The Baron paled at this sight. Candide, seeing his beautiful Cunegonde embrowned with blood-shot eyes, withered neck, wrinkled cheeks, and rough, red arms, recoiled three paces, seized with horror, and then advanced out of good manners. Cunegonde did not know she had grown ugly, for nobody had told her of it.
Candide, Voltaire, 1759
‘My least favourite bit is my boobs – without a doubt. They’re practically non-existent. I don’t feel womanly. I hate them. I despise them. If I could get rid of them and have them replaced, then I would. If I could sell a kidney to pay to get them done, then I would. Taking my top off with my boyfriends has always been a definite no-no. I somehow feel that if I keep my top on they won’t notice.’
Anonymous interviewee ‘C’
During the last century, female reproduction changed profoundly. In developing countries girls now undergo puberty at a much younger age than they did one hundred years ago. Estimates vary, but over the course of that century, puberty may have been hastened by between three and four years, from an average age of fifteen to eleven. This rate of change is equivalent to roughly twelve days of ‘hastening’ for each year of that century – breakneck speed from the biological point of view, and certainly too fast to be an evolutionary change. What is causing this novel reproductive precociousness?
For some time, scientists have pointed out that this rapid hastening of puberty has occurred during the same period and in the same societies in which children have become better and more consistently nourished, less diseased and less drained by the pernicious effects of parasitism. And because of this, the suspicion has arisen that female fertility is largely controlled by some combination of diet, weight, fatness or body shape.
Most of us have heard of female athletes or women with eating disorders who have stopped having periods, and we often take for granted the idea that women need a certain amount of body fat before they can be fertile. This idea was first proposed in the 1970s and went a long way to explaining the apparent pro-fertility effects of fatness. It provided an explanation for the hastening of fertility in girls, and it could also explain why women with little fat often stop menstruating. It even accorded with the widely held assumption that curvier women – with ‘childbearing hips’ – were more fertile. In early versions of this theory, it was suggested that girls had to reach a threshold weight of 46kg before puberty could start, although later the threshold became more fat-focused – with a 17 per cent body fat content required for puberty, and 22 per cent needed for regular periods.
The fatness-threshold theory fitted the existing data well. Childhood obesity is indeed linked to precocious puberty and being underweight is linked to delayed puberty, and more moderate variations in pre-puberty body weight also cause similar, but more subtle effects. The theory also seemed to tie in with socio-economic factors relating to diet – a study in South Africa showed that girls raised in relatively privileged families underwent puberty earlier than their less fortunate peers. And indeed, malnutrition, eating disorders and large amounts of physical exercise do delay puberty, and also suppress reproduction in adult women: they stop cycles (‘starvation amenorrhoea’), reduce the chances of conception and increase the risk of stillbirth.
Recently, researchers have shifted away from simply measuring body weight, mainly because it is a poor indication of slimness or fatness as it takes no account of height. The most common replacement measurement is the well-known ‘body mass index’ which is calculated as a ratio:
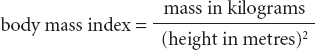
Yet even body mass index is not a perfect assessment of fatness or thinness, mainly because it does not distinguish between muscle and fat – very muscly people can be classed as ‘obese’ according to the ratio. However, it is certainly better than just using weight as a measure, and it will do for now. And indeed, the timing of puberty correlates well with body mass index in girls, although notably this correlation does not hold up well for boys.
The fatness-threshold theory remains appealing, and since it was proposed we have discovered possible mechanisms by which it might work. We no longer think of adipose tissue as an inert, passive blob, but rather as a metabolically active organ which produces and modulates the effects of oestrogen, secretes a wide variety of chemical messengers which influence distant tissues, and also secretes leptin which appears to be precisely what the theory requires – a hormonal barometer of the amount of fat in the body. In addition, the fatness-threshold theory also makes evolutionary sense. We have already seen that human females face extremely high energy demands associated with pregnancy and lactation, so it would be eminently sensible if the biological decision whether to breed was based on the amounts of energy-rich fat stored in the body.
However, this is where problems in the theory start to appear. ‘Current’ fatness is all very well as an indicator of a girl or a woman’s past ability to acquire and store calories, but it does not predict the future. This is especially true of girls at the start of puberty, because so much will have changed for them by the time they become fully fertile – they will have doubled in size and their family and social situation may be completely different. Another problem is that the theory has no ‘upper cut-off’ to prevent girls and women conceiving if they are obese, despite the fact that being overweight increases the risk of miscarriage, stillbirth and diseases of pregnancy. Finally, it has even been claimed that the theory may have got the whole thing the wrong way round, and that it is early puberty which causes girls to accumulate body fat rather than the converse (although the theory’s supporters point out that body mass index at the age of three, or changes between three and six, do correlate well with the timing of puberty).
All in all, the accumulating evidence now fails to support a simple, direct effect of fat on female fertility. Instead, more complex forces seem to be at work. For example, teenage girls who frequently go running are more likely to stop menstruating – yet of those girls, the ones who stop their periods do not have lower levels of body fat than girls who do not stop them. In fact, many biologists now agree that there is so much natural variation in women’s weight, fat content and fertility that there cannot be a simple, predictable link between them.
This complexity may reflect the possibility that fertility responds to fatness in different ways at different weights. For example, a direct, causative link between body fat content and reproduction may only take effect at extremely low fatness levels rarely seen in healthy women in developed societies. Thus, according to this argument, a minimum threshold amount of fat may indeed be required to permit fertility, but beyond that minimum requirement, fatness has little effect on most women’s month-to-month fertility.
Another possibility is that we have not been sufficiently specific about the type of fat we are measuring. If curvaceous buttock-and-thigh, ‘gluteofemoral’ adipose tissue is as important for reproduction as I claimed in the last chapter, then perhaps we should be measuring that instead, and ignoring fat elsewhere. And indeed, puberty occurs earlier in girls with more gluteofemoral fat, whereas it may occur later in girls with more fat around their waist. Certainly, such discrepancies could explain why whole-body fatness does not reliably predict the timing of puberty. Also, girls who undergo puberty despite being underweight tend to possess more gluteofemoral fat than underweight girls who do not start menstruating.
The gluteofemoral fat depot may be directly measured by various elaborate means, but a convenient estimate of its relative size may be calculated in the form of the ‘waist–hip ratio’. The waist–hip ratio is just what it sounds like – the circumference of the waist divided by the circumference of the hips – so hourglass, wasp-waisted and wide-hipped women have low ratios and men with narrow hips and fat bellies have high ratios. And intriguingly, women with low waist–hip ratios have higher levels of oestrogen during their cycles, ovulate more frequently and are more likely to conceive. Thus distribution of fat – body shape, not size – is probably more important than total fatness in human female fertility.
As well as the jealously guarded, lactation-earmarked gluteofemoral fat stores, there is increasing evidence that the female reproductive system also responds to day-to-day fluctuations in energy availability. Energy comes into the body as food, it can be stored in, or liberated from, adipose stores, and it may be expended when women move around, keep warm, pump their heart, or do innumerable other things. This complexity of the female energy budget has led to the idea that fertility is not only dependent on fat stores, but also the interconnecting flows of energy between diet, storage and utilisation. Indeed, it would make sense for fertility to respond to the entire energy budget, rather than just one part of it, because it is probable future availability of calories that is important if a child is to be successfully gestated, born and fed. If the energy budget is tight – and it does not matter if that is because of inadequate diet, poor fat stores or excessive expenditure – then the female body interprets this as a sign that it should not breed.
In recent years, physiologists have started to investigate how the energy budget controls female fertility. A region on the underside of the brain, the hypothalamus, and the glandular appendage which dangles from it, the pituitary, have long been known to be master coordinators of reproductive hormones and behaviour, and it now seems likely that they receive many inputs which ‘tell’ them what is going on in the world of energy metabolism. And this information may come via nerve connections from the rest of the brain, or it may come in the form of hormones.
One such hormone is leptin, which is secreted by adipose tissue. Because it is made by fat cells, it is thought that the hypothalamus and pituitary might use it as an indicator of the body’s fat content. With this in mind, it is notable that mice which cannot synthesise leptin are infertile – as if their brains think they are fat-less – but their fertility can be restored by leptin injections. Similarly, puberty may not occur in girls who cannot make leptin normally. Thus leptin probably feeds information about fatness into the brain’s decisions about the timing of puberty. Indeed, girls’ circulating leptin levels increase before the start of puberty and usually continue to rise until it is complete. And intriguingly, as puberty progresses, buttock and thigh fat produces a disproportionate amount of leptin. The curves are ‘speaking’ to the brain.
So our understanding of the interactions between body shape and fertility has become much more intricate in recent years – no longer do we think crude fatness dictates whether female reproduction is switched on or off, but instead the brain uses the subtle, quantitative information it receives to increase or decrease its pressure on the reproductive throttle. In studies in the developing world, reduced diet during the pre-harvest season, or the physical exertion of the harvest itself, has been shown to reduce women’s circulating oestrogen and progesterone levels, shorten menstrual periods and lengthen the intervals between ovulations. In developed societies, there is evidence that it is weight loss, rather than unchanging slimness, which reduces hormone levels, and it has even been suggested that something as innocuous as regular jogging could do the same.
Once a child is conceived, a woman has little choice but to support and nurture it – effectively, she is biologically committed to it. Because of this, pregnancy and breastfeeding are relatively unaffected by poor diet or low fat stores (except in extreme circumstances). Thus the initial ‘decision’ to conceive is absolutely crucial, and it is at this all-important decision-point that the reproductive system ‘listens’ most intently to what the curves have to say.
For most of our evolutionary history, women often did not know where their next meal was coming from, so the biology of the entire body slowly became more and more focused on energy acquisition and storage. Women alive today are, by definition, the descendants of individuals who made the right metabolic decisions during those difficult times, and because of this, a complicated system of whole-body metabolic checks and balances has become etched into their genes.
Today that system still controls women’s metabolism, but one important thing has changed. Humans in the developed world now have access to more food than they know what to do with, and this is a situation for which their metabolic control system is not prepared. This is why modern humans so easily become overweight, and we suffer several major diseases as a result. Yet once again, and especially in women, it is often the location of fat rather than its total mass which dictates susceptibility to disease. Having a high body mass index increases a human’s chances of dying within the next few years, but having a high waist-hip ratio – deviating from the ‘classic’ hourglass shape – reduces the chance of survival even more. Thus women’s body shape controls not only their fertility, but also their susceptibility to disease and even their longevity.
Of all the diseases caused by being overweight, type 2 diabetes is perhaps the most thoroughly studied (although smoking, lack of exercise and genetic factors are other causes too). It is extremely common in developed countries, affecting people of all ages but especially older people: perhaps one in ten people over seventy years of age has the disease. For reasons we do not entirely understand, in type 2 diabetes, most of the cells in the body stop responding to insulin – the hormone which usually makes them remove fats and sugars from the blood and store them away. The result of this is that blood concentrations of fats and sugars creep upwards, and the pancreas churns out more insulin to exhort body cells to soak up these fats and sugars. However, the body is not responding to insulin any more, so the extra insulin achieves little, and eventually the pancreas simply gives up, exhausted.
Obesity is a major cause of type 2 diabetes, so body mass index can be used to predict the likelihood of someone getting the disease. However, as we have already seen, all fat is not created equal, and the location of excess fat is just as important as its quantity. In women of all ages, abdominal fat – fat held around the waist – seems to be particularly damaging. Both before and after the menopause, large waist measurements and high waist–hip ratios are strongly linked to insulin-insensitivity, abnormal patterns of lipids in the bloodstream and the onset of full-blown diabetes. As a result, pre-menopausal women are relatively protected from type 2 diabetes because they store little fat in their abdomen – completely the opposite of men, in whom the belly is the main store.
The dangers of abdominal fat are only slowly being elucidated, but there is clearly something sinister about this fat depot. Much of the fat in the abdomen is held in a membranous sheet called the ‘omentum’ which hangs down from the stomach. In abdominal obesity the omentum becomes stuffed with hugely distended adipocyte cells which start to disgorge a variety of damaging substances into the blood – including chemicals more usually seen at sites of inflammation, or free fat molecules overflowing from the swollen fat cells. Because of the anatomical arrangement of the omentum, this toxic mix is delivered by the bloodstream directly to the liver, where it damages liver cells and immune cells. From there, yet more noxious substances now spew into the circulation, from which they enter other body cells and destroy the mechanisms by which insulin exerts its effects.
Thus, by this tortuous route, waist-fatness can destroy the body’s delicate energy control mechanisms. Losing weight can help a lot, but some of the damage may already be irreversible. This biological response to obesity may seem entirely counterproductive as far as health is concerned, but it is important to bear in mind that obesity was very rare in humans until the last hundred years or so. Because of this, natural selection has never had a chance to rid us of our self-defeating responses to it.
There is an upside to this sad story of modern life, however, and it particularly benefits women. Just as abdominal fat causes type 2 diabetes, recent research suggests that gluteofemoral fat may actually protect against the disease. Both before and after the menopause, having a ‘feminine’ low waist–hip ratio, and having high thigh and hip measurements are indeed strongly linked to insulin-responsiveness, normal patterns of lipids in the blood and a low likelihood of getting overt type 2 diabetes. And surprisingly, womanly curves do not prevent diabetes simply by providing a safe alternative to storing fat in the abdomen – instead, the statistics suggest that gluteofemoral fat actively prevents the disease in women (but not, perhaps, in men). Not only does thigh fat not release the same toxic cocktail as omental fat, but it may even release substances which promote healthy energy metabolism.
So if a woman is overweight, her health prospects are much better if she retains a pear or hourglass shape. This is no cause for complacency, however, as age brings with it a tendency to shift that previously beneficial fat to a new dangerous, abdominal location.
Having a dysfunctional insulin system can have serious knock-on effects and can cause several other important diseases, especially cardiovascular disease – which accounts for roughly one-third of all deaths in developed countries. Cardiovascular disease comes in two overlapping forms – damage to the heart and brain due to blockage of the coronary or cerebral blood vessels, and high blood pressure – and both of these conditions are strongly related to body shape. There is a clear link between body mass index and cardiovascular disease, but having a non-curvy waist–hip ratio predicts susceptibility to the disease more accurately, suggesting that abdominal fat is once again the villain of the piece.
Type 2 diabetes increases the chances of blockages forming in the coronary vessels because it inflames their inner vessel lining, and it also fills the blood with the fatty building blocks of the clotted plaques of atherosclerosis. In addition, diabetes can damage the nerves which regulate the activity of the heart and the springiness of blood vessels. Body mass index and especially waist–hip ratio are also clearly linked to long-term kidney disease, which can further damage the body’s ability to regulate blood pressure.
Yet again, the feminine pattern of fat distribution comes to the rescue. There is evidence that gluteofemoral fat can protect against cardiovascular disease, and it is associated with a healthy balance of lipids in the blood. Indeed, there seems to be no limit to its protective effects – studies show that having a ‘womanly’ low waist–hip ratio can also protect against gall bladder disease, some forms of breast cancer, and dementia.
The story is not so straightforward when it comes to skeletal disease. It may come as no surprise that arthritis is strongly linked to obesity, because being heavy obviously puts more strain on the joints. However, fat also releases chemicals and hormones which probably directly accelerate the degeneration of joints. At present, there is no clear evidence that distribution of fat in different locations makes women either more or less likely to suffer from arthritis, but I expect that this evidence will appear within the next few years.
However, there are other skeletal problems of particular relevance to women, and which are affected by body shape. One of these is osteoporosis, the loss of bone mineral which occurs later in life in both sexes but is more likely to lead to fractures in women because their bones are less robust to start with. Osteoporosis rather bucks the trend of the rest of this chapter, as studies suggest that having a high waist–hip ratio correlates with good bone density – so having a male-like silhouette may actually protect against this disease.
Another set of female orthopaedic problems clearly related to body shape occurs in women with large breasts, or gynaecomastia. The weight of large breasts can completely unbalance the musculoskeletal system, and lead to upper back pain, lower back pain, shoulder pain, neck pain, arm pain, bra-strap abrasions, and may even compromise women’s ability to breathe. Considering its adverse effects, gynaecomastia is surprisingly common, and it is hard to imagine why natural selection has not expunged it from the human population. However, modern surgical breast-reduction techniques offer an almost complete cure.
Many women with larger breasts also worry that they are more likely to develop breast cancer, and at first sight this fear seems reasonable. After all, the last few decades of oncology research have shown that tumours develop from single, aberrant cells which slip free from the restraints which usually prevent their uncontrolled proliferation. So because some women have many times more breast tissue than others, it might seem sensible to assume that those women are many times more likely to develop breast cancer – simply because they possess more individual cells which might grow into tumours. Indeed, this is often assumed to be the case, even by evolutionary biologists, who have sometimes proposed it to be one of the selection pressures which prevented women’s breasts evolving to be even larger.
It should be easy to show a link between breast size and cancer risk – woman’s breast sizes vary dramatically, and breast cancer is a common disease, and variation and commonness usually make demonstrating something statistically straightforward. In fact it has proved surprisingly difficult to demonstrate such a link. Initial studies suggested that such a simple correlation did indeed exist, but this may have been due to the confusing influence of body weight. Being heavier is already known to be linked to an increased incidence of mammary cancer, but heavier women also tend to have larger breasts – so simple correlations do not mean that breast size in itself increases the risk of cancer. However, in women in general, breast tumours are more common in the left breast, which is on average larger than the right. More recent studies have provided evidence of a link between breast size and mammary cancer, but it seems surprisingly subtle – one study suggested that women with larger breasts at the age of twenty were more likely to develop breast cancer later in life, but the correlation only held true for women with lower body mass indices. Another complicating factor in all this is breastfeeding, which changes the shape and size of the breast, but is also suspected of protecting against the development of mammary cancer. However, it now appears that any protective effect of breastfeeding may be lost after the menopause, so it may only actually reduce a woman’s chances of getting mammary cancer early in adulthood when she is relatively unlikely to get the disease anyway.
Intriguingly, genetic research carried out in the last few years has now suggested mechanisms by which breast size and breast cancer may be linked. Breast size is a trait which can be inherited, and breast cancer risk is often heritable too – but only recently has evidence appeared hinting that the two may be inherited together. First, scientists discovered seven genetic markers linked to breast size, but then found that three of them are inherited along with, or are physically adjacent on the chromosome to, genetic markers of breast cancer. Three out of seven is too many to be a coincidence, so it now seems very likely that mammary size and mammary cancer are indeed genetically linked in some way. These results do not tell us what these genes actually do, nor unequivocally prove that breast size increases tumour risk in the real human population, but they are certainly intriguing.
Yet still, all of this may seem strangely vague. Some women’s breasts are at least ten times larger than others’, so why, if a link between size and cancer risk exists, has it been so difficult to find? One possibility is that most of a mammary gland is fat, while relatively little consists of the gland cells which can grow into tumours – so breast size may be a poor measure of the pool of potentially tumour-forming cells. It is also likely that breasts and breastfeeding are so important for human survival that a complex system of checks and balances exists to control cell growth, proliferation and activity in mammary cells. After all, breast cells are unusual because they are meant to undergo periodic episodes of frantic cell replication – in preparation for lactation – and I suspect it will be some time before we fully understand how this natural, healthy proliferation is normally prevented from escalating into something more malign. Evolution can produce amazing things, but those things can be fiendishly difficult to understand.
Our journey through the biology of female shape has shown that the elements which make the female body look so distinctive are not just superficial adornments, added to the human form as an irrelevant afterthought. Instead femininity is spread throughout the body, sometimes in places where you might not expect to find it, such as the morphology of fat cells or the angle of the elbow. And it has had, and continues to have, profound effects on human evolution, development, fertility, health and disease.
Many women feel that being a larger or curvier shape is an essential part of their self-confidence, self-belief and self-determination, whereas others feel guilty about being that same shape because they live in a society which often seems to equate fat not with a state of healthy nourishment, but with disease. Of course, the longing to be free to inhabit a body of any shape is usually tempered by a wish to be healthy, but only recently has it become clear just how important it is to treat shape and size differently. After all, we have now seen that rounded hips, buttocks and breasts could even be beneficial for women’s health. Millions of years of evolution have ensured one thing – it’s not the size of the curves which is important: it’s where they are.
Although my intention in the first third of this book was to stick to the basic biology of women’s body shapes, that biology has already spilled over into psychology and emotion – confidence, guilt, happiness and desire. In the second part of this book, I will surrender to the inevitable lure of the mind and broaden our story to consider how it interacts with the female body. I will show how all the biology is only our first step on the way to understanding our strange relationship with women’s bodies – and as we will now see, one of the strangest stories in human evolution has been the remarkable ascent to primacy of a single organ: the brain.
The girl stood up and stretched her long straight limbs. She felt the distant heat of the ochre sun warm her dark skin and smelled smoke from the previous night’s fire in her nostrils. She cupped her breasts in her hands. They seemed to be getting slowly larger ever since the wriggling thing in her belly had appeared. She could not explain why, but this made her laugh out loud.