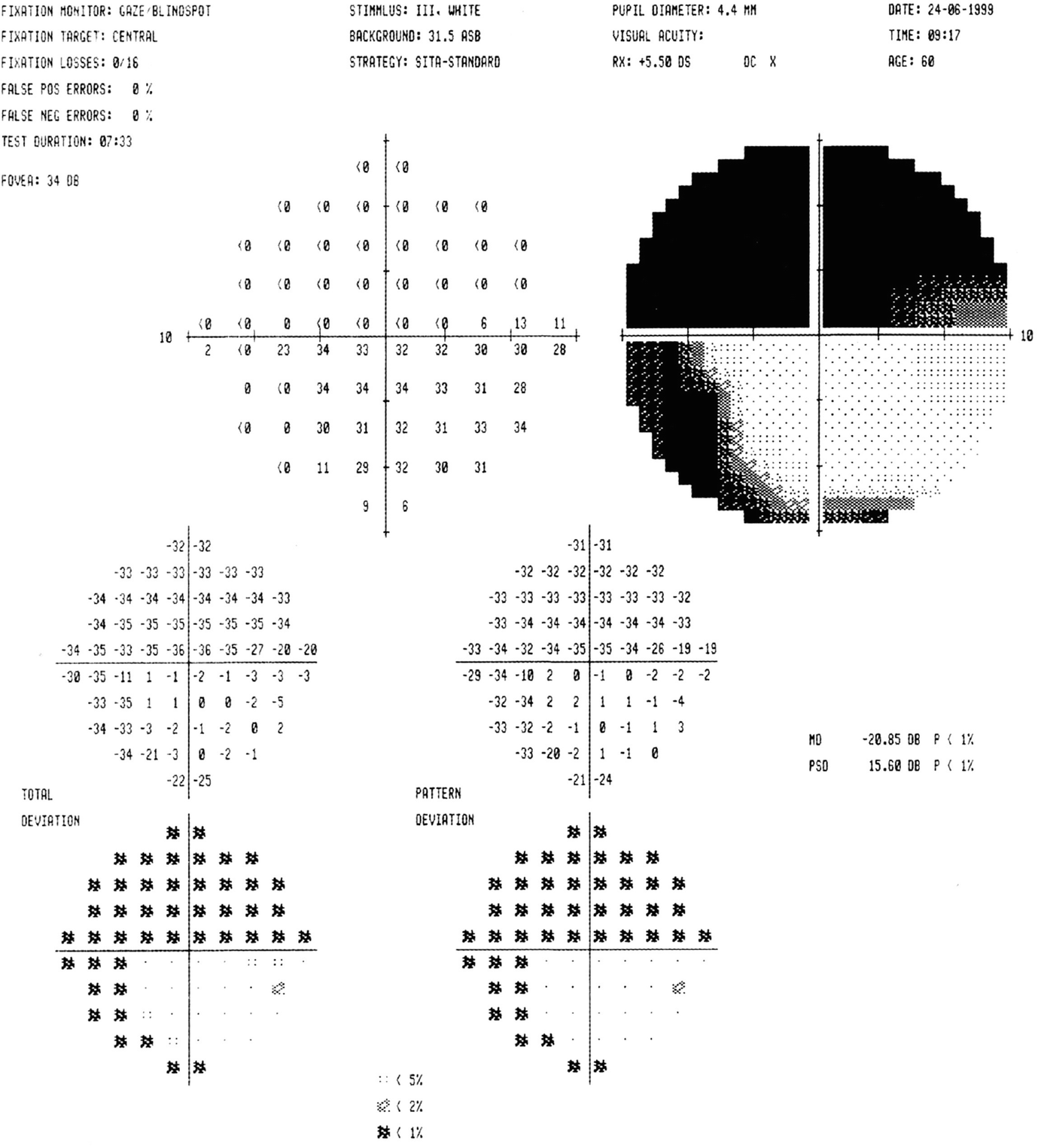
Humphrey visual field 24-2 testing in a patient with severe glaucoma, with dense peripheral constriction and defects approaching central fixation point [2]

(a) Representative diagram of the thinning of the nerve fiber layer and deepening of the optic nerve cup. (b) Glaucoma-related enlarged cup-to-disc ratio of the nerve, with nasalization of central vasculature [3]

Enlarged cup-to-disc ratio of the optic nerve secondary to long-standing glaucoma. Note significant thinning of upper and lower aspects of optic nerve [4]
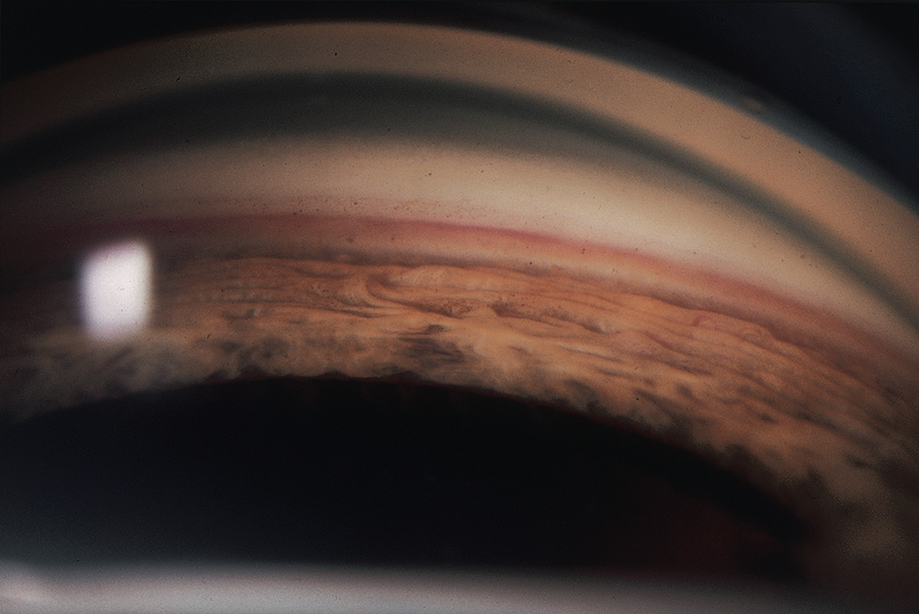
Gonioscopic evaluation reveals angle structures, which is helpful in identifying etiology or classifying type of glaucoma [5]
The loss of nerve fiber layer cells is manifested initially as diminished or absent peripheral vision but can ultimately result in complete loss of central vision. Researchers have not yet identified all of the triggers linked with POAG but have found multiple factors that put individuals at risk for development of the disease. These factors include older age, African American race, positive family history, decreased central corneal thickness, increased vertical cup-to-disc ratios of the optic nerve, and elevated IOP. Of these, the only modifiable risk factor is IOP, which is the target of modern-day glaucoma therapy.

Schematic diagram of aqueous production and outflow pathways within the human eye. Red arrow denotes the trabecular meshwork outflow pathway, while green arrow denotes the uveoscleral outflow pathway [6]
When there is excessive aqueous humor production or insufficient drainage, the IOP will rise. While the relationship between elevated IOP and glaucomatous optic nerve damage is well-supported by research, the exact causative mechanism is unclear.
Effective treatment of glaucoma, achieved with oral medication or topical eye drops, laser, or surgery, classically translates to sustained, continuous control of IOP. Oral carbonic anhydrase inhibitors or topical ocular antihypertensive drops are affordable solutions that are dosed once or twice a day and alter IOP by acting at different sites of the aqueous humor formation and drainage pathways. The mechanisms are well-described in other resources and are beyond the scope of this chapter. If IOP remains uncontrolled or if the patient does not tolerate oral or topical therapy because of systemic side effects, an ophthalmologist may offer laser therapy targeting the trabecular meshwork to increase aqueous humor outflow. Alternatively, there are surgical options for controlling elevated eye pressure, which include the use of drainage tube implants, scleral flaps, or, more recently, implantation of devices made of heparin-coated titanium and other materials to safely shunt fluid away from the anterior chamber toward other regions of the eye. If none of the above achieves sufficient IOP control and there is evidence of disease progression, the ciliary body epithelium can be destroyed with laser or cryotherapy to reduce aqueous humor production.
Despite these efficacious treatment options, there has been increasing public interest to find alternative, natural remedies to treat glaucoma, as well as other ocular diseases. The plant Cannabis sativa, from which marijuana is derived, has long been recognized to have medicinal properties [7]. In 1964, the active component of marijuana, delta 9-tetrahydrocannabinol (THC), was isolated and defined structurally [8]. The cannabis plant contains more than 100 unique chemical components classified as cannabinoids. These ingredients actively bind to specific endocannabinoid receptors in the brain and many other organs of the body, including the eye. Of note, the two most prevalent components of the cannabis plant are delta-9-tetrahydrocannabinol (THC), which creates a psychotropic effect in users, and cannabidiol (CBD), which has minimal psychotropic influence.
There are numerous ongoing studies to evaluate the efficacy of endocannabinoid (ECB)-based therapeutics for treatment of glaucoma, as well as other ophthalmic diseases including uveitis, ischemic retinal disease, diabetic retinopathy, and age-related macular degeneration. At a molecular level, endocannabinoids are endogenous lipids that act as neuromodulators targeting the same receptors within the body that bind THC and CBD. Endocannabinoids are different than other neuromodulators in that they are not synthesized in advance and stored in vesicles, but rather exist in cell membranes and are cleaved by specific enzymes on an as-needed basis [9].
ECB molecules such as 2-arachidonoylglycerol (2-AG), anandamide (N-arachidonoyl ethanolamine) (AEA), and N-palmitylethanolamide (PEA)
Classical and nonclassical receptors such as CB1 and CB2, GPR18, GPR55, TRPV1, and PPAR alpha/beta/gamma
Enzymes that synthesize and degrade ECBs such as diacylglycerol lipase (DGL), fatty acid amide hydrolase (FAAH), and many others [10]
The concentration of ECB receptors varies by the specific structure of the eye. For example, the retina and trabecular meshwork each contain many more receptors than the crystalline lens [11].
A recent study published by a group at Indiana University looked specifically at two cannabinoids found in marijuana, CBD and THC (materials supplied by the National Institute on Drug Abuse drug supply program, dissolved in Tocrisolve™solvent), and their effects on IOP [12]. The researchers found that a single topical CBD (5 mM) eye drop in mice increased IOP by 18 percent for at least 4 hours after administration compared to mice treated with the vehicle in the contralateral eye. This effect disappeared if the CB1 receptor was knocked out prior to drop use. This finding implicates the CB1 receptor as a key target for these molecules. On the contrary, the group also found that a single topical THC (5 mM) eye drop decreased eye pressure by up to 30 percent within 8 hours. Interestingly, THC drops affected IOP differently in male and female rats, with males having a much larger decline in pressures secondary to THC compared to females. These results highlight that the IOP effects of THC and CBD may counteract one another in a complex mechanism, with a biologic variable potentially involved as well. Ultimately, this study shows that additional research is necessary to further understand the effects of these compounds on IOP (including translational research in human beings, using well-tolerated formulations) and that marijuana cannot be safely or reliably used as monotherapy for diseases like glaucoma.
A review of current literature reveals that many synthetic cannabinoids, which bind to CB1 receptors similar to THC or CBD, are being studied for their effects on various aspects of the ocular system. Specifically, IOP can be reduced by synthetic aminoalkylindoles (e.g., WIN 55212-2) via a mechanism similar to topical beta blockers that are used for glaucoma, but without a sustained effect [10, 13]. Additionally, studies have shown that CB1 agonists appear to have a neuroprotective effect on retinal ganglion cells in ischemia-reperfusion settings, with the co-administration of CB1 antagonists blunting this beneficial effect. Nevertheless, the potency or duration of effect remains unspecified [10].
Recent studies have also shown CB2 receptor agonists temper inflammation within the eye, as is commonly seen with disease states like anterior, intermediate, and posterior uveitis. Specifically, studies have shown that these molecules decrease inflammatory cell infiltration in the retina, reducing overall cellular infiltrates and granulomas in a dose-dependent manner [14]. Toguri et al. (2014) similarly demonstrated that the topical CB2 receptor synthetic agonist, HU-308 (dissolved in Tocrisolve solvent for topical application at 1.5%), reduces leukocyte-endothelial adhesions within iridal microcirculation of rats, dampening inflammation more significantly than comparable treatments (e.g., topical steroids or nonsteroidal anti-inflammatory medications) [15]. This effect was dampened with the co-administration of CB2 receptor antagonist, AM630, administered by intravenous route. These findings suggest a role for the CB2 receptor pathway in moderating intraocular inflammation.
Studies have also demonstrated potential therapeutic benefits of CB1 and CB2 receptor agonists on corneal nociceptors causing pain in acute corneal pathology, such as alkali burns. Yang et al. showed that receptor activation by synthetic cannabinoids like WIN 55212-2 appears to stimulate corneal wound healing and stromal thickening at a more rapid rate [16]. Furthermore, the study showed that CB1 −/− mice had more significant CD11b staining, consistent with upregulated monocyte and neutrophil stromal infiltration associated with increased corneal fibrosis compared to WT mice, suggesting the necessity of the ECB system for this healing process without overactivation of the innate immune response. On an unrelated note, disease states like diabetic retinopathy and age-related macular degeneration are associated with lower levels of ECB-degrading enzymes, suggesting a role for the ECB system in the pathophysiology of these blinding conditions [10].
Finally, in 2007, the oral synthetic cannabinoid, dronabinol (7.5 mg Marinol, Unimed Pharmaceuticals, Chicago, IL, USA), was studied in its effects on lowering IOP as well as influencing retinal hemodynamics [17]. Eight healthy medical doctors underwent IOP testing, blood pressure measurement, and fluorescein angiography with scanning laser ophthalmoscopy before and 2 hours after ingestion of the substance. There was a statistically significant decrease in IOP (13.2 to 11.8 mmHg, p = 0.038) as well as a reduction in retinal arteriovenous passage time (on average, 1.77–1.57 seconds, p = 0.028), without a significant effect on blood pressure or heart rate. The researchers concluded that dronabinol could be potentially useful in ocular circulatory disorders including glaucoma, where decreased retinal circulation is often noted. On the contrary, in a study published in 1978, researchers found that this same substance increased blood flow through the iris, ciliary body, and choroid, but not the retina in rabbits [18].
While these may be interesting discoveries in various animal models, there remains a large gap between our understanding of the ocular endocannabinoid system and the implementation of ECB-based therapy to successfully treat human ophthalmic disease. Furthermore, there is a large difference between the purified, synthetic agonists used in these studies and dispensary cannabis ingested by patients who are intending to self-treat ophthalmic disease, the latter of which often contains over 480 chemicals including at least 66 cannabinoids that can vary greatly in concentrations and safety profiles depending on the source [19]. The lack of consistency counters the requirements of regulatory bodies like the FDA with regard to standardized and accepted treatment options for human disease. Future research will likely be directed toward identifying biased agonists or allosteric modulators with increased receptor affinity that can decrease the required drug dose necessary to treat disease, as well as isolating inhibitors of ECB-degrading enzymes. This could potentially increase duration of drug effect while reducing behavioral and systemic side effects associated with marijuana use. Future research must also transform from animal models into more reliable human clinical trials before any ECB-based therapies could be safely recommended for patients with glaucoma and other ocular disease.
Proponents of marijuana use for glaucoma treatment cite a study from the early 1970s, where Hepler and Frank showed both marijuana and one of its ingredients, THC, could reduce IOP [20]. Over the years, other studies have confirmed the IOP-lowering effect of THC by various modes of administration including inhalational [21], oral [22], intravenous [23], sublingual [24], and topical routes [25].
However, in order to achieve therapeutic levels of marijuana in the bloodstream to treat glaucoma, an individual would need to smoke approximately six to eight times a day [26]. At this frequency, an individual would likely be physically and mentally unable to perform tasks requiring attention and focus (e.g., studying, working, driving). This dosing frequency could be even higher with the pharmacologic tolerance that a patient would develop with chronic use of this substance [27, 28]. Studies also indicate a direct relationship between drug concentration and decrease in IOP from baseline; higher concentrations result in greater reductions in pressure. This would translate to reliance on extremely potent forms of marijuana for adequate treatment of disease. These studies did not find a relationship between potency and duration of effect, so one may still need to smoke every 3–4 hours around the clock, regardless of the substance potency, to achieve any purported therapeutic effect [19].
Oral and sublingual consumption is associated with variable systemic absorption as well as poorly tolerated global side effects [22, 26]. Given this, the topical eye drop approach would appear to be an optimal route of administration. However, delta-9-tetrahydrocannabinol (THC) has been investigated in the form of topical application, and these forms were found to be less effective compared to inhalational, oral, or intravenous marijuana [29]. This is may be because ocular penetration with THC compounds is poor due to the highly lipophilic and poorly hydrophilic nature of the cannabinoid extracts. Furthermore, these substances commonly create intolerable ocular irritation, conjunctival hyperemia, decreased lacrimation, and corneal damage [29]. Overall, several studies have failed to demonstrate a substantial hypotensive effect of topical THC within the eye [29, 30].
The American Glaucoma Society position statement in 2010 declared that there is no scientific basis for the use of marijuana in the treatment of glaucoma “unless a well-tolerated formulation…with a much longer duration of action is shown in rigorous clinical testing to reduce damage to the optic nerve and preserve vision.” In addition to marijuana failing to achieve sustained IOP-lowering effects, the organization notes that the drug is associated with tachycardia and systemic hypotension, a potentially devastating effect on a glaucomatous optic nerve that may already have inadequate blood supply [26, 31]. While the organization expresses optimism that a locally administered, well-tolerated drug targeting the ocular ECB system can potentially augment IOP control, it expressly disavows the use of marijuana in any form as a primary or sole treatment for glaucoma.
The American Academy of Ophthalmology issued statements in 2009 and 2014, from their Complementary Therapy Task Force, stating that “no scientific evidence has been found that [marijuana] demonstrates increased benefit or diminished risk in the treatment of glaucoma compared with the wide variety of pharmaceutical agents now available” [32]. Similarly, in 2010, the Canadian Ophthalmological Society of Eye Physicians and Surgeons stated it “does not support the medical use of marijuana for the treatment of glaucoma due to the short duration of action, the incidence of undesirable psychotropic and other systemic side effects and the absence of scientific evidence showing a beneficial effect on the course of the disease. This is in contrast to other more effective and less harmful medical, laser, and surgical modalities for the treatment of glaucoma” [33]. In November 2018, the Canadian Ophthalmological Society issued another position statement discouraging medical use of cannabis for dry eye disease, “due to its undesirable side-effects, including dry eye symptoms if smoked, and the absence of scientific evidence showing any beneficial effect at this time” [34].
Navigating the complex and intricate web of factors that influence public perception with respect to glaucoma and medical marijuana will require an equally intricate patient-centered approach if physicians who treat glaucoma patients are to effectively address false perceptions and transcend the clash between scientific evidence and truth versus popular culture [35]. This undeniable clash places unprecedented importance on considering the patient perspective. It is not enough to simply detect patient misconceptions on the topic of marijuana and glaucoma. We must mold our practices to correct these errors in understanding using a patient-centered compassionate and evidence-based approach.
In 2015, Belyea and colleagues published a study that investigated the attitudes of glaucoma patients in Washington, DC toward the legality of marijuana and its use as a potential treatment agent [36]. This study was performed in the context of legalization of the drug in the city as well as 21 other states for medicinal purposes. Approximately 59.8% of study participants (122 of 204) reported awareness about the potential use of marijuana to treat glaucoma. The authors identified that the following factors contribute to patient intention to use marijuana for treatment of their glaucoma: younger age, lower level of education, and prior marijuana use. Newer factors contributing to this decision now include increasing legalization of marijuana, false beliefs regarding marijuana’s efficacy in glaucoma treatment, and disregard for the associated costs to purchase and use the drug. The authors stressed that the best way to alter these perspectives is to place greater emphasis on evidence-based reasoning and to offer a thorough explanation of FDA-approved glaucoma therapies.
Interestingly, Belyea et al. found that patient satisfaction with current glaucoma management options was dependent on a combination of personal, cultural, socioeconomic, and health-related factors that were all superimposed on past experiences with healthcare services. In their study, the physician’s ability to convey respect and empathy for patient needs and values, engagement of family members, and capacity to provide emotional support alongside high-quality, easy-to-comprehend educational materials were the key ingredients to improved patient-centered care and outcomes.
Perhaps this is a reminder for physicians to sit down and spend greater time with each of our patients and their family members in our clinics, listen more attentively, and care more deeply about their concerns regarding their ocular health. This will establish a greater degree of trust in the physician-patient relationship as we move forward together toward newer treatment options and scientific discoveries.
The clinical utility of marijuana for the treatment of glaucoma and other ocular diseases is limited by the short duration of effect on intraocular pressures, propensity for tolerance, and inability to separate reproducible therapeutic action and benefit from the undesirable physical, neuropsychological, and behavioral effects of the drug.
Delta-9-tetrahydrocannabinol (THC) appears to lower intraocular pressure, but recent studies show cannabidiol (CBD) may increase intraocular pressure; both are found in marijuana and can cause tachycardia and systemic hypotension, which can compromise vascular flow to an otherwise unhealthy optic nerve in glaucoma.
Patients should be counseled regarding the lack of reputable scientific evidence demonstrating superiority of cannabinoid-containing products over FDA-approved and currently used medications, laser, and surgical interventions to treat glaucoma and other ocular disease.