Figure 1. (a) XRD patterns of type B TiO2, before and after beads milling (b) TEM micrograph of type B TiO2

The deterioration, caused by surface corrosion occurring at high temperatures, of the shell of casting mold costs the manufacturing industry great losses annually. When the demand for finding new materials for the shell is crucial, the yttria-based ceramic Y2Ti2O7 possessing superior hardness, thermal-creep and corrosion resistance is a potential candidate. In this study, Y2O3 and TiO2 powders were mixed in 1:2 of mole ratio to synthesize Y2Ti2O7 powder, which was then used to sinter ceramic plates by hot-pressing method. Depending on the particle size of starting material, various types of Y2Ti2O7 were sintered with variation in density, hardness, flexural strength and fracture toughness. The one having the best mechanical properties was employed as ceramic molds for casting metal like aluminium (Al) or iron (Fe) over their molten temperature. The micro-structural observation on the interface of ceramic/metal was conducted using analytical electron microscopy and the diffusion of metallic particles into Y2Ti2O7 was investigated using energy dispersive X-ray spectroscopy.
The deterioration, caused by corrosion occurring at high temperatures, of the casting mold or the crucible is consuming the manufacturing industry a high maintenance cost. In recent years, in order to prevent corrosion, highly corrosion resistant ceramics such as alumina (Al2O3), chrome-alumina (Cr2O3-Al2O3), etc. were used as the coating layers, but some studies1,2 have revealed the corrosion still happens at high temperatures (1000 °C or higher). When the demand for finding new materials is crucial, Y2Ti2O7, which is known as a reinforcing phase in the oxide particle dispersion strengthened steel (ODS steel), has attracted attention of many researchers. ODS steel dispersed with nanoscale Y2Ti2O7 oxide particles has shown the great improvements in high tensile, fatigue strength, creep resistance, swelling resistance, and radiation resistance3. On the other hand, the fact that Y2Ti2O7 is a phase which precipitated in ODS steel4 suggests the poor reaction between Y2Ti2O7 and steel. From this key point, Y2Ti2O7 has been considered as a promising material with high molten-metal corrosion resistance.
In this study, we firstly synthesized a composite consisting of a metal layer sandwiched between two ceramic plates. The sandwich was heated over the melting points of metal and then cooled down to room temperature. Finally the sandwich was cut into cross-section and brought to analysis to investigate the resistivity of ceramics against diffusion and corrosion of molten metal.
Y2Ti2O7 plates were synthesized using hot-pressing method described by He et al.5. TiO2 and Y2O3 were chosen as the starting materials to synthesize the Y2Ti2O7 ceramics. The powder mixture was wet ball-milled in anhydrous ethanol for 24 h followed by evaporation and drying in an air oven at 80 °C before bring it to dry ball-milling for 12 h. Afterwards, the powder mixture was calcined at 1200 °C for 5 h and then dry ball-milled again for 12 h to break up agglomerates. Finally, the powder was sieved and then hot-pressed (25 MPa, argon (Ar), 1500 °C, 1 h) in a high-temperature-furnace (Haimaruchi 5000, Fujidempa Kogyo) to form sintered ceramics. The hot-pressing process was monitored by a digital program controller (KP1000, Chino).
To investigate the relationship between grain size and mechanical properties of as-synthesized Y2Ti2O7 ceramics, three types of TiO2 with different particle size were used as starting materials; type A is the micrometer size of TiO2 powder purchased from Wako, type B is the nanometer size powder pulverized from type A using beads milling (Ultra Apex Mill, Kotobuki Industries) and type C is nanometer size powder provided by Sigma Alrich. The XRD patterns of type A and type B powder shown in Fig. 1a confirm that there is no contamination by beads milling in type B. The TEM micrograph (Fig. 1b) reveals that the average particle size of this type is around 100 nm, which is much smaller than 5 μm of type A, but still bigger than that 25 nm of type C. Table I summarizes the information of starting materials and corresponding synthesized ceramics.
Figure 1. (a) XRD patterns of type B TiO2, before and after beads milling (b) TEM micrograph of type B TiO2

Table I. Summary of starting materials and corresponding synthesized ceramics.
| Starting materials | Synthesized ceramics | |
| Y2O3 particle size, origin | TiO2 particle size, origin | Y2Ti2O7 |
| 5 μm, Wako (type A) | YTO_A | |
| 430 nm, Shin Etsu | 100 nm, beads milling (type B) | YTO_B |
| 25 nm, Sigma Aldrich (type C) | YTO_C |
The synthesized ceramics were then employed as ceramic plates for casting metal at high temperatures. The synthesized sandwich of ceramic and metal was then brought into characterization to investigate the diffusion of metallic particles and corrosion resistance of the sintered Y2Ti2O7. In order to confirm that the sintered Y2Ti2O7 ceramic has excellent corrosion resistance against a variety of metals, the experimental process are carried out with aluminium (Al) foil and coarse-grained pure iron (Fe), with a variation of heating temperature. Three types of Y2Ti2O7-Al sandwiches synthesized at 560 °C, 660 °C (melting point of aluminium), 800 °C, are denoted as SA1560, SA1660 and SA1800, respectively. Similarly, two types of Y2Ti2O7-Fe sandwiches synthesized at 1400 °C (below melting point) and 1600 °C (over the melting point of iron), are denoted as SFe1400, and SFe1600, respectively. Table II summarizes the types of sandwich and corresponding synthesizing conditions.
Table II. Summary of sandwich and corresponding synthesizing conditions
Characterization and Measurements:
Bulk density of fabricated ceramics was measured in high purity toluene by the Archimedes method. Porosity of bulk samples was investigated by an X-ray CT Scan (SkyScan 1172, highest resolution 0.5 μm, Broker microCT). Phases of both powder and bulk samples were indentified using X-ray diffractometer (RINT 2500PC, Rigaku). The microstructures of samples were studied using a field-emission scanning electron microscope (JSM-6700F, JEOL). To investigate the grain size and grain boundary, the as-sintered and polished sample were thermally etched at 1400 C for 1 h before scanning electron microscopy.
The flexural strength of as-sintered ceramics was measured according to industrial standard (JIS R 16016). For measurement, sintered ceramic plates were cut into bar samples (36 × 4 × 3 mm) and tested in a universal testing machine (MODEL-1311 VRW, Aikoh Engineering) with outer and inner spans of 30 and 10 mm, respectively. The Vickers hardness Hv was measured according to standard JIS R 16107 by a hardness testing machine (HM-200, Mitutoyo) at different loads with a dwell time of 15 s. The fracture toughness KIC was determined by the indentation method according to the Niihara equation8:
To investigate the diffusion of metallic particles into ceramic layer in the as-synthesized sandwich, the sandwich was cut into cross-section and analyzed using X-ray diffraction (XRD) and energy dispersive X-ray spectroscopy (EDS) (JSM-6700F, JEOL).
Mechanical properties of sintered ceramics
Fig. 2 shows the particle morphology observed by TEM and the particle size distribution of three types of Y2Ti2O7 powders. While most particles of all three types are in agglomerates, it is clear that the average particle size of type C(YTO_C) is around 60 nm and much smaller than that of type A (YTO_A) and type B (YTO_B).
Figure 2. Particle morphology (top) and Particle size distribution (bottom) of Y2Ti2O7 powders

Fig. 3 compares the XRD patterns of the as-synthesized Y2Ti2O7 bulks and powders. It is obvious that the as-synthesized Y2Ti2O7 ceramic is single phase. The similar patterns of the bulk and powder materials confirm that there is no preferred orientation in the sintered ceramics.
Figure 3. XRD patterns of YTO_A (left), YTO_B (center) and YTO_C (right)

Fig. 4 (top) shows the microstructure of the thermally etched surface of three types of Y2Ti2O7 ceramics with equiaxed grains. It is obvious that the grain size of YTO_C is much smaller than that of the two others ceramics. This result can be attributed to the smallest particle size of YTO_C powder mentioned above. The grain size distribution of three types of sintered Y2Ti2O7 is shown in Fig. 4 (bottom).
Figure 4. Microstructure (top) and Grain size distribution (bottom) of Y2Ti2O7 ceramics

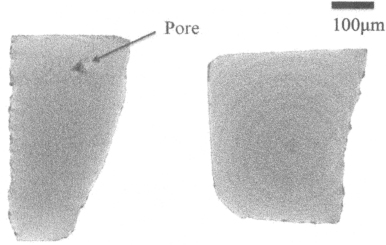
The measured density of all samples summarized in Table III, in which YTO_A’ sample was prepared by the same conditions (ambient, temperature, time) with YTO_A, but pressure was reduced to 5 MPa. While all three samples sintered at 25 MPa reach a density higher than 99%, the sample YTO_A’ sintered at 5 MPa only has a density lower than 97% of the theoretical one (4.98 g/cm3). At 25 MPa, even the sample sintered from coarse powder like YTO_A can reach a density as high as 99.3%, and has no pore bigger than 0.5 μm in diameter as confirmed by the X-ray CT scan microtomograph (see Fig. 5). Meanwhile, it can be seen that there are some big pores (around 5 μm in diameter) presenting inside the sample YTO_A’. It is believed that the optimized conditions of hot-pressing process (25 MPa, 1 h) resulted in reducing the pore’ size and in increasing the high density of sintered sample considerably.
Table III. Bulk density of sintered ceramics

Figure 5. X-ray CT Scan microtomographs of YTO_A (right) and YTO_A’ (left). There is a big pore inside the sample YTO_A’
The mechanical properties including bending strength, Vickers hardness and fracture toughness of the Y2Ti2O7 ceramics are summarized in Table IV. These results are comparable to the works of He et al.5. Out of three types of Y2Ti2O7, the YTO_C having the finest grain size shows the highest bending strength and Vickers hardness up to 185.55 MPa and 10.52 GPa, respectively. The phenomenon that mechanical properties like hardness and strength are improved with the shrinkage of grains was also found in other yttria-based ceramics9,10. It is believed that the denser ceramic with fewer and smaller pores are more durable under effect of Vickers indentation or loading force. However, the fracture toughness of YTO_C is slightly lower than that of YTO_A and YTO_B. The reason for the decrease of fracture toughness when the grain size shrinks is still not understood completely. Nonetheless, the SEM observation of cracks induced by Vickers indenter can give us some clue for this phenomenon (see Fig. 6). In the case of sample YTO_C, thee crack induced by indenter was developed longer than that of sample YTO_A. On the way of crack developing from the indentation, there is a vast of boundaries parallel to the direction of crack propagation. These boundaries became an avenue for crack developing far from the indentation (Fig. 6a). On the contrary, there are not many such paralleled-boundaries from the indentation of YTO_A due to the bigger grain size, as a result the crack just propagated in a short distance (Fig. 6b). From eq. (1), one can deduce that the fracture toughness KICwill decrease when the crack-length c increasing. Consequently, the longer crack of YTO_C sample leads to its lower KIC.
Table IV. Measurements of mechanical properties of Y2Ti2O7 ceramics

Figure 6. Propagation of crack from the indentation

Fabrication and analysis of ceramic-metal sandwiches
The analyzed results indicated that YTO_C is the sample having highest density, hardness and strength. Thus, it can be inferred that the quantity and size of pores inside YTO_C is very small comparing to the two other ceramics. Generally, porosity has been considered as a very important factor in determining the liquid metal corrosion resistance of a ceramic, because it can encourage the the diffusion of external metallic atoms/molecules into the ceramic structure. Therefore, YTO_C was chosen to synthesize the ceramic-metal sandwiches.
The sandwich is synthesized by the following method: both surfaces of YTO_C ceramic plate were grinded and polished until 5 μn of roughness. Two plates were used to clamp the metal component (11 μm-thick-aluminium foils or 5mm-iron grains) to form a sandwich, which was then put into a vacuum chamber to be annealed for 1 hr in argon atmosphere. Depending on the annealing temperature, which was varied from under- to over-melting point, corresponding sandwich was synthesized as summarized in Table II. Fig. 7 shows photographs of aluminium foils, iron grains and a synthesized sandwich. The sandwich consists of two black ceramic plates at top and bottom, and a bright thin layer of aluminium (Al) clamped in between them.
Figure 7. Aluminium foils (left), iron grains (center) and a synthesized sandwich (right).

In the case of sandwich SA1560, because the annealing temperature was much lower than the melting point (660.3 °C), the aluminium foils was totally not molten and stuck on the plates. When the annealing temperature was increased to the melting point (sandwich SA1660), the foils started to be molten and attached on the plates’ surfaces. However, it is very simple to detach the plates from each other, as shown in Fig. 8. It can be seen that there are some traces of molten Al remaining on the surface of the plates, however, from the XRD pattern, the crystallinity of the surface was confirmed Y2Ti2O7, which means the plates did not react with molten Al in this case.
Figure 8. Ceramic plates after detachment and their XRD pattern

When the annealing temperature changed to 800 °C (higher than the melting point), the molten foils strongly stick to the plates and cannot be detached by hand. Though they stick to each others, the XRD pattern (Fig. 9) confirms that the interface of the sandwich has two separated phases of Al and Y2Ti2O7. This indicates that Y2Ti2O7 did not react with molten Al during the heating process.
Figure 9. XRD pattern and XRD test specimen of sandwich SA1800. The thin bright part located in center of specimen is Al layer.

The EDS analysis reveals that there are small amount of Y2Ti2O7 existing in Al layer and vice versa. It is believed that under the effect of grinding process, Y2Ti2O7 particles were moved to the Al domain and trapped there. On the other hand, the presence of pores inside the ceramics leads to the diffusion of metallic particles into the ceramics, as shown in Fig. 10.
Figure 10. EDS results taken from (a) metal and (b) ceramic domains in SA1800 sandwich

In order to investigate the behavior of Y2Ti2O7 towards molten metal at higher temperatures, the sandwich was also synthesized by pure iron (Fe) grains and ceramic plates at 1400 °C and 1600 °C (under and over melting point of iron, which is 1530 °C). Similar to the ceramic-aluminium sandwich, after being synthesized at 1400 °C (under melting point) the ceramic plates can be easily detached from each other. The X-ray diffractogram of the detached plates’ surface (see Fig. 11) only shows the Y2Ti2O7 phase proving that the ceramic did not react with the iron grains during the heat treatment.
Figure 11. XRD pattern of Y2Ti2O7 plate after detached from SFe1400 sandwich

In the case of SFe1600 sandwich, synthesized at 1600 °C, although the molten Fe have solidly stuck to ceramic plates, it is confirmed by XRD pattern (see Fig. 12) that the ceramic and the iron absolutely did not react with each other.
Figure 12. XRD pattern of SFe1600 sandwich

The EDS result also indicates that there is some amount of Fe diffused into Y2Ti2O7 domain. Comparing to diffusion level of Al into the ceramic (0.65 %) in the case of SA1800 sandwich, the diffusion level of Fe (4.43 %) in SFe1600 sandwich is a bit higher (both two EDS analysis are performed at position situated in the ceramic domain, far from interface around 20 μn). It should be noticed that 1600 °C is higher than sintering temperature of Y2Ti2O7 (1500 °C). The heat-treatment over this optimal sintering temperature possibly has increased the pore sizes inside the ceramic, resulting in the higher diffusion level of external molten metallic particles.
Figure 13. EDS result taken from Y2Ti2O7 domain in SFe1600 sandwich

The results of this study can be summarized as follows: 1. Y2Ti2O7 single phase sintered body was successfully fabricated by optimizing the sintering conditions and calcination conditions. 2. The sintered body synthesized from the nanometer-size calcined powder shows the highest mechanical properties. 3. The sandwich of metal (aluminium foils or iron grains) and Y2Ti2O7 sintered body was heated and molten to test the corrosion under the high temperature. Because of the existence of the pores in Y2Ti2O7 sintered body, a small amount of molten metal has invaded Y2Ti2O7 phase. However, it is confirmed from the phase analysis results that Y2Ti2O7 did not react with molten aluminium and iron.
1 T. Hirata, K. Akiyama and H. Yamamoto, Corrosion resistance of Cr2O3-Al2O3 ceramics by molten sodium sulphate-vanadium pentoxide, J. Mater. Sci., 36, 5927–34 (2001).
2 T. Hirata, T. Morimoto, A. Deguchi and N. Uchida, Corrosion Resistance of Alumina-Chromina Ceramic Materials Against Molten Slag, Mater. Trans., 43, 10, 2561–67 (2002).
3 S. Yamashita, N. Akasaka, S. Ukai and S. Ohnuki, Microstructural development of a heavily neutron-irradiated ODS ferritic steel (MA957) at elevated temperature, J. Nucl. Mater., 367–370, part A, 202–7 (2007).
4 M. Klimiankou, R. Lindau and A. Möslang, TEM characterization of structure and composition of nanosized ODS particles in reduced activation ferritic–martensitic steels, J. Nucl. Mater., 329–333, 347–51 (2004).
5 L.F. He, J. Shirahata, T. Nakayama, T. Suzuki, H. Suematsu, I. Ihara, Y.W. Bao, T. Komatsu and K. Niihara, Scr. Mater., 64, 548–51 (2011).
6 JIS R 1601:1995, Testing method for flexural strength (modulus of rupture) of fine ceramics, Japan Standards Association (2005)
7 JIS R 1610:2003, Test method for hardness of fine ceramics, Japan Standards Association (2005)
8 K. Niihara, A. Nakahirai and T. Hirai, The Effect of Stoichiometry on Mechanical Properties of Boron Carbide, J. Am. Ceram. Soc., 67, 1, C-13–14 (1984).
9 M. Trunec, Effect of Grain Size on Mechanical Properties of 3Y-TZP Ceramics, Ceram–Silikaty, 52, 3, 165–71 (2008).
10 R. Chaim and M. Hefetz, Effect of grain size on elastic modulus and hardness of nanocrystalline ZrO2-3 wt% Y2O3, J. Mater. Sci, 34, 3057–61 (2004).