Figure 1 Outline of study of PCS coating and corrosion test of B4C joined samples

This paper describes the experimental results on microstructural observations and tensile tests of Al joined boron carbide (B4C) bulks coated by silicon carbide (SiC). For the formation of SiC coating, polycarbosilane (PCS) was applied and pyrolyzed on the bulk surface in Ar gas. Then, the reaction of B4C, Al and PCS mixed powders in air was investigated by phase identification and thermogravimetric analysis of the heat treated powders.
Boron carbide (B4C) has many excellent characteristics such as high hardness (the third hardest ceramics), good abrasion resistance, and a light weight. Thus, B4C is a very important material in the field of engineering.1 In particular, its high hardness and light weight are remarkably useful because saving weight in moving or shaking machines is effective at saving energy.2 Thus, methods have been investigated for making B4C ceramics with large and complex shapes such as the stages of semiconductor processing machines. Sekine et al. reported that aluminum can intrude into any tiny cracks in B4C ceramics and suggested that assembling B4C parts by using aluminum was a useful method.3
However, the use of the joined B4C is restricted, because aluminum can be easily eroded away by a corrosive environment. This restriction is not acceptable, because it spoils the good resistance of B4C to a corrosive environment.
To remove this restriction, we proposed the use of a ceramic coating on the joined B4C samples and utilized polycarbosilane (PCS) for this coating. Some researchers have reported that PCS can play a role in ceramic surface modification.4,5 Moreover, PCS can be transformed into SiC, which has a good resistance to a corrosive environment.6–8 Therefore, coating the joined B4C with PCS will be effective at providing protection against a corrosive environment.
In this study, we coated B4C joined samples with SiC by using PCS and performed corrosion testing after this coating by using liquid NaOH. In addition, the detailed mechanism of the PCS transformation process into SiC on B4C was also investigated.
We used PCS to coat B4C samples that had been joined by aluminum metal and performed corrosion tests on the coated samples. An outline of this study is shown in Fig.1. B4C can diffuse into PCS polymer (NIPUSI-Type A, Nippon Carbon, Japan) by heating.9 Therefore, it was considered that the effect of the combination of PCS and B4C could be confirmed by this coating and corrosion test. The B4C joined samples were dipped into a PCS solution that was dissolved in toluene to a concentration of 0.1 mol/L, and the coated B4C joined samples were cured by using thermal oxidation, with a heating rate of 8 K/h up to a temperature of 485 K under flowing air. After curing, the samples were pyrolyzed for 1 h at 1073 K or 1473 K under an Ar flow (purity of Ar gas: 99%). The PCS could be transformed into SiC by this pyrolysis. After the pyrolysis, a corrosion test was carried out by soaking the coated samples in NaOH solvent at 2 mol/L. After the corrosion test, the samples were examined using optical microscopy and a four-point bending test, along with JIS R1601:2008.
Figure 1 Outline of study of PCS coating and corrosion test of B4C joined samples

To clarify the mechanism of the process for transforming PCS into SiC on B4C, pyrolyzed mixtures that included 10 vol% B4C were investigated. The outline of this experiment is shown in Fig.2. Commercially available PCS, with 10 vol% B4C, was dissolved in a cyclohexane solvent. The solvent was removed using a freeze-drying method, and the mixture powder was obtained. The mixture was cured using thermal oxidation, with a heating rate of 8 K/h up to a temperature of 485 K under flowing air. After the curing, the powder was pyrolyzed for 1 h at 1073 K or 1473 K under an Ar flow. The TG-DTA curve of the mixture after curing was obtained using a thermo-gravimetry analyzer, and the XRD pattern of the mixture after pyrolysis was obtained using a diffractometer (RINT2500, Rigaku Corporation, Japan).
Figure 2 Outline of study of pyrolyzed mixtures containing PCS and B4C powders

Before the corrosion test, we attempted to observe cross sections of the joining areas of these samples to clarify the detailed structure of the coated samples. Figure 3 shows the SEM image, and Fig.4 shows EDS mappings of the cross section of the joining area of the B4C joined sample after the SiC coating was pyrolyzed at 1473 K. The bottom half of each image shows the B4C joined sample. Silicon atoms (Si) mainly existed over the sample, suggesting that the layer over the sample was derived from PCS and transformed into SiC. The center of the B4C joined sample was an aluminum layer that joined the individual B4C pieces.3,10 Some papers have shown that some Al transforms into Al3BC and AlB2 after joining, whose volume is considered to be increased by heating.3,10 Therefore, it would be difficult for the aluminum to flow out. There were some holes in the aluminum layer. These holes were considered to be the result of grinding, because similar holes also existed in B4C. No definitive cracking could be observed between the B4C and the SiC coating layer. Therefore, it appears that there was no exfoliation between the B4C and the membrane. In the EDS mappings, the boron atom (B) of the mapping shows that boron atoms existed in the area of the SiC coating layer, and that B4C-derived boron diffused in the SiC coating layer. Aluminum atoms (Al) resulting from the B4C joining did not reach the coating layer surface, and the EDS mapping also revealed that the SiC coating layer completely covered the aluminum.
Figure 3 SEM image of cross section of joining area of B4C joined sample after SiC coating pyrolyzed at 1473 K

Figure 4 EDS mappings of cross section of joining area of B4C joined sample after SiC coating pyrolyzed at 1473 K

Figure 5 shows cross-sectional photographs of the joining area for the B4C joined samples after corrosion testing, and Table 1 lists the average tensile strength and the remaining aluminum in the B4C joined samples after the corrosion test. Only 41% of the joining area of the B4C joined sample without coating remained. The rest of the area was dissolved by the NaOH solution, and the B4C in the joining area was clearly exposed. In the case of the B4C joined sample with the SiC coating at 1073 K, the area that was dissolved by NaOH decreased compared with the bare sample. However, 18% of the joining area still dissolved. For the B4C joined sample with the SiC coating at 1473 K, almost all of the joining area remained intact. It appears that the SiC coating protected the joining area from dissolution by NaOH.
Figure 5 Cross-sectional photographs of joining area for B4C joined samples after corrosion testing (left: B4C joined sample without coating, center: B4C joined sample with SiC coating at 1073 K, right: B4C joined sample with SiC coating at 1473 K)

Table 1 Average tensile strength and remaining aluminum in B4C joined samples after corrosion testing

The number of samples used in the tensile strength test was fixed at five. In the case of the B4C joined sample without coating, the average tensile strength could not be measured because it was below the tensile-strength-measurement threshold of the testing machine. This means that the average tensile strength of this sample was close to 0 MPa. The average tensile strength of the B4C joined sample with the SiC coating at 1073 K was approximately 106 MPa, and that of the B4C joined sample with the SiC coating at 1473 K was approximately 304 MPa, which indicated that the tensile strength was directly proportional to the rate of remaining aluminum of the joining area. This corrosion test showed that the SiC coating derived from PCS could effectively provide protection against a corrosion environment.
To shed light on how the different results obtained from the corrosion test depended on the temperature during pyrolysis for the PCS transformation into SiC, we performed an investigation using the mixture of PCS and 10 vol% B4C powder. The rate was derived from the point analysis of the EDS mappings. Liquid NaOH can dissolve excess materials such as carbon content or organometallic polymer before decomposition. PCS includes numerous carbon atoms. Therefore, many organic compounds remained after pyrolysis.11 Thus, the reason the organic compounds were decreased by the addition of B4C should be investigated.
Figure 6 shows the XRD patterns of these mixtures pyrolyzed at 1073 K or 1473 K. For comparison, the patterns of B4C and PCS pyrolyzed at 1473 K are also shown in this figure. The pattern of the mixture of PCS/B4C powder pyrolyzed at 1073 K shows that boron oxide (B2O3) peaks mainly occur at 27.5°, with a set of small peaks around 14.5°, 18.9°, 30.7°, and 40.1°. This B2O3 was derived from the oxidation of B4C, and it was considered that the oxide was derived from the cured PCS and impurities in the argon gas. In particular, B4C can easily be oxidized by heating at more than 873 K, and it was considered that some oxygen in the polymer reacted with B4C during pyrolysis. As for the mixture pyrolyzed at 1473 K, the XRD pattern shows that the boron oxide (B2O3) peak occurred at 27.5°. However, the set of small peaks of B2O3 were not found, except the peak at 14.5°, but the SiC peaks at 35.6° and 59.9° existed. In the pattern for just the PCS pyrolyzed at 1473 K, the sharp peak of SiC cannot be observed because the SiC consists only of the amorphous phase. However, the mixture pyrolyzed at 1473 K shows the sharp peaks of SiC. The broad peak at around 35° in an XRD pattern suggests the existence of amorphous SiC. The patterns of the SiC fibers pyrolyzed at 1473 K show the same broad peak.12 They reveal that PCS pyrolyzed at 1473 K usually becomes amorphous SiC. In addition, the sharp peaks of the SiC crystal can be observed from the PCS pyrolyzed at 1773 K.12,13
Figure 6 XRD patterns of B4C, PCS pyrolyzed at 1473 K, and mixtures of PCS-B4C powder pyrolyzed at 1073 K and 1473 K

In these XRD patterns, it is interesting to note that the remarkable SiC crystal peaks can be observed in the pattern for the mixture of PCS/B4C powder pyrolyzed at 1473 K, which reveals that SiC crystal in this sample grew in spite of the 1473 K pyrolysis.
Figure 7 shows that the weight and heat flow curves of B4C, PCS, and the mixture of PCS and 10 vol% B4C measured by TG-DTA, where the temperature range for this measurement was from 473 K to 1573 K. The temperature curves of the PCS and the mixture decreased from 873 K to 1073 K, and this decrease was derived from the PCS transformation into SiC, which was accompanied by decomposition and gas generation. In the case of B4C, the weight was increased from 773 K to 1373 K, and decreased beyond 1373 K. This increase was considered to be derived from oxidation, whereas the decrease was caused by B2O2 gas generation. B4C can easily be oxidized by heating at temperatures greater than 873 K, and some papers have shown that B2O2 gas can be generated by the decomposition of B2O3 by heating at more than 1373 K under an inert atmosphere.14 Therefore, this result is consistent with the results of the XRD patterns.
Figure 7 Weight and heat flow curves of B4C, PCS, and mixture of PCS and 10 vol% B4C measured by TG-DTA

It is important to note that the heat flow curve of the mixture beyond 1373 K was increased despite the mass being maintained beyond 1373 K. This phenomenon shows the continuation of the crystallization of SiC. The heat flow curve of PCS shows a gain beyond 1473 K, and this result is acceptable because the remarkable peak of SiC could not be observed in the XRD pattern of PCS pyrolyzed at 1473 K.
The generation of B2O2 gas is related to the SiC crystal growth. Active B2O2 gas can generate CO gas under an inert atmosphere, with the following chemical formula:15
(1) 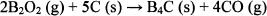
CO gas is required for SiC crystal growth, because the silicon oxide over the SiC crystal must be removed by an active gas such as CO gas.12 Therefore, it was natural that the SiC crystal could grow at 1473 K by the addition of B4C. Moreover, this phenomenon consumes a large quantity of carbon, and it was considered that the organic compounds derived from PCS were consumed by the B2O2 gas. As a result, the SiC coating layer over the B4C joined samples pyrolyzed at 1473 K had good resistance to liquid NaOH.
The B4C joined samples with a SiC coating layer derived from PCS pyrolyzed at 1473 K had good resistance to a corrosive atmosphere. The tensile strength of a sample after a corrosion test involving submerging it into 3 mol/L of liquid NaOH for 72 h was almost the same as its strength before the corrosion test. The good resistance was caused by the consumption of the excess organic components in the PCS after pyrolysis by the B2O2 gas derived from B4C oxidation and the decomposition of the oxidized B4C.
1 F. Thevenot, Boron carbide – A comprehensive review, J. Eur. Ceram. Soc., 6, 205–225 (1990)
2 M. A. Kuzenkova, P. S. Kislyi, B. L. Grabchuk and N. I. Bodnaruk, The structure and properties of sintered boron carbide, J. Less-Common Met., 67, 217–223 (1979)
3 K. Sekine, T. Kumazawa, W.–B. Tian, H. Hyuga, and H. Kita, Low-temperature joining of boron carbide ceramics, J. Ceram. Soc. Jpn., 120, 207–210 (2012)
4 K. Kita, N. Kondo, H. Hyuga, Y. Izutsu and H. Kita, Study of modification on alumina surface by using of organosilicon polymer, J. Ceram. Soc. Jpn., 119, 378–381 (2011)
5 K. Kita, N. Kondo, Y. Izutsu and H. Kita, Investigation of the properties of SiC membrane on alumina by using polycarbosilane, Mater. Lett., 75, 134–136 (2012)
6 P. Colombo, T. E. Paulson, and C. G. Pantano, Synthesis of Silicone Carbide Thin Films with Polycarbosilane (PCS), J. Am. Ceram. Soc., 80, 2333–2340 (1997)
7 R. A. Wach, M. Sugimoto, M. Yoshikawa, Formation of Silicone Carbide Membrane by Radiation Curing of Polycarbosilane and Polyvinylsilane and its Gas Separation up to 250°C, J. Am. Ceram. Soc., 90, 275–278 (2007)
8 K. Kita, M. Narisawa, H. Mabuchi, M. Itoh, M. Sugimoto, M. Yoshikawa, Formation of continuous pore structures in Si-C-O fibers, J. Am. Ceram. Soc., 92, 1192–1197 (2009)
9 K. Kita, N. Kondo, K. Sekine and H. Kita, Silicon carbide coating of the aluminum joined boron carbide by using polycarbosilane, Mater. Lett., 112, 8–11 (2013)
10 K. Sekine, T. Kumazawa, W.–B. Tian, H. Hyuga, and H. Kita, Influence of joining time and temperature on the flexural strength of joined boron carbide ceramics, J. Ceram. Soc. Jpn., 120, 393–399 (2012)
11 E. Bouillon, D. Mocaer, J. F. Villeneuve, R. Pailler, R. Naslain, M Monthioux, A. Oberlin, C. Guimon and G. Pfister, Composition-microstructure-property relationships in ceramic monofilaments resulting from the pyrolysis of a polycarbosilane precursor at 800 to 1400 °C, J. Mater. Sci., 26, 1517–1530 (1991)
12 T. Shimoo, F. Toyoda, and K. Okamura, Thermal Stability of Low-Oxygen Silicon Carbide Fiber (Hi-Nicalon) Subjected to Selected Oxidation Treatment, J. Am. Ceram. Soc., 83, 1450–1456 (2000)
13 T. Shimoo, T. Maeda, and K. Okamura, Pyrolytic behavior of Si-C-O fiber in alumina powder under N2 atmosphere, J. Ceram. Soc. Jpn., 104, 169–173 (1996)
14 L. T. Su, S. S. Xie, J. Guo, A. I. Y. Tok and O. Vasylkivb, A novel non-catalytic synthesis method for zero- and two-dimensional B13C2 nanostructures, Cryst. Eng. Comm., 13, 1299–1303 (2011)
15 J. Tian, X. -J. Wang, L.–H. Bao, C. Hui, F. Liu, T.–Z. Yang, C.–M. Shen, H.–J. Gao, Boron Carbide and Silicon Oxide Hetero-nanonecklaces via Temperature Moduation, Cryst. Growth Des., 8, 3160–3164 (2008)