15
Biocultural Coconstruction of Brain Plasticity across the Life Span:
From Cognitive Training to Neurotransmitters
Shu-Chen Li
Plasticity … means the possession of a structure weak enough to yield to an influence, but strong enough not to yield all at once. Each relatively stable phase of equilibrium in such a structure is marked by what we may call a new set of habits. Organic matters, especially nervous tissues, seem to be endowed with a very extraordinary degree of plasticity of this sort…
—William James, The Principle of Psychology (1890)
Mental exercise facilitates a greater development of … the nervous collaterals in the part of the brain in use. In this way, preexisting connections between groups of cells could be reinforced…
—Ramón y Cajal, Croonian Lecture to the Royal Society (1894, cited in Squire and Kandel 1999)
What seemingly was often overlooked is that the brain itself is a dependent variable, something that is co-shaped by experience and culture, something that does not operate within an environmental vacuum, but that at any moment is subject to environmental constraints and affordances.
—Paul B. Baltes, Patricia A. Reuter-Lorenz, and Frank Rösler, Lifespan Development and the Brain (2006, italics added)
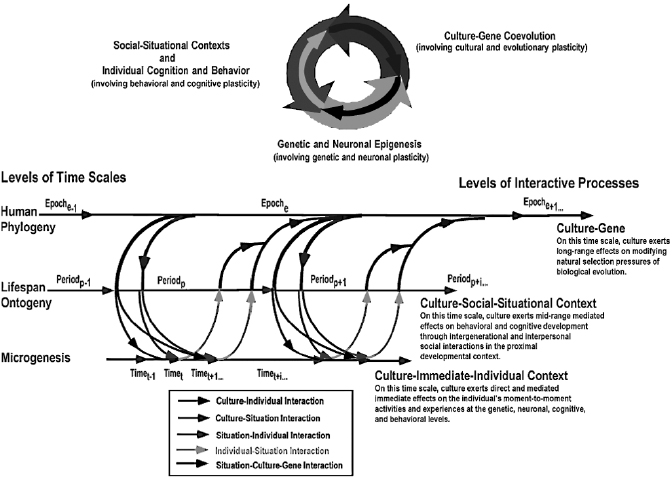
For more than a century, the leading forerunners of modern neuroscience and psychological science have recognized that the brain is an open, adaptive system that is endowed with much plasticity, which makes it malleable by contextualized experiential tuning through development across the life span. The general notion of the brain as the modifiable neurobiological faculty of the mind was clearly advocated as early as the days of Ramón y Cajal and William James. However, only until recently could cognitive neuroscientists undertake empirical research to investigate questions of how socioculturally contextualized experiences may exert reciprocal influences on neurobiological mechanisms. In light of advancements in brain-imaging and genome-sequencing techniques, and the public’s enthusiasm about brain or genetic determinism as the consequences thereof, it is necessary to generate a variant of coconstructivism that highlights reciprocal interactions between brain, behavior, and sociocultural contexts. To this end, a metatheoretical framework, termed “cross-level dynamic biocultural coconstruction” (Li 2003), was proposed to highlight the reciprocal dynamics between biological and sociocultural contextual influences on brain and cognitive development across the life span (figure 15.1). Viewed through this framework, human development is embedded in three nested time scales (i.e., human phylogenetic, ontogenetic, and microgenetic times) and across multiple levels (i.e., sociocultural, behavioral, cognitive, neuronal, and genetic). Biology and culture coconstruct human development through a series of reciprocal interactive processes, interlinking developmental plasticity across the different levels (e.g., cultural, behavioral, and brain plasticity).
Brain Is a Dependent Variable: It Has Plasticity!
With the rapid new developments in brain imaging methods, the misconception of the brain being “the independent determinant” of behavior and cognition is commonly found in interpretations of empirical findings in popular and academic media. Statements such as “…part of the brain determines self-control” (United Press International, August 23, 2007) or “…single spot in brain determines size of visual scratch pad” (news@nature, April 15, 20041) are just two examples. Contrary to this notion of a unidirectional influence from brain to behavior, much of the accumulated empirical evidence shows that the brain is endowed with substantial experience-dependent plasticity at the anatomical, neurochemical, and functional levels. Such evidence is very much in line with the general conceptions James and Cajal conceived of in the late 1800s, which were then further elaborated by Lashley (1930) and Hebb (1949) in the first half of the twentieth century.
Furthermore, not only are younger organisms endowed with such plasticity for new learning, an appreciable amount of plasticity is still available in adulthood and old age (e.g., Baltes & Kliegl, 1992; Li et al., 2008; see Li 2003; Lövdén et al. 2010; Hertzog et al. 2009 for reviews). In other words, with plasticity the brain itself is a dependent variable: the fine details of its anatomical structure, neurochemical mechanisms, and functional circuitry depend on various kinds of experiential tunings that unfold in ontogeny over an individual’s life span. Selected empirical findings of functional brain plasticity in the sensorimotor cortices and in regions of higher cognitive functions are highlighted below. More in-depth reviews about brain plasticity at different levels and with respect to different functions can be found in other sources (Hensch 2005; Li 2003; Lövdén et al. 2010; Pascual-Leone et al. 2005; for topics specific to adult neurogenesis, see Kempermann 2008).
This chapter focuses on the emerging trend of interdisciplinary research that aims at exploring the effects of socioculturally contextualized experiences and learning on human brain functioning. An integration of multiple levels of empirical evidence indicates that brain processes are dynamic and plastic and could be scaffolded by enrichments of the developmental context, such as providing socioculturally contextualized cognitive interventions or skill acquisitions. The cognitive and brain plasticity in response to the enriched developmental context is in turn a resource for further development. Two classes of empirical evidence are reviewed to highlight brain plasticity with respect to sensorimotor and memory process. The first set of evidence shows that the functions of the brain’s sensory and motor cortices adapt to environmental inputs and professional skill acquisition, and the second line of findings reveals that the brain networks implicating memory functions are shapeable by mnemonic trainings. Using memory plasticity as an illustration, multiple levels of evidence will be integrated to track the scaffolding effects of culturally derived mnemonic training on (1) memory performance, (2) brain functional circuitries supporting memory functions, and (3) the neurotransmitter systems that modulate these brain networks.
Plasticity of the Sensory and Motor Cortices Shaped by Environmental Inputs, Expertise, and Age
Cortical regions involved in sensory (e.g., visual and auditory cortices) and motor (e.g., primary motor cortex) processes are the first candidates when looking for evidence of experience-dependent plasticity, given that they interact directly with environmental inputs through afferent sensory and motor pathways. Since Wiesel and Hubel’s (1963) classic study, the experience-dependent nature of circuit fine tuning in the visual cortex and the importance of certain time windows (the sensitive period) for plasticity through environmental inputs have been studied vigorously in animal research. Surgical closure of one eyelid of the kitten, thus precluding external visual inputs during the sensitive period, causes a permanent loss of visual acuity in that eye and alters the physical structure of the visual cortex profoundly (see Hensch 2005 for a detailed review). Whereas animal studies yield findings of such experience-dependent plasticity that is primarily a function of the developing brain’s interaction with the physical environment (visual inputs), human experience usually takes place in sociocultural contexts or through learning with culturally developed materials, customs, and tools. In human studies, cross-modal plasticity of the visual cortex that functionally reorganizes itself to process tactile information in congenitally blind individuals who are skilled in Braille reading is also a well-established phenomenon (see Pascual-Leone et al. 2005 for review).
Cortical plasticity is not only limited to situations where certain aspects of experience are severely limited due either to physical deficits or impoverished developmental contexts. Musical training as a form of culturally contextualized human experiences also affects the functional organization of the primary motor cortex relevant for the playing of a particular instrument. For instance, Elbert and colleagues showed that cortical representations in the primary motor cortex of the fingers of the left playing hands of expert violinists are enhanced in comparison to those of naive controls. Furthermore, the enlargement is modulated by the age of the inception of music training. The effect is clearer in violinists who have started to learn to play the string instruments in early childhood (i.e., at around seven years of age) than those who started learning in adolescence (Elbert et al. 1995). Independent of instrument types, musicians as a group in general show increased auditory cortical representations compared to nonmusicians (Pantev et al. 1998). Daily activities that are part of the individual’s professions, such as driving and navigating in complex spatial environments, have also been shown to increase the volume of the posterior hippocampi, a brain region that is involved in storing the spatial representation of the environment (Maguire et al. 2000). Taken together, these findings indicate that cortical plasticity associated with sensory and motor processes is, to some extent, age sensitive. Optimal plasticity operates within certain time windows and is reciprocally influenced by the individual’s activities and experiences. Life span age differences in memory plasticity will be discussed further later.
Plasticity of Brain Regions Supporting Effective Information Processing Depends on Cognitive Training
Brain regions implementing higher-order cognitive functions also express plasticity, with their functional activations being modifiable by cognitive training. For instance, remediation training programs that focus on enhancing auditory and language processing with adaptive exercises of nonlinguistic and acoustically modified linguistic speech have been shown by Temple and colleagues to improve dyslexic children’s reading performance and the related functional activities in relevant brain regions. Compared to normal children, brain activations during rhyming in the left temporal–parietal as well as the frontal regions are much weaker in dyslexic children. However, after one month of daily language exercises (about 100 minutes long per day) brain activations in these two regions are increased in dyslexic children, resulting in functional activities more similar to those observed in normal children. The magnitude of activation increase in the left temporal–parietal region is also correlated with improvement in reading performance (Temple et al. 2003).
As for brain plasticity in adulthood, one study with younger adults showed that training enhances the functional circuitry supporting working memory (Olesen, Westerberg, and Klingberg 2004). After five weeks of training in three visuospatial working memory tasks, younger adults’ spatial working memory capacity increased (from level 3 to level 6 of task demands) and the reaction time decreased (from about 2,000 milliseconds to about 1,500 milliseconds). Furthermore, brain activities in the left middle frontal region as well as laterally in the superior and parietal regions all increased after the memory training and were correlated with increased working memory capacity.
An important question is the extent to which cortical plasticity is still retained in the aging brain. The evidence to date seems to suggest that, though being more limited than in other life periods, training-induced changes in brain activity are still present in old age. As an example, Eirkson and colleagues showed that dual-task training increases older adults’ performance both in accuracy and reaction times. Furthermore, training also increases brain activation in the left ventral prefrontal cortex, a region that may be associated with the use of verbal inner speech as a strategy to switch between tasks. At the same time, training decreases activation in right ventral prefrontal cortex, a region that might be associated with reduced reliance on response selection strategies (Erickson et al. 2007).
Linking Levels of Memory Plasticity: From Training to Transmitters
The previous section illustrates that functional brain plasticity responsive to skill acquisition or cognitive training in a variety of functional domains is present, though to varying degrees, in different life periods. This section focuses specifically on plasticity in two aspects of memory, associative and working memory, to highlight the multiple levels of mechanisms involved in biocultural coconstruction of memory plasticity from behavioral to functional brain activity and neurochemical levels. From Aristotle to British associationists to modern researchers of associative learning and memory, it has been assumed that elements of perception and memory are associated (bound together) in the course of experience. According to this view, associative learning and memory constitute the basic mechanisms of “experiencing” or, put differently, the elementary modes through which neurobehavioral systems construct, accumulate, and modify their internal representations as a function of experiential tunings. Basic mechanisms of associative memory and learning share close analogues at various levels of the neurobehavioral system; thus they are good candidates to track links from behavior to neuromodulation (cf. Squire and Kandel 1999).
Neurochemical Mechanisms of Memory Plasticity
Evidence accumulated in neuroscience throughout the twentieth century suggests that experience-dependent changes in the efficacy of synaptic plasticity across distributed neuronal cell assemblies are fundamental mechanisms of behavioral and memory changes. For instance, Hebb (1949) considered that even the perception of simple Gestalt figures requires construction from simpler elements, and that such construction initially requires associative learning. He further formulated that associative learning at the neuronal level takes the form of a long-lasting increase in synaptic strengthening (termed long-term potentiation; LTP) between simultaneously firing pre- and postsynaptic neurons. There is general agreement that LTP corresponds to associative memory formation at the level of synaptic plasticity.
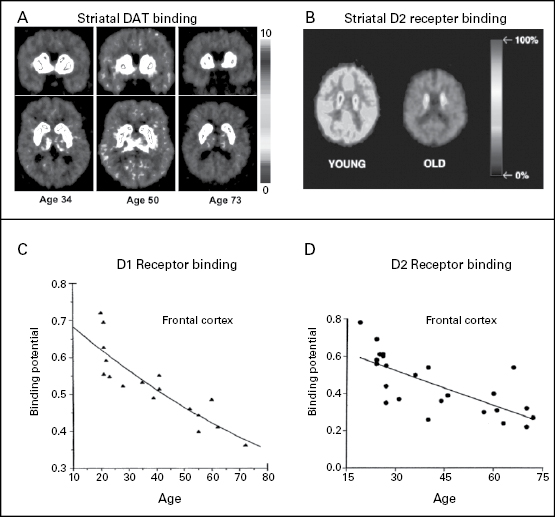
A number of relevant neurochemical substances and mechanisms have been identified. For instance, neurotransmitters such as acetylcholine, norepinephrine, and dopamine are implicated in the modulation of LTP by regulating the ease of inducing synaptic potentiation (see Squire and Kandel 1999 for reviews). Moreover, the relative ease or difficulty of LTP induction may be associated with differences in the efficacy of various transmitter systems in different life periods as well (e.g., Sweatt 1999). Given that dopamine and other neurotransmitters are involved in affecting synaptic plasticity, life span age differences in the efficacy of neuromodulation thus would have direct implications for experience-dependent tuning of synaptic connections. Over the past two decades, studies investigating the impact of aging on the brain’s neurochemical processes have yielded a consensus regarding age-related declines in the efficacy of various neurotransmitter systems. Of particular interest here are aging-related declines in different components of the dopaminergic system (see figure 15.2; see also Bäckman et al. 2006 for recent reviews). Declines in dopaminergic function are present both with respect to presynaptic dopamine transporter (DAT) mechanisms as well as postsynaptic receptor binding mechanisms. For instance, molecular imaging work reveals age-related losses of both striatal dopamine D1 (e.g., Suhara et al. 1991) and D2 receptors (Kaasinen et al. 2000; Kaasinen and Rinne 2002). Similarly, an age-related decrease in DAT density in the caudate and the putamen was shown in a recent positron-emission tomography study on dopamine and cognitive aging (Erixon-Lindroth et al. 2005). In terms of the extent of decline, two recent cross-sectional studies (Inoue et al. 2001; Kaasinen et al. 2000) show that, on average, there is about a 10% decline in receptor binding efficacy across a variety of brain regions.
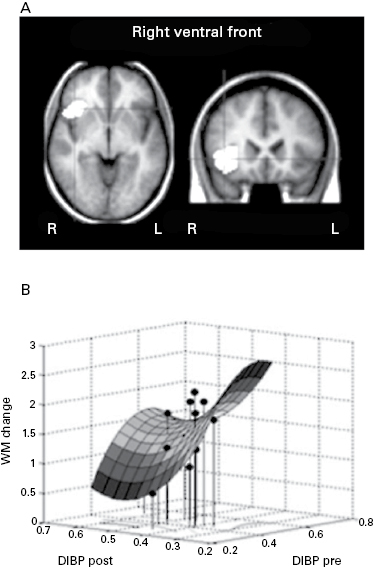
A recent receptor imaging study by McNab et al. established a first empirical link between memory training and changes in dopamine signaling in various brain regions implicating working memory. In young adults fourteen hours of working memory training over five weeks was associated with changes in dopamine D1 receptor binding potential in the prefrontal and parietal cortex. Furthermore, individuals who showed larger performance improvements as a function of training also exhibited a greater change in receptor binding potential (see figure 15.3a,b; McNab et al. 2009). Similar plasticity in response to working memory training has been found for striatal dopamine D2 receptor as well (Bäckman et al. 2011). A recent behavioral study compared working memory plasticity in younger and older adults across forty-five days of training. Although performing at a lower level, older adults also benefited from training and showed substantial working memory plasticity (see figure 15.4d; Li et al. 2008).
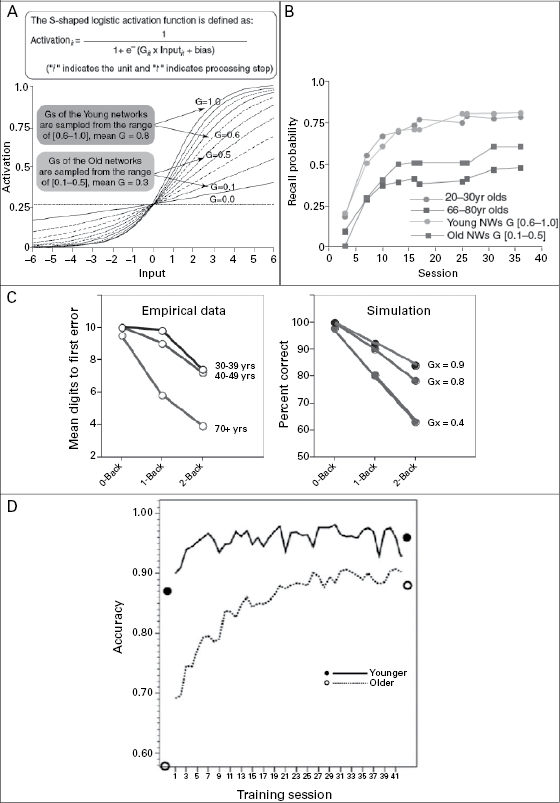
The direct effect of aging-related declines in dopaminergic modulation on memory plasticity has thus far rarely been investigated empirically, but a theoretical link has been made. Aging-related decline in dopaminergic neuromodulation can be computationally modeled by stochastically attenuating the gain control (G) of the sigmoidal activation function that models presynaptic to postsynaptic input-response transfer. Computational simulations modeled as such accounted for the reduced associative memory plasticity (figure 15.3a, b) and working memory capacity (figure 15.3c) in old age as observed in the human data. Specifically, analyzing the internal activation patterns of simulated neural networks suggest that aging-related reduction in memory plasticity can arise from less distinctive neuronal representations of memory items as well as associations between items that are the consequence of noisier pre- and postsynaptic information transmission due to deficit neuromodulation (see figure 15.4; Li, Lindenberger, and Sikström 2001; Li and Sikström 2002; Li, Naveh-Benjamin, and Lindenberger 2004).
Memory Plasticity as a Function of Culturally Derived Mnemonic Training
Associative mechanisms in learning and memory in humans have mostly been studied with paired associates. Although plasticity is commonly regarded as the hallmark of youth, memory plasticity has been studied more extensively among adults than among children of different ages. In behavioral studies of adult age differences in memory plasticity, the effects of using culturally derived cognitive support to augment the “reserve developmental plasticity” (Baltes 1987) that is still available in old age have attracted some research attention. Specifically, Paul Baltes and colleagues (e.g., Baltes and Kliegl 1992) applied the so-called “testing-the-limits” procedure, which involved instructing and providing adults of differing ages with opportunities for extensive, deliberate practice in using the method of loci. In this paradigm, individuals are trained to remember a long list of memory items by associating each item with the famous landmarks in a city they are familiar with. Evidence based on this paradigm yielded a dual picture. On the one hand, the associative memory of cognitively healthy older adults (ranging from sixty to eighty years of age) can be significantly improved after the mnemonic training, thus showing a sizeable amount of plasticity. On the other hand, the extent of plasticity in old age is much reduced compared to younger adults in their twenties and thirties. This aging-related constraint of developmental plasticity was further accentuated among individuals beyond the eighth decade of life. For instance, individuals older than eighty-five years only showed small instruction-related gains but no further training-related gains (Singer, Lindenberger, and Baltes 2003). One recent memory training study extended procedures similar to those applied in adult development to cover the age periods from middle childhood to old age (Brehmer et al. 2007). This study provides the first empirical evidence that compares associative memory plasticity in young children and older adults. Specifically, younger children aged nine to ten years were found to possess a greater extent of developmental reserve plasticity (indicated by performance gain after extensive practice and training) than older adults around seventy years of age. The greater extent of developmental reserve plasticity in children than in old adults reveals that cognitive and neuronal mechanisms associated with implementing the memory strategy are more sensitive to training experience during childhood than in old age. Taken together, behavioral evidence indicates that the magnitude of associative memory plasticity changes across the life span. Relative to young adulthood and middle childhood, old age is marked by a clear reduction in plasticity in general.
Brain Plasticity as a Function of Culturally Derived Mnemonic Training
Recent findings from human brain-imaging studies provide evidence for the effects of memory training on functional neural circuitry. Parallel to the behavior memory training studies on memory plasticity reviewed above, Kondo et al. (2005) compared brain activity patterns before and after instructing their subjects (twenty to twenty-six years of age) to use the method of loci mnemonic strategy to improve their memory for serial associations. Other than showing improved memory recall at the behavioral level, Kondo et al. also showed that functional brain circuitry involved in episodic encoding and retrieval of associative information was modifiable by mnemonic training. Relative to the activities before instruction, encoding after learning to use the method of loci as a mnemonic was associated with activity increase in the right inferior frontal gyrus, bilateral middle frontal gyrus, and posterior cingulate gyrus, for instance. Retrieval after instruction was associated with activity increase in the parahippocampal gyrus, cingulate gyrus, and a few other regions. Whereas Kondo et al. studied neural correlates of strategy-related memory plasticity only in younger adults, another study by Nyberg et al. (2003) investigated adult age differences in the functional plasticity of brain circuitry as a function of the method of loci training. Similar to Kondo et al.’s (2005) findings, Nyberg et al. showed that encoding after strategy instruction was associated with activity increase in frontal as well as occipitoparietal regions in their younger adult sample (around twenty-five years of age). In contrast, accompanying their reduced memory plasticity as indicated by poor memory performance even after memory strategy training, older adults (around seventy years of age) in the Nyberg et al. study did not show training-related increase in frontal activity, and only those older adults who benefited from the memory training showed increased occipitoparietal activity (see figure 15.5). It should be kept in mind, however, that the low-performing older adults in this case might not have used the mnemonic they were taught properly. With more suitable mnemonic strategies and other training regimes perhaps some of the relevant functional brain circuitry in the poor-performing older adults could be activated to improve memory performance.
Outlook: Research Paradigms for Biocultural Coconstructive Dynamics
Although this endeavor is still at an embryonic stage, new conceptual and empirical opportunities are emerging. The studies and evidence reviewed above indicate that culturally derived experiences of learning to use effective mnemonics or other forms of skill acquisitions exert reciprocal influences on cortical processes. The effect of such reciprocal tuning may be affected by individual differences as well as age-related differences in the efficacy of neuromodulation. Facing aging-related declines at the neuroanatomical as well as neurochemical levels, compensatory processes at the behavioral and cortical levels as well as sociocultural supports may be invoked to compensate such losses (e.g., Cabeza et al. 2002; see Hertzog et al. 2009; Kramer & Willis, 2002; Park and Reuter-Lorenz 2009 for reviews). In current discussions about compensatory mechanisms of the aging brain, further effort needs to be devoted toward understanding the relations between the causes (e.g., anatomical or chemical losses) for compensations, on the one hand, and the compensatory outcomes expressed at the brain function level on the other hand.
Currently, findings regarding sociocultural influences on life span neurocognitive development are still very limited and leave many gaps between the different levels of analyses. Nevertheless, the fact that there are emerging coconstructive views and empirical evidence of developmental plasticity at various levels as reviewed in this chapter indicate that the possibilities for bridging these gaps are gradually arising.
Future research will need to focus more on individual differences and life span differences in training-related modifiability of brain–behavior mapping. For instance, by integrating behavior-genetic analyses with cognitive neuroscience research of aging in genomic imaging paradigms, the coconstructive links between genetic influences on neuromodulation, on the one hand, and age-related differences in cognitive and brain plasticity as a function of environmental support, on the other hand, can be investigated directly (cf. Bäckman et al. 2006; Lindenberger, Li, and Bäckman 2006). Recent findings of dopamine transporter gene association with individual differences in working memory plasticity (Brehmer et al. 2009) suggest that the scaffolding effect of memory training or other cognitive intervention may differ between individuals of different genetic predispositions. In other words, a given intervention method may yield different outcomes in different individuals. Some individuals may benefit from one type of training whereas others learn better with a different method. This calls for further research on personalized cognitive supports in order to cultivate developmental contexts that would be beneficial and optimal for individual development.
Furthermore, given the highly interactive nature of neurocognitive processes involving constant information exchanges with the experiential contexts, future research needs to consider more explicitly information exchanges between the individual and the context, and how such interactions may affect the functional aspects of neurocognitive processing. For instance, it is of interest to investigate how the sociorelational context (e.g., Singer et al. 2004) and dynamic, online social interactions (King-Casas et al. 2005) affect the functional circuitry involved in different aspects of social cognition. So far, we have only focused on findings showing adaptive neural plasticity induced by supportive sociocultural contexts. However, as Nelson (2000) pointed out, plasticity can be a two-edged sword. Thus, the influences of dysfunctional social contexts that cause individuals highly stressful experiences or even trauma also need to be investigated.
In conclusion, the existing empirical evidence of developmental plasticity at different levels presents a warning against the “purely reductionist approach” to the genetic and neuronal bases of mind and behavior that ignores influences from cultural, experiential, and cumulative developmental contexts. The reason is clear: genetic activities and neural mechanisms themselves possess remarkable plasticity awaiting sociocultural contexts to exert reciprocal influences on them and to be the “coauthors” of mind and behavior. People are more than mere biological organisms; human mind and behavior need to be understood by situating them properly within a brain in a body that lives in an eventful world abounding with objects, other creatures, and sapient colleagues!
Note
References