5
Hops
What Are They?
Hops are the strobili—the cone-like female reproductive structures—that constitute the flower of the hop plant (Humulus lupulus). The hop plant is a climbing vine that is native to the temperate regions of North America, Europe, and Asia. The species has separate male and female plants. Only the female vines (known as bines) produce the cones. The vines will climb 30 ft. (9 m) or more up any available support and are commonly trained onto strings or wires when grown commercially. The leaves of the hop plant resemble grape leaves, while the cones of the hop itself vaguely resemble pinecones in shape, but are light green, thin, and papery. At the base of the bracts (the thin, papery outer leaves of the hop) are the yellow lupulin glands that contain the essential oils and resins that are so prized by brewers for their bittering and aroma qualities. The hop oils and resins also inhibit bacterial growth, and this natural preservative property is one reason hops were first used in beer.
Hops have been cultivated for use in brewing for over 1,000 years. Hops are most productive between 35° and 55° latitude in both the northern and southern hemispheres. The earliest known cultivation was in central Europe, and by the early 1500s cultivation had spread to western Europe and Great Britain. At the beginning of the twentieth century, only a couple dozen varieties of hop were being used for brewing worldwide; today, there are over two hundred. The focus of breeding programs has been to increase the alpha acid bittering compounds, while improving yield and disease resistance.

Figure 5.1. Hop cones.

Figure 5.2. Lupulin glands at the base of the bracts.
Hop Bitterness
The primary bittering agents in hops are derived from the alpha acids, called humulones, which are present in hop resin. These alpha acids are neither bitter-tasting or soluble in water until they are isomerized by boiling. Isomerization means that the configuration of the molecules has changed, but not the chemical formula. In other words, the molecules are made of the same atoms but in a different arrangement, which alters the properties. When the hops are boiled in the wort, isomerization makes the alpha acids—now called iso-alpha acids (iso-humulones)—intensely bitter and more water-soluble. The longer the boil, the more alpha acids are isomerized, the more iso-alpha acids end up dissolved in the wort, and the more bitter the beer will be. Typically, after an hour of boiling hops, around 25%–30% of the alpha acid content is isomerized.
Hop resin also contains beta acids, called lupulones, but these beta acids do not isomerize in the boil. Instead, beta acids oxidize during storage, with the oxidized beta acids becoming more water soluble and bitter. This is the main source of bitterness in “aged hops.” Alpha acids also oxidize if stored at room temperature. While these oxidation products are not the same as isomerized alpha acids, they are bitter. However, oxidized alpha acids are not as bitter as oxidized beta acids, and oxidized beta acids are not as bitter as iso-alpha acids.1 Although oxidized beta acids are more bitter than oxidized alpha acids, modern hop varieties contain two or three times the level of alpha acids than beta acids. This affects the overall contribution of each type of hop acid to perceived bitterness.
Hop polyphenols (a different type of compound from hop acids) from the hop cones are thought to influence the perceived bitterness of a beer, particularly beers that are dry-hopped. However, a study that combined chemical analysis with the results from a trained tasting panel showed that the perceived bitterness in selected commercial beers correlated almost entirely with levels of iso-alpha acids and oxidized alpha acids.2 So, while hop polyphenols may affect perceived bitterness, their effects are variable and not as significant as oxidized alpha acids.
To summarize, here are the key points to remember when considering hop bitterness:
Compounds that impart bitterness
- • Iso-alpha acids: These compounds are intensely bitter, and are the most prevalent bittering agent in hopped beer.
- • Oxidized alpha acids: These are less bitter than oxidized beta acids, but present in greater amounts. Most prevalent in old or aged hops.
- • Oxidized beta acids: These are more bitter than oxidized alpha acids, but present in lower amounts. These are the primary bittering agent in aged hops, such as those used in sour (lambic-style) beers.
- • Hop polyphenols: The bittering effects of this class of compounds are variable and not as significant as hop acids.
Compounds that do not impart bitterness
- • Raw alpha acids3: These are the non-isomerized and non-oxidized, water-insoluble acids that naturally occur in hop resin.
- • Raw beta acids: These are the non-oxidized, water-insoluble acids that naturally occur in hop resin.
- • The decomposition products of the bittering compounds described above.
Hop Aroma and Flavor
The aromas associated with hops come from their essential oils (table 5.1), and these oils contain roughly 500 different chemical compounds that have been identified so far. Many exist in only the barest of trace amounts, while others, such as myrcene, can make up 50% of the total essential oil content. The interaction of these compounds is very complex. Brewing scientists have attempted to recreate the aroma of a particular hop by combining the primary individual oils in the appropriate proportions and failed miserably. Of course, there is nothing wrong with adding hop oils to a beer to enhance its hop character, but the whole of hop aroma is greater than the sum of its parts.
Hop flavor is a combination of hop bitterness, resin (mouthfeel), and aroma. Hop aroma from the essential oils is hard enough to describe; flavor is much more difficult. Suffice to say that middling short boil times that contribute a combination of isomerization and residual oil and resins to the wort can be perceived as flavor in the final beer. Generally, these are boil times of less than 20 minutes. Hop steeps, where the hops are soaked in hot wort before cooling, will also contribute hop flavor to the beer.
Table 5.2 lists seven primary characters that are often used to describe hop aroma: floral, fruity, citrus, vegetal, herbal, resinous, and spicy. Of course, this listing is subjective, and if you ask three different experts you will get three different lists. Some characterization plots break the aroma down into 12 or more different characters, but these seven get the point across. It is important to understand that aroma is subjective, and different people can perceive a particular aroma differently. For example, some people might describe mint as herbal and others would describe it as spicy, and they both would be correct.
For more discussion of the essential hop oils, see Stan Hieronymus’s book, For the Love of Hops, from Brewer’s Publications (2012).
Table 5.1—Aromas of the Main Essential (Aromatic) Oils Found in Hops
| Oil | Aroma description |
|---|---|
|
Myrcene |
Sweet carrot, celery, green leaves |
|
Humulene |
Herbal, woody, spicy clove |
|
Caryophyllene |
Spicy, cedar, lime, floral |
|
Farnesene |
Woody, citrus, sweet |
|
β-Damascenone |
Honey, berry, rose, blackcurrant, Concord grape |
|
β-Ionone |
Raspberry, violets |
|
Linalool |
Floral, lavender |
|
Geraniol |
Floral, rose, marigold, geranium |
|
Nerol |
Floral, wisteria |
|
Citronellol |
Citrus, lemon, citronella oil |
|
Terpineol |
Citrus, fruity |
|
Humulenol |
Spicy, pineapple, cedar, sagebrush |
|
Humulol |
Spicy, herbal, hay |
|
4MMPa |
Muscat grapes, blackcurrants, onion |
a 4MMP = 4-mercapto-4-methylpentan-2-one
Table 5.2—Categorization of Hop Aroma
| Floral | Fruity | Citrus | Vegetal | Herbal | Resinous | Spicy |
|---|---|---|---|---|---|---|
|
Geranium |
Apple |
Grapefruit |
Celery |
Tarragon |
Pine |
Fennel |
|
Rose |
Berries |
Orange |
Tomato leaves |
Marjoram |
Juniper |
Black pepper |
|
Jasmine |
Peach |
Lemon |
Green pepper |
Lavender |
Heather |
Nutmeg |
|
Lily of the Valley |
Melon |
Lime |
Cabbage |
Dill |
Tobacco |
Clove |
|
Lavender |
Passionfruit |
Bergamot |
Hay |
Sage |
Woody |
Mint |
Hop Variety Categories
Today, hops are cultivated in many different countries. European, English, American, and Pacific hop varieties are readily available on the open market. In addition, there are Chinese and South African varieties as well, but these are seldom seen outside of their own regions due to high domestic demand. The European varieties were the original brewing hops, and these are often referred to as “landrace” or “noble” hops. These early hops have delicate floral, spicy, and resinous aromas that for centuries were the definition of what hops should smell like. The English varieties developed next and these have traditionally had more of an herbal, earthy, and fruity aroma compared to the European varieties. The American varieties have a predominately citrus character, with undertones of herbal, resinous, and spicy aromas. The Pacific varieties (i.e., New Zealand and Australia) have a strong tropical fruit character with citrus and floral notes.
There was a time when each region could be described by one word: European, spicy; English, herbal; American, citrus; and Pacific, fruity. But, with the craft beer movement accelerating since the early 1990s, hop breeding and development has exploded. Today it is common to find German varieties, such as Mandarina Bavaria and Hüll Melon, that smell of fruit; American varieties, such as Citra and Mosaic, that smell tropical; and English and Pacific varieties that smell like citrus.
There are some hop varieties that help define particular styles, such as the minty flavor of Northern Brewer for California common beer, the floral character of Spalter and Hersbrucker for German lagers, Cascade for American pale ale, and Fuggle and East Kent Goldings for British pale ale. As you are learning to brew, and learning what defines the character of particular beer styles, you will probably attempt to brew classic recipes faithfully by using the prescribed malts, hops, and yeast, and that is a good idea. If you want to get a great foundation in brewing and tasting different beer styles, I heartily recommend brewing all of the recipes in Brewing Classic Styles by Jamil Zainasheff and John Palmer (Brewers Publications, 2007).
However, one of the first substitutions any brewer will make is for a hop that is not readily available, or for one that they just don’t particularly like. In fact, I would suggest that new brewers consider the hop callouts in any beer recipe to be mere guidelines for a couple of reasons. First, because bitterness is bitterness—there is very little difference in the flavor of that bitterness across different hop varieties. Now, that being said, the flavor and aroma hop selections of a recipe are a bit more important, because the dominant character of those hops may indeed be characteristic of the style. There is still room for substitution though within that hop’s variety category. For example, if a recipe for American pale ale calls for Cascade, a good substitute would be Centennial or Amarillo. If you need to substitute for German Tettnang in a German Pilsner recipe, you could readily use Spalt Select or Saaz.
Don’t be afraid to substitute hops, just be reasonable about it. You would probably not want to substitute Cascade or Chinook for a German hop if you are trying to faithfully brew a German Pilsner style, because the characters are just too different. But brew what you like; everyone’s flavor preferences are different, some prefer fruity, others prefer floral, and still others prefer resinous. Gradually you will find the hop varieties you prefer, and as you fine tune your favorite recipe(s) you will probably decide on particular hops that have to be in there. That’s the purpose of a recipe.
Table 5.3 lists some common hop varieties and suggested substitutions. I used to try to keep this book up to date on all varieties, but the hop world is moving far too fast. Get online and find out what’s new.
Table 5.3—Common Hop Varieties and Suggested Substitutions
| Category | European Hop Varieties | English Hop Varieties | American Hop Varieties | Pacific Hop Varieties |
|---|---|---|---|---|
|
General Character |
Floral, Spicy, Resinous |
Resinous, Fruity, Spicy |
Citrus, Herbal, Resinous |
Fruity, Citrus, Floral |
|
Substitution: European-like |
Hallertauer Mittelfrüh Tettnang Spalt Select Saaz Hersbrucker |
Target Challenger Northdown Progress |
Crystal Mt. Hood Horizon Cluster Wakatu |
Helga Pacifica Sylva Ella |
|
Substitution: English-like |
Magnum Opal Merkur Smaragd |
East Kent Goldings Fuggles West Goldings Variety Sovereign |
Glacier Columbia Willamette Galena |
Green Bullet Fuggle Wye Challenger Pacific Gem Super Pride |
|
Substitution: American-like |
French Triskel Hallertau Blanc |
Admiral Pioneer Epic Pilgrim |
Amarillo Cascade Centennial Ahtanum |
Dr. Rudi Waimea Sicklebract Galaxy |
|
Substitution: Pacific-like |
Hüll Melon Mandarina Bavaria |
Archer Olicana Jester |
Mosaic Citra Simcoe Amarillo |
Nelson Sauvin Riwaka Motueka Topaz |
Note on using this table: Hops are arranged according to region of origin and principle characters. Hops may be substituted within a subgroup, and across the categories in the same row. Have fun exploring the similarities and differences!
Using Hops
Each of the following hopping methods will develop a different hop character in the beer. Some of the methods differ by temperature, some by time, and some by both. Several of the methods will often be used in the same recipe. In general, the longer you boil the hops in the wort the more bitterness, less flavor, and less aroma you will produce. Alpha acid isomerization can occur when the wort temperature reaches 185°F (85°C), but is most active during the boil.
Mash Hopping
Mash hopping is putting hops in the mash, and it is said to contribute some aroma and flavor to the beer. Other possible benefits are that the alpha acids lower the mash pH by a small amount, and that the hop cones can act to loosen up the grain bed for better lautering. Hop pellets, on the other hand, would in principle have the opposite effect and potentially impede lautering. (All-grain techniques, including mashing and lautering, are covered in section 2 of this book.)
An experiment conducted for the 2014 National Homebrewers Conference by David Curtis and the Kalamazoo Libation Organization of Brewers indicated that the bitterness contribution from mash hopping was about 30% of that from a 60 min. boil using the same amount of hops.4 In addition, the overall hop character (bitterness, flavor, and aroma) was generally perceived as being less than that of a 60 min. boil.
My opinion is that mash hopping is a waste of hops, but there are many brewers who swear by it for contributing more character to their India pale ales (IPAs). Your mileage may vary.
First Wort Hopping
First wort hopping (FWH) consists of adding hops to the boil kettle as the wort is received from the lauter tun. The hops steep in the hot wort as the boil kettle is being filled, which may take a half hour or longer. The essential (aromatic) oils are normally insoluble and tend to evaporate to a large degree during the boil. The idea is that, by letting the hops steep in the wort prior to the boil, the oils have more time to oxidize to more soluble compounds, resulting in more flavor and aroma being retained during the boil. However, it is important to understand that the original German study5 only examined bittering additions, so we are only talking about the difference in hop character between first wort hopping and 60 min. bittering. Late hop addition character would most likely overwhelm any FWH character.
An experiment conducted for the 2014 National Homebrewers Conference6 indicated that the bitterness contribution from FWH was about 110% of that from a 60 min. boil using the same amount of hops. There wasn’t a significant increase in overall hop character as perceived by the participants.
I often use FWH for my beers, because it doesn’t seem to hurt anything and I get more utilization from my hops.
Bittering
The primary use of hops is for bittering. Bittering hop additions are boiled for 45–90 min. to isomerize the alpha acids; 60 min. is most common. Typically, a maximum of 30% of the alpha acids are isomerized during a 90 min. boil. Most of this occurs within the first 45 min., and between 45 and 90 minutes the isomerization only increases by about 5%, and only a little (<1%) at longer boil times. The aromatic oils of the bittering hops tend to boil away if boiled for too long, leaving little hop flavor and no aroma.
If you consider the cost of bittering a beer in terms of the amount of alpha acid per unit weight of hop used, it is more economical to use a half ounce of a high-alpha acid hop rather than one or two ounces of a low-alpha acid hop. You can save your more expensive (or scarce) aroma hops for flavoring and finishing. A more detailed discussion of hop utilization follows later in this chapter, where you can refer to table 5.5 for the percent utilization of hops as a function of boil time and boil gravity.
Flavoring
Adding flavoring hops midway or later in the boil allows both isomerization of alpha acids and evaporation of light aromatics. This yields moderate bitterness and retains residual oils that are perceived as hop flavor. These flavoring hop additions are added at any time within the final 30 min. of the boil. Any hop variety may be used, but usually the lower alpha acid or mid-range alpha acid varieties are chosen. However, high alpha acid varieties that have pleasant flavors, such as Galena and Challenger, can be used. Often small amounts—0.25–0.5 oz. (7–15 g)—of several varieties will be combined at this stage to create a more complex character.
Finishing, Hop Bursting, and Hop Steeping
When hops are added during the final minutes of the boil, less of the aromatic oils are lost to evaporation and more hop aroma is retained. One or more hop varieties may be used, in amounts varying from 0.25–4.0 oz. (7–120 g), depending on the character desired. A total of 1–2 oz. (30–60 g) is typical. These finishing or aroma hops are usually added 15 min. or less before the end of the boil, or are added at “knockout” (when the heat is turned off) and allowed to steep 10–30 min. before the wort is cooled.
Hop bursting is a practice that is common with American IPA brewers, where most of the bitterness of the beer comes from finishing hop additions added in the last 15 minutes before knockout. This allows some isomerization and more retention of oils. This technique uses much more hops to achieve the desired bitterness, which also produces a lot more hop flavor in the beer than traditional hopping schedules.
Hop steeping, or whirlpool hopping, comes from commercial brewing practice where the wort is directed to a whirlpool after the boil to separate spent hops and trub before going through the plate heat exchanger for chilling. The hot wort will often spend 30–60 min. in the whirlpool before being chilled, and brewers would use this opportunity to add more hop oil to the wort. The temperature in the whirlpool is hot (>185°F [>85°C]) but not boiling. This causes some isomerization to occur, but also preserves more of the essential oils than finish hopping or hop bursting. It is important to understand that whirlpool hopping is a necessity that grew out of commercial brewers having to separate the trub. Hop steeping is a good way to improve hop flavor and aroma in a beer, but the whirlpool action is not necessary.
If you are attempting to clone a commercial beer that uses whirlpool hopping, you need to find out how long the hops are in the whirlpool and at what temperature. You can estimate the bitterness contribution by taking 40% of the calculated bitterness for boiling for that amount of time. While perceived bitterness is more than just the level of isomerized alpha acids, 40% of calculated bitterness is a good starting point. This is based on a study by Malowicki and Shellhammer (2005) that showed the amount of isomerization at 194°F (90°C) is roughly 40% of that at 212°F (100°C); this fell to about 15% at 176°F (80°C). If maximum hop aroma and flavor is your goal, you may be better off boiling some of your hop-standing hops for an equivalent time to get all of the bitterness contribution, and then adding the rest (and maybe some extra) at knockout, chilling them quickly to capture all of the oils.
In some setups, a “hop back” is used—the hot wort is run through a small chamber full of fresh hops before the wort enters a heat exchanger or chiller. This is essentially the same as hop steeping, or whirlpool hopping; in effect, you are using the kettle or whirlpool as your hop back.
A word of caution when adding hops at knockout or using a hop back—depending on several factors relating to the hops you are using (e.g., the amount, variety, and freshness), the beer may take on a grassy taste, due to hop polyphenols that would normally be neutralized by the boil. If the hop character ends up too grassy, then I would suggest boiling your hop addition for a little more time. If there is not enough fresh hop character, then I would suggest using dry hopping.
Dry Hopping
Dry hopping, where hops are added to the beer at the end of fermentation, is probably the best way to get fresh hop aroma into a beer. As the name implies, the hops are added dry. Many varieties of hop are appropriate for dry hopping, and several varieties can be combined to give the beer a more complex character. While you may be tempted to use a large amount of low-alpha acid aroma hops, you need to consider that this will also add a lot of plant material, which can contribute tannic or grassy flavors. Even though the tannic quality will probably subside after a few weeks, many craft brewers use higher-alpha acid varieties instead, such as Centennial, Galaxy, and Citra, because these hops generally have higher oil content by weight, which means less vegetative matter in the tank. Choose your variety with care because not every hop is appropriate for every beer style.
Dry hops for IPAs are generally added to give 3–5 days of contact time at temperatures of 50–70°F (10–21°C). Allow less contact time at warmer temperatures. The hops should be removed after this period to reduce the grassiness that can come from the cones. Historically, dry hopping rates for British pale ales were 0.5–1 lb. per barrel (note: these are imperial units). By contrast, British IPAs were historically hopped at 5–9 lb. per barrel (imperial units), but you have to remember that the typical hop then was half the strength of a typical hop today, and that the beers were often held in the casks for a year or more before serving, which greatly decreased the bitterness and aroma of the hops. Today’s IPAs are generally dry hopped at 1–2 lb. per US beer barrel (31 gal.), which works out to 0.5–1 oz./gal. (7.5–15 g/L). And remember, not every beer is an IPA; just because you can dry hop any style doesn’t necessarily mean you should.
Dry hopping actually does add bitterness to the beer (usually 1–5 IBU), but that bitterness comes from oxidized alpha and beta acids, and hop polyphenols, not from isomerized alpha acids.
Note: Dry hopping means what it says, that is, you add them dry. You don’t have to pre-boil or sanitize them. Contamination and beer spoilage from hops just doesn’t happen. However, dry hopping re-introduces oxygen to the beer, and this can cause oxidation and staling. Therefore, many brewers add dry hops toward the end of fermentation while there is still active yeast able to scavenge the oxygen, rather than afterward when the beer is off the yeast. Another method for reducing the potential for oxidation is to boil some water to drive out the oxygen, refrigerate it to cool, and then carefully disperse the hops into that water and pour the resulting slurry into the beer. A third method, when dry hopping in kegs, is to put the hops into the keg first, pressurize and purge the keg with carbon dioxide, and then rack the beer into the purged keg.

Figure 5.3. Clockwise from left, dried whole cones, fresh whole cones, and pelletized hops.
Hop Forms—Pellets, Plugs, and Whole Hops
It’s rare for any group of brewers to agree on the best form of hops. Each of the common forms—whole, plug, or pellet—has its own advantages and disadvantages. What form works best for you will depend on where in the brewing process the hops are being used, and will probably change as your brewing methods change.
Whichever form of hops you choose to use, freshness is important. Fresh hops smell fresh, herbal, and spicy, like resinous needles, and have a light green color like freshly-mown hay. Old hops, or hops that have been improperly stored, are often oxidized and smell like pungent cheese and may have turned brown. Ideally, hop suppliers pack hops in oxygen-barrier bags and keep them cold to preserve the freshness and potency. Hops that have been stored warm or in non-barrier (thin) plastic bags can easily lose 50% of their bitterness potential in just a few months. Most plastics are permeable to oxygen; so when buying hops at a homebrew supply store, check to see if the hops are kept in a cooler or freezer and if they are stored in oxygen-barrier containers. If you can smell the hops when you open the cooler door, then the hop aroma is leaking out through the packaging and they are not well protected from oxygen. If the stock turnover in the brewshop is high, non-optimum storage conditions may not be a problem. Ask the shop owner if you have any concerns.
Table 5.4—Hop Forms and Their Relative Merits
| Hop form | Advantages | Disadvantages |
|---|---|---|
|
Whole |
Easy to strain from wort. Best aroma, if fresh. Good for dry hopping. |
Will oxidize faster than pellets or plugs. They soak up wort, resulting in some wort loss after the boil. Bulk makes them more difficult to weigh. |
|
Plugs |
Retain freshness longer than whole hops. Convenient 0.5 oz. (15 g) units. Plugs behave like whole hops in the wort. |
Can be difficult to break apart into smaller amounts Soak up wort just like whole hops. |
|
Pellets |
Easy to weigh Small increase in utilization due to shredding. Best storability. |
Turns into hop sludge in bottom of kettle that is difficult to strain. Aroma content tends to be less than other forms due to amount of processing. Hard to contain when dry hopping—creates floaters. |

Figure 5.4. Cascade hops on the vine.

Figure 5.5. Measuring hops on a digital scale
How to Measure Hops
Hops used by homebrewers are typically measured by weight in either ounces or grams (pounds and kilograms for commercial brewers). Hop callouts in recipes specify the weight of the addition, the percent of alpha acids (% AA), and the time of the addition. The hop addition times are measured from the end of the boil, that is, the amount of time that they are boiled before the heat is turned off.
The % AA value is usually listed on the bag. If the hops have been stored cold, that number is usually fairly accurate. However, if the hops have been stored warm, the actual % AA may be significantly lower. Hops can lose as much as 50% of their bitterness potential in six months if stored poorly. Oxidized alpha acids are still bitter, but they are only about 66% as bitter as iso-alpha acids, and they will not isomerize in the boil.
Hop Utilization and (International) Bitterness Units
Hop resins containing the alpha acids act like oil in water. Heating the hops causes isomerization of the alpha acids, and this change in their molecular geometry makes the alpha acids more soluble in the wort (which is, after all, mostly water). The percentage of the total alpha acids from the hops that are isomerized and survive into the finished beer as iso-alpha acids is termed the hop “utilization.”
The isomerization rate for alpha acids is solely dependent on temperature. Isomerization starts at about 175°F (80°C), and the rate reaches a maximum at the temperature of the boil. Therefore, the total amount of isomerization for any hop addition depends mainly on boiling time. Higher elevations lower the boiling temperature of the wort, and therefore lower the isomerization rate and total isomerization as well.7 Under homebrewing conditions, hop utilization generally tops out at about 30%. Many factors, like boil temperature, affect hop utilization, but the poor solubility of the hop resins is always a problem (the resins consist of the bittering compounds). Most of the losses that affect hop utilization are due to the iso-alpha acids and other bittering compounds being carried out of solution. This is due to these bittering compounds sticking to the trub and the walls of the boil kettle. Think of the oil and water analogy, the hop resins are going to stick in a thin layer to every surface they contact in the wort, which, in addition to the kettle and trub, includes the fermentor, the chiller, the tubing, and the yeast.
Therefore, one of the more significant factors for utilization is the batch size. The reason is simply the surface area-to-volume ratio of the kettle and fermentor: the larger the batch size, the less surface area per unit volume for the resins to stick to. This is probably the main reason why commercial brewers get better utilization than homebrewers, because the batch sizes are 10 to 100 times larger.
The other important factors are the amount of trub generated in the wort and the yeast mass in the fermentor. Both of these effects can be estimated by looking at the OG of the beer. The wort gravity doesn’t affect the solubility of the iso-alpha acids and other bittering compounds directly. Instead, the higher the wort gravity, the more protein in the wort, and the more solid material will be generated during the hot and cold break. A higher OG also means that more yeast has to be pitched to ferment it, leading to more losses. Of course, the wort composition also affects utilization; for example, a high-adjunct, low-protein wort will generate less break material than an all-malt wort.
The last factor affecting utilization is the hop form, that is, whether you use pellets, plugs, or whole hops. Hop pellets seem to give better utilization because the processing has crushed and squeezed the lupulin glands and made the compounds inside more accessible. Pellets seem to have about 10%–15% better utilization than the rate for whole cones or plugs, but all this means is in relation to what you would achieve anyway for a particular boiling time, all else being equal. For example, if boiling whole hops for 50 min. gives you 20% utilization, then pellets may give you 22%–23%, that is, a 10%–15% increase on what you could achieve with whole hops. Pellets are not going to increase your total hop utilization from, say, 20% to 30% over whole hops, all else being equal.
There are several different models for calculating bitterness units (BUs) currently in use among homebrewers. The difference between them all is how the utilization is calculated. The Tinseth model8 is probably the most commonly used and is presented in the section below under “Hop Utilization Equation Details.” This utilization model consists of a boil time function and wort gravity function. These two functions are multiplied together to give the combined utilizations found in table 5.5.
A final note on bittering and utilization. It is nearly impossible to model every factor that will affect the utilization across everyone’s equipment and brewing process. The key to using the following equations is to understand that the results are a benchmark, a number you can use to measure how much hop bitterness you intend the beer to have. Calculate your recipes using the model, taste your beers, then adjust your hop additions based on the numbers and your perception of those numbers as realized in the beer. Experienced brewers understand that different brewers brewing the same recipe will make different beers. The beers may be very similar or they may be very different—there are a lot of variables at play. Also, don’t get obsessed trying to calculate utilization or BUs to three decimal places. People have a bitterness resolution of about five bitterness units, which means that you can readily taste the difference between a 20 and a 25 BU beer, but not between a 28 and 31.
Calculating Hop Bitterness Units
Alpha acid units simply tell you how much alpha acid is going into the wort. The BUs estimate how much of that alpha acid will be isomerized and make it into the final beer. The equation for calculating BUs is:
For oz./gal.,
BU = weight of hops × % AA × % utilization × (75 / final volume).
For g/L,
BU = weight of hops × % AA × % utilization × (10 / final volume).
The proper units for BUs are milligrams per liter (mg/L), so to convert from oz./gal. a conversion factor of 75 (actually 74.89) is needed. For the metric world, to convert from g/L, the conversion factor is 10. (For those of you paying attention to the units, the missing factor of 100 was taken up by the % utilization, i.e., multiplying as 0.28 as opposed to 28%.)
Let’s do an example with the following recipe for Joe Ale.
Joe Ale
To make 5 gal. (19 L).
| Extract | Gravity Points |
|---|---|
|
5.5 lb. (2.5 kg) amber DME |
|
|
Boil gravity for 6 gal. (23 L) |
1.038 |
| Hop schedule | Boil time (min.) |
|
1.0 oz. (30 g) Mt. Hood 8% AA |
60 |
|
1.5 oz. (45 g) Hersbrucker 4% AA |
15 |
There are three steps to calculating the BUs for this recipe.
- 1. Calculate the boil gravity based on the initial boil volume.
- 2. Calculate the utilization for each addition based on the boil gravity and boil time.
- 3. Calculate the BUs for each addition using the final volume after the boil.
Note that there are two gravity and volume factors in the utilization and BU calculations. The utilization factor depends on the gravity of the wort before the boil, because that is what predicts the amount of trub in the kettle. The final volume factor for the BU equation is the volume of wort after the boil, because now we are calculating the final concentration of the isomerized alpha acid. The hop additions added X amount of alpha acids to the wort. The utilization is the amount of alpha acid isomerized divided by what was lost to the trub and environment. The final bitterness is the concentration of what’s left in the volume of wort after the boil. Any dilutions you do in the fermentor will change the concentration and the BUs.
Calculate the boil gravity. As we saw in chapter 4, the boil gravity of your wort can be calculated by rearranging the mass gravity volume equation. This allows the boil gravity points to be calculated by multiplying the weight (mass) of extract by the extract potential (in PPG or PKL) and dividing this value by the wort volume before the boil.
gravity points = (mass of extract × PPG) / volume of wort
In this case, the boil volume is 6 gal. (23 L). From table 4.1, we know dry malt extract typically yields 42 PPG (350 PKL), and the Joe Ale recipe above calls for 5.5 lb. (2.5 kg) of extract. So, for US standard units:


Now we can use the known gravity of the boil (i.e., 1.038) to figure out the utilization.
If you have a smaller pot and wanted to use the Palmer method to carry out a 3 gal. (11.4 L) boil, you can still follow the same procedure. Calculate the gravity of the boil based on how much extract you are adding to the water, for instance, 3 lb. into 3 gal. Your boil gravity would be (3 × 42) / 3 = 42, or 1.042, but remember that the final volume you use for the BU equation would be total volume of wort in the fermentor after you have added the extra extract and water.
Utilization. The utilization is a combination of two factors: one is the isomerization rate and the other is the loss of isomerized alpha acids to the environment as a function of wort gravity. The utilization numbers that Tinseth published are shown in table 5.5. To find the utilizations for the “in-between” boil gravity values, simply interpolate the value based on the numbers for the bounding gravity values at the given time.
For this example, to calculate the utilization for a boil gravity of 1.038 at 60 min., look at the 60 min. utilization values for 1.030 and 1.040, which are 0.276 and 0.252, respectively (table 5.5). There is a difference of 24 between the two, and 8/10ths of the difference is about 19, so the adjusted utilization for 1.038 would be 0.276 − 0.019 = 0.257. The utilization for the 15 min. hop addition is calculated the same way and equals 0.127.
Going back to our Joe Ale recipe and calculating BUs for both additions:

and

In grams and liters, the calculations would be:
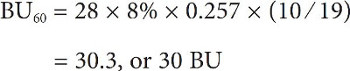
and
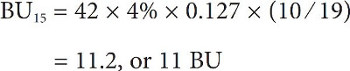
Giving a grand total of 41 or 42 BUs depending on how you round it, but, in all honesty, I would probably just call this 40 because you can’t taste a difference less than 5 BU and these calculations tend to overestimate the actual BUs anyway.
Please understand that these equations are just an estimate. Each brew is unique; the equipment, temperature of the boil, pH, and losses during fermentation; all of these factors make it nearly impossible for two people to arrive at the same amount of bitterness in their beer for any given hop addition. However, these equations allow us to consistently plan our hop additions, to be consistent within our own setup, and make calculated changes from batch to batch to improve our beers. And that is a good reason to use them.
Hop Utilization Equation Details
For those of you who are comfortable with the math, the following equations were determined by Tinseth (see table 5.5) from curve fitting a lot of test data while working on his PhD at the University of Oregon. The degree of utilization is composed of a gravity factor and a boil time factor multiplied together. The gravity factor accounts for reduced utilization due to higher wort gravities. The boil time factor accounts for the change in utilization due to boil time:
Utilization = ƒ(G) × ƒ(t)
where
ƒ(G) = 1.65 × 0.000125(GB − 1),
ƒ(t) = [1 − e(−0.04 × t)] / 4.15,
and
GB is the boil gravity; t is the time in minutes.
The numbers 1.65 and 0.000125 in ƒ(G) were empirically derived to fit the boil gravity (GB) analysis data. In ƒ(t), the number −0.04 controls the shape of the curve for utilization versus time (t). The factor 4.15 controls the maximum utilization value. This number may be adjusted to customize the curves to your own system. For example, if you feel that you are having a very vigorous boil or generally get more utilization out of a given boil time for whatever reason, you can reduce the number a small amount to 4.0 or 3.9. Likewise, if you think that you are getting less out of your boil, then you can increase it to 4.25 or 4.35. These adjustments will alter the utilization value for each time and gravity in table 5.5.
Bitterness Units Nomograph for Hop Additions
To use a nomograph for deriving BU (fig. 5.6 and 5.7), start on the right and draw a straight line from the %AA of your hop through the Weight of the addition, to arrive at the AAUs for that addition. Next, draw a line from the AAUs through the Recipe Volume to arrive at the AAUs per gallon. Now move to the left hand side of the chart and draw a line from your Boil Gravity, through your Boil Time, to determine the Utilization. Finally, draw a line through the points from the Utilization and AAUs per gallon lines to determine the BUs of that hop addition.
Table 5.5—Utilization as a function of Time versus Boil Gravity
| Boil gravity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Boil time (min.) | 1.030 | 1.040 | 1.050 | 1.060 | 1.070 | 1.080 | 1.090 | 1.100 | 1.110 | 1.120 |
|
0 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
5 |
0.055 |
0.050 |
0.046 |
0.042 |
0.038 |
0.035 |
0.032 |
0.029 |
0.027 |
0.025 |
|
10 |
0.100 |
0.091 |
0.084 |
0.076 |
0.070 |
0.064 |
0.058 |
0.053 |
0.049 |
0.045 |
|
15 |
0.137 |
0.125 |
0.114 |
0.105 |
0.096 |
0.087 |
0.080 |
0.073 |
0.067 |
0.061 |
|
20 |
0.167 |
0.153 |
0.140 |
0.128 |
0.117 |
0.107 |
0.098 |
0.089 |
0.081 |
0.074 |
|
25 |
0.192 |
0.175 |
0.160 |
0.147 |
0.134 |
0.122 |
0.112 |
0.102 |
0.094 |
0.085 |
|
30 |
0.212 |
0.194 |
0.177 |
0.162 |
0.148 |
0.135 |
0.124 |
0.113 |
0.103 |
0.094 |
|
35 |
0.229 |
0.209 |
0.191 |
0.175 |
0.160 |
0.146 |
0.133 |
0.122 |
0.111 |
0.102 |
|
40 |
0.242 |
0.221 |
0.202 |
0.185 |
0.169 |
0.155 |
0.141 |
0.129 |
0.118 |
0.108 |
|
45 |
0.253 |
0.232 |
0.212 |
0.194 |
0.177 |
0.162 |
0.148 |
0.135 |
0.123 |
0.113 |
|
50 |
0.263 |
0.240 |
0.219 |
0.200 |
0.183 |
0.168 |
0.153 |
0.140 |
0.128 |
0.117 |
|
55 |
0.270 |
0.247 |
0.226 |
0.206 |
0.188 |
0.172 |
0.157 |
0.144 |
0.132 |
0.120 |
|
60 |
0.276 |
0.252 |
0.231 |
0.211 |
0.193 |
0.176 |
0.161 |
0.147 |
0.135 |
0.123 |
|
70 |
0.285 |
0.261 |
0.238 |
0.218 |
0.199 |
0.182 |
0.166 |
0.152 |
0.139 |
0.127 |
|
80 |
0.291 |
0.266 |
0.243 |
0.222 |
0.203 |
0.186 |
0.170 |
0.155 |
0.142 |
0.130 |
|
90 |
0.295 |
0.270 |
0.247 |
0.226 |
0.206 |
0.188 |
0.172 |
0.157 |
0.144 |
0.132 |
Source: Glenn Tinseth, “Glenn’s Hop Utilization Numbers,” 1995, accessed November 15, 2016, http://www.realbeer.com/hops/research.html.
Nomograph for IBU Calculations

See instructions.
Figure 5.6. IBU nomograph in ounces and gallons
Nomograph for IBU Calculations Grams and liters
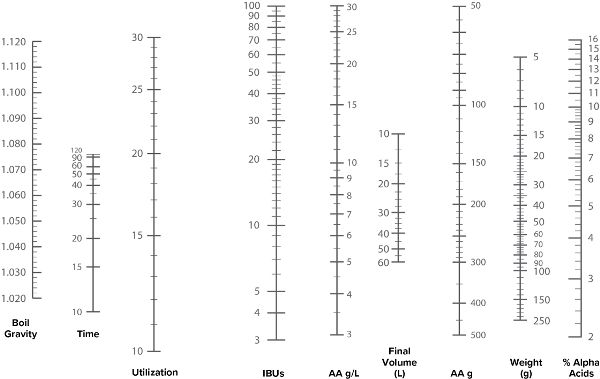
See instructions.
Figure 5.7. IBU nomograph in grams and liters.
1 Algazzali and Shellhammer (2016) reported that oxidized beta acids are about 84% (±10%) as bitter as iso-alpha acids, whereas oxidized alpha acids are about 66% (±13%) as bitter as iso-alpha acids.
2 Hahn, Lafontaine, and Shellhammer, “A holistic examination of beer bitterness” (abstract presentation, World Brewing Congress, Denver, CO, August 17, 2016).
3 Fritsch and Shellhammer (2007).
4 Curtis (2014).
5 Preis and Mitter (1995).
6 Curtis (2014).
7 However, using a pressure-cooker to increase the isomerization rate is a bad idea, because it will change the Maillard reactions and subsequently affect the flavor or the beer.
8 Glenn Tinseth, “Glenn’s Hop Utilization Numbers,” 1995, accessed November 15, 2016, http://www.realbeer.com/hops/research.html.