according to some, egg yolk is the best sauce in the world—I could not agree more. Who does not love the silky, salty, warm taste of a runny egg yolk? More people than I would have thought, regrettably (for them). What are the reasons behind the polarizing attitudes toward runny egg yolk? Texture? Taste? Food safety? Ignorance?
Runny yolks find their way onto our tables in one of three ways: as poached, soft-boiled, or sunny-side-up eggs. In these preparations, the culinary objective (or more correctly, my objective) is to cook the white long enough that it sets, while warming up the yolk to a point that it develops some viscosity, or a thickened mouthfeel, but remains free to flow. Getting the right texture is easier said than done. At least in the case of a poached or fried egg, we can see the color and texture developing before our eyes, but what about a soft-boiled egg? There is no way to know until it is already—hopefully not—too late.
Before we jump into deeper deliberations, let me first reflect on the term soft-boiled. What does it describe—the cooking conditions or the desired texture? Cooking in boiling water implies that the cooking ing temperature is about 212°F (100°C). It follows that “soft” would refer to the texture of the egg, but it is still unclear to what part of the egg—the yolk or the white? To complicate things even more, some may argue that “soft boiling” is a synonym for simmering, also known as the “lazy bubble stage,” in which water temperature can be anywhere between 180 and 195°F (80–90°C). I hereby propose that when we refer to shell-on eggs cooked by immersion in hot water, we are speaking of the desired texture of the yolk. This is because, as I will demonstrate, any yolk texture is achievable by selecting the right time-temperature combination.
But what is an egg yolk, exactly?
According to Harold McGee (2004), the word yolk comes from the Old English word geolu (yellow), which in turn, derives from an Indo-European root meaning “to gleam or glimmer.” Technically speaking, raw egg yolk is simply an emulsion of fat in water. It represents approximately 32 percent of the weight of an intact egg and it is composed of about 50 percent water, 32 percent fat, and 17 percent protein. Much of its color, an element of quality, comes from a family of compounds known as carotenoids, which are normally absorbed from the animal’s corn or grass feed. Many of the proteins and lipids contained in an egg yolk provide much of its known functionality as a foaming, emulsifying, or texturizing agent. It is then not surprising to find it as part of a wide range of dishes, savory and sweet alike, in which the cook takes advantage of one, two, or all three functionalities in a single dish. Its emulsifying power is obvious in the making of mayonnaise, hollandaise, and gribiche sauces. Crème anglaise and crème brûlée are possible thanks to the egg yolk’s water-holding capacity, whereas sabayon relies on the yolk’s thickening and foaming properties. Although I have a deep personal interest in all these functions, I thought that I should begin with the thickening process that the yolk undergoes when eggs are simply immersed in hot water.
There are countless ways of preparing soft-cooked eggs, probably as many as there are home cooks. What is your way? Mine is as follows: I boil water; add the eggs directly from the refrigerator (up to five eggs at once, provided there is enough water to immerse the eggs by at least ¼ inch [0.5 cm]); keep them in boiling water for about 6 minutes; remove them and cool them under cold running water—the usual and rather gratifying result is a set, soft, and pliable white married to a yolk that is almost as viscous as honey. Regardless of the method, what matters is the outcome: your desired outcome.
Some professional cooks have taken a relatively new approach to the cooking of eggs. They realize that cooking eggs in boiling water allows for little or no control of the speed at which the eggs are cooked. The use of a temperature-controlled water circulator, or water bath, allows for new possibilities. Using this device, chefs have now ventured into cooking eggs at relatively low temperatures (140–160°F [60–70°C]) for relatively long periods of time (at least 1 hour). As a result, it is rather common to find the so-called 6X°C egg on many menus around the world. I call it the 6X°C egg because the second digit varies, depending on the restaurant (from 1 to 5, but a 7 can be found as well). To make matters more complicated, the cooking time for the 6X°C egg also varies. It might seem reasonable to assume that the texture of the eggs cooked under these rather variable conditions should be different. But is it?
Some scientists and chefs familiar with this technique suggest that once the desired internal temperature of the yolk has been reached, the egg can remain at the temperature for a long time, its texture will not be influenced. I found this hard to believe and, to some degree, an oversimplification of the physical chemistry involved: egg cooking is all about texture development, a process driven by protein dena-turation and the rate at which it occurs. In this context, the time element is equally as important as the temperature element. Everybody knows that an egg cooked in boiling water for 5 minutes tastes and feels completely different from one cooked for 15 minutes! Several questions are key to my gallinaceous enterprise: Are there significant textural differences among all these 6X°C eggs? How can the texture of these eggs be better described? How does the chef conclude which time-temperature relationship is optimal? How versatile is the technique (that is, can we get the same results by using different time-temperature combinations)? Only a systematic and experimental approach can shed light on the matter.
To learn a bit more, I called a few chefs to find out under what conditions they cooked their 6X°C eggs, and this is what I found: Chef 1 cooked the eggs at 62°C for 2 hours; Chef 2 at 62.5°C for 1 hour; Chef 3 at 61°C for 1 minute per gram of egg; Chef 4 at 63.5°C for 55 minutes; Chef 5 at 63°C for 1 minute per gram of egg; and Chef 6 at 62.5°C for 40 minutes. How different was the texture of the eggs cooked under these different conditions? I had to find out.
I decided to study the cooking of the yolk and white separately because their composition and location within the egg pose different practical challenges. My focus—based on my proposal that the texture of egg yolk indicates the degree of doneness of the egg—was then on the texture development, or thickening, of the yolk. To grasp the effect of temperature and time on egg yolk thickening, I first needed to measure the increase in temperature of the yolk once the egg was placed inside the water bath. After all, the thickening process is primarily dependent on temperature. I found that the temperature increased linearly with time until it reached about 120°F (50°C), after which it started to slow down as it approached thermal equilibrium. Knowing the “heating rate” of the yolk enables the mimicking of such conditions in the texture analyzer and accounts for the overall cooking time. The experiments were conducted using eggs weighing 2½ ounces (70 g).1
Avoiding oversimplification, I divided the heating process of the yolk into four distinct steps. Step 1 is the time it takes for the yolk, at a fixed water bath temperature (for example, 147°F [64°C]), to reach an internal temperature of 140°F (60°C), which is the point at which the most labile protein in egg white, ovotransferrin, denatures.2 Step 2 is the elapsed time between 60 and 6X°C (where X is the second digit of the desired cooking temperature, in this case 4), in which nonnegligible changes in the overall egg texture occur. This is because the heating rate is now considerably slower, and 10 minutes or more can elapse before the desired temperature is reached. Step 3, the isothermal step, corresponds to the time the egg is cooked at 6X°C. Step 4 is the time it takes to cool the egg, in ice water, back to room temperature, which stops the cooking process (and thereby the protein denaturation process).
Recall that the aim of my gallinaceous enterprise was to be able to quantify and describe the textural changes of egg yolk as a function of time and temperature. This meant I had to evaluate how sensitive egg yolk is to small changes in either cooking time or temperature. In this context, is there a significant textural difference in yolks cooked for 15 or 30 minutes at 149°F (65°C) or by holding them for 30 minutes at 145 and 147°F (63 and 64°C)? To answer this question, I tested a total of sixty-six different time-temperature combinations.

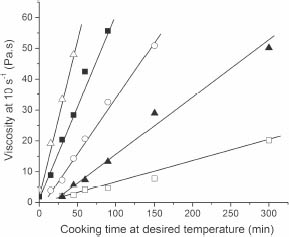
Figure 29 Viscosity (at 10 s-1 and 23°C) of cooked egg yolks as a function of isothermal cooking time at different temperatures: 60 (?), 62 (A), 63 (O), 64 (?). and 65°C (A). The lines represent the fit of the linear regression of the data.
I was so impressed by the results! It seemed that, no matter what the temperature the egg was cooked at, it continued thickening linearly with time (figure 29). This is the same as saying that for every minute spent at 6X°C, the viscosity increased by the same amount, which suggests that we might actually be able to predict or, better yet, design the final texture of an egg yolk!
What was equally fascinating was how an increase of only 1.8°F (1°C) in cooking temperature made a tremendous difference in the final texture of the yolk. Figure 29 shows the final viscosity of egg yolks cooked at different and constant temperatures (each set of points) for different lengths of time. You can observe that, for instance, cooking an egg for 30 minutes at 140 or 144°F (60 or 62°C) makes absolutely no difference in the yolk’s final texture (2 Pa.s). (For reference, a viscosity of 1 Pa.s is already one thousand times more viscous or thicker than water.) The relative and physical meaning of these numbers will become clearer later. If the temperature is raised by just 1.8°F (1°C) to 146°F (63°C), the viscosity increases by almost a factor of four (7 Pa.s). Take the temperature up a notch to 147°F (64°C), and the viscosity shoots to 20 Pa.s; at 149°F (65°C), it is 33, and at 151°F (66°C), it is 54 Pa.s (not shown). I also cooked the eggs at 152, 154, 156, and 158°F (67, 68, 69, and 70°C), but the yolks were way too viscous for the measurement to have any practical meaning. I was now convinced that something very useful would come out of this.
What do all these Pa.s units really mean to you, me, or the chef, anyway? How can we translate these changes in viscosity into a language we all can understand and apply during cooking? There is a need, at least in my mind, for descriptors of texture for cooked egg yolk. Terms like viscous, runny, or thick remain inadequate and ambiguous for the wide range of textures that can be achieved. Perhaps the key in refining our description of texture is to associate yolks with other food products, making it possible to describe—and even better, demand—a sought-after eating experience. So, what other foods does thickened yolk look or feel like? Table 1 lists the viscosity of a variety of foods that are often perceived as thick or viscous. The values were measured at conditions that resemble the deformation that foods undergo within the mouth. The significance of the table is that it represents our own mini library of textures that can be used as a tool to describe the texture of cooked egg yolk. The library spans from relatively fluid foods, like pancake syrup, to the more solid toothpaste and Marmite (the British yeast spread).
So, what is the texture you look for when cooking, or more important, eating egg yolks? We now have a series of model viscous foods from which to choose. Say you like egg yolk with a honey-like texture. What does this mean in terms of time-temperature settings? From the texture library, we can observe that this corresponds to a viscosity of roughly 18 Pa.s. Next, you would need to plan ahead and decide whether you have time to spare (that is, at least 1 hour) or whether you are cooking “à la minute,” which means you would need to plate the dish within the next 10 to 20 minutes. If you were to use figure 30 to find out how long you would need to cook your eggs to achieve a honey-like texture, you would either hold them for about 300 minutes at 140°F (60°C), 60 minutes at 146°F (63°C), or 15 minutes at 149°F (65°C). Now, if you stop and reflect for a moment (but please, not while the eggs are in the pot!), you might notice that figure 29 plots the isothermal time only. Therefore, it does not include the time it takes for the eggs to reach the desired cooking temperature. As it happens, I have created a master plot that not only accounts for this but also eliminates the need for dealing with rheo-logical units (figure 30).
Figure 30 Isoviscosity lines that relate the holding temperature to the total cooking time needed to develop a characteristic texture in egg yolks (by immersion of eggs in a water bath). Each line represents a desired texture—any combination of time and temperature that falls on or close to any line would render the texture assigned to that line: (O) sweetened condensed milk, (?) chocolate pudding, and (?) cookie icing. The inset shows a close-up of the high-temperature region, where cooking occurs at a faster rate.
Figure 31 Visual cue into the viscosity of egg yolks cooked in the rheometer at a constant temperature of 63°C. The cooking times from left to right are 15, 45, and 90 minutes, respectively. The photos were taken once the samples were cooled to room temperature and 15 seconds after removing the bottom cap of the sample holder (held horizontally).
Once the chef or home cook is clear on the sought-after egg yolk texture, or mouthfeel, this plot becornes the tool to define what the optimal cooking conditions will be. If we are looking for a thick yolk, like cookie icing, we would need to cook the egg for either 8 hours at 140°F (60°C), a bit more than 5 hours at 142°F (61°C)—3 hours saved by only 1.8°F (1°C)!—or about 45 minutes at 151°F (66°C). The lower the cooking temperature, the more forgiving the process becomes. The corollary is that cooking at 153°F (67°C) or higher and having the desire to create significantly different yolk textures will require an expert familiarity with the technique (see inset in figure 30). To visualize how the structure, or viscosity, and hence the mouthfeel of the yolk changes as a function of time at a constant temperature, see figure 31.
I believe that this study is a good practical approximation of texture design for a simple thermally treated egg yolk. It has a place in kitchens that practice science-based cooking as a smart tool that, ironically, stops the cook-and-look approach. The obvious challenge before us is to apply this analysis to the textural metamorphosis of the egg white. We can then combine the results and predict or design the texture of the perfect soft-cooked egg. If such a journey proves to be a platonic one, I would just scramble and cook the egg inside its shell and worry about only one thing: How will it taste?
Gadsby, Patricia. 2006. “Cooking for Eggheads.” Discover Magazine, February. Available at http://discovermagazine.com/2006/feb/cooking-for-eggheads.
McGee, Harold. 2004. On Food and Cooking: The Science and Lore of the Kitchen, 70. New York: Scribner.
This, Hervé. 2006. Molecular Gastronomy: Exploring the Science of Flavor, 29–31. Translated by Malcolm DeBevoise. New York: Columbia University Press.
Vega, C, and R. Mercadé-Prieto. 2011. “Culinary Biophysics: On the Nature of the 6X°C Egg.” Food Biophysics 6:152–159.
1 Significant differences can be expected when using eggs that are at least 5 grams more or less heavy than the ones used in this experiment.
2 Even though the focus of the study was on egg yolk, it was important to include the temperature sensitivity of all proteins because eventually they will be integrated into one “whole egg” scenario.