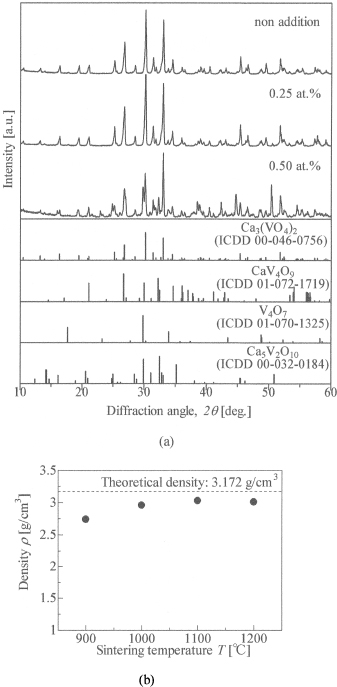
Figure 1. (a)XRD patterns of Ca3(VO4)2 consisted of a CaCO3 and V2O5. (b)Density of Ca3(VO4)2 consisted of a CaCO3 and V2O5.

Recently, ferroelectric of high-temperature curie point (Tc) has attracted a lot of interest because of growing interest in high temperature sensing. This study, focuses on Ca3(VO4)2 (Tc=1110°C), which has been studied as host of the phosphor. The purpose of this study is to optimize the normal sintering of Ca3(VO4)2 for densification, and to measure high temperature dielectric characterization. Ca3(VO4)2 was prepared by the normal sintering with CaO and V2O5 as raw material. The dielectric properties of Ca3(VO4)2 were measured in detail as functions of temperature (R.T. to 800°C) and frequency (1 kHz to 300 kHz). The results revealed that the relative density was raised up to 98.4% when V2O5 was 0.25% higher than the stoichiometric composition. It was also confirmed that the relative permittivity at room temperature was 17.5 and the dielectric relaxation was around 300 kHz. Furthermore, the dielectric properties were frequency dependent in high temperature range. The results of this study are expected to be applicable in non-destructive sensor such as Acoustic Emission (AE) used on high temperature and low frequency. This study also suggested that the composition and the variation of Ca3(VO4)2 density was induced by raw materials and sintering conditions.
Dielectric materials have been used for many applications, such as in capacitive components like capacitors, resonators, and memories. In recent years, ferroelectric materials which can be used in extreme environments such as high-temperature and low temperature, have attracted may interests. For monitoring in incinerators, we are considering the applicability of detecting acoustic emissions occurred from clacks of metals by any sensor. Ferroelectric materials which have high curie temperature were required to realize this. There have been a number of studies on these ferroelectrics materials such as Li systems and Bi systems, however, it has mostly been put to practical use in the single crystal using the optical properties. Therefore, we focused on Ca3(VO4)2 (Curie temperature Tc = 1100°C) as a ferroelectric material.
Ca3(VO4)2 has been reported as a high curie temperature ferroelectric by A.M.GLASS1 et al. in 1978. It was the single crystal made by the czochralski method. In addition, there have been a number of studies such as solid state laser element2, luminescent3 and host of phosphor4. However, there has been no study that tried to made the polycrystalline Ca3(VO4)2 to assume as a dielectric. Therefore, the purposes of this study are to get a high density polycrystalline Ca3(VO4)2 ceramics by optimizing sintering process and to reveal the high temperature dielectric properties.
The raw materials and the mixing composition were varied to investigate the optimal sintering process. Calcium carbonate (Ca2CO3, Wako purity chemical, 99.8%), calcium oxide (CaO, high purity chemical lab., 99.9%), and vanadium oxide (V2O5, high purity chemical lab., 99.9%, <75μm) were used as the raw materials. They were mixed by ballmilling in an ethanol solution according to the stoichiometric ratio with the nominal compositions of Ca3(VO4)2 ceramics and 0.25 to 0.50 at.% V2O5 higher than Ca3(VO4)2. After being ball-milled for 12 h, the mixed powders were dried. The dried powders were packed into an Al2O3 crucible, calcined and crushed on two times (470°C, 80h and 580°C, 10h). The synthesized powders were then compacted into discs that are 10 mm in diameter and 2.0 mm in thickness, then followed by cold-isostatic pressing (CIP) at 100 MPa. The compacted powders were heat treated at 750°C in air for 1h. These compacts were sintered at 800°C to 1200°C in air for 1 h. The calcined temperature, heat treatment temperature and sintering temperature were decided by using thermogravimetry-differential thermal analysis (TG-DTA : DTG-60/60H, SHIMADZU, Kyoto, Japan) and phase diagram. The calcined powders and sintered samples were characterized by X-ray diffractometer (XRD, Rigaku, RINT-2500PC, Tokyo, Japan). The bulk density was measured by the Archimedes method. The micro structures of as-sintered samples were observed by a scanning electron microscope (SEM, JSM-6700F, JEOL, Tokyo, Japan). For measuring dielectric properties, disk-shaped samples were coated with Au on both surfaces. Dielectric characteristic was measured using a LCR meter (3522–50, HIOKI, Tokyo, Japan) and electric furnace (HPM-1N, AS ONE, Tokyo, Japan) in 100 Hz to 1 MHz of frequency range, over 25°C to 800 °C of temperature range.
Fig. 1 (a) shows the XRD patterns of Ca3(VO4)2 consisted of CaCO3 and V2O5. The patterns revealed that the main phase was Ca3(VO4)2. However, there was the secondary impurity phase of identified Ca7V4O17. Fig. 1 (b) shows the density of Ca3(VO4)2 consisted of CaCO3 and V2O5. Measured densities were lower than the theoretical density. It was caused due to a CO2 gas occurred during the sintering. Formula (1) shows the chemical reaction formula at the sintering.
Figure 1. (a)XRD patterns of Ca3(VO4)2 consisted of a CaCO3 and V2O5. (b)Density of Ca3(VO4)2 consisted of a CaCO3 and V2O5.

(1) 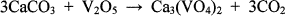
In order to investigate the temperature for including the calcination process, the reaction temperature between CaCO3 and V2O5 was measured by the TG-DTA.
Fig. 2 (a) shows TG-DTA curves of the mixed powder of CaCO3 and V2O5, which was heated at 4°C/min in air from room temperature to 1000 °C. Fig. 2 (a) shows Mass analysis curves. From the DTA curve, endothermic peaks were observed at 470°C, 580°C, 750°C and 880°C. It is considered that CaCO3 was completely decomposed, because there wasn’t difference between DTA curves at 750°C. Also, it seems that the crystallization proceeds by heat-treating above 880°C, because a slope of the DTA curve was changed without mass decreasing. Therefore, calcined temperatures are decided at 470°C, 580°C and 750°C.
Figure 2. (a)Temperature properties of TG and DTA. (b)Result of mass analysis.

Fig. 3 (a) shows XRD patterns of Ca3(VO4)2 consisted of CaCO3 and V2O5 with calcination processes. Fig. 3 (b) shows results of density at the same condition. From Fig. 3 (b), the density was increased due to the introduction of calcination processes. However, CaO was confirmed as the secondary phase at each temperature condition. Also, synthesized crystal phases at each temperature were Ca2V2O7 at 470°C, Ca7V4O17 at 580°C and Ca3(VO4)2 at 750°C. Therefore, it is also concerned that vanadium which has highest vapour pressure was lost during processes of calcination and so on.
Figure 3. (a)XRD patterns of Ca3(VO4)2 consisted of a CaCO3 and V2O5 with calcination processes. (b)Densities of Ca3(VO4)2 consisted of a CaCO3 and V2O5 with calcination processes.

Ca3(VO4)2 was made by CaO and V2O5. Fig. 4 (a) shows XRD patterns of Ca3(VO4)2 consisted of CaO and V2O5. XRD patterns revealed that the single phase of Ca3(VO4)2. Fig. 4 (b) shows the density of Ca3(VO4)2 consisted of CaO and V2O5. In the case of sintering at 1100°C, the relative density was 98.0%. However, the mass loss was examined, 2.94 wt.% of mass lost on sintering back and forth was confirmed. This result also suggests the possibility of lost of vanadium.
Figure 4. (a) XRD patterns of Ca3(VO4)2 consisted of a CaO and V2O5. (b) Density of Ca3(VO4)2 consisted of a CaO and V2O5.
As shown in previous results, samples were prepared by adding more V2O5 than the stoichiometric composition. Fig. 5 (a) shows the XRD patterns of Ca3(VO4)2 consisted of a CaO and V2O5 with excess V2O5. From the Fig. 5 (a), in the case of 0.25 at.% excessively adding V2O5, single phase of Ca3(VO4)2 was confirmed, however, in the case of 0.50 at.%, some impurity phases were confirmed. It seems that the stoichiometric composition was deviated by the addition of V2O5 than the volatilization volume. In addition, Fig. 5 (b) shows densities of Ca3(VO4)2 consisted of CaO and V2O5 with and without excess addition of V2O5. Regarding densities, samples with excessive addition of V2O5 show higher density than samples without. In the case of, sintering at 1100°C, the relative density was 98.4%.
Figure 5. (a) XRD patterns of Ca3(VO4)2 consisted of a CaO and V2O5 with excess V2O5. (b) Densities of Ca3(VO4)2 consisted of a CaO and V2O5 with excess V2O5.

Fig.6 (a) shows the relative permittivity of Ca3(VO4)2 at room temperature. The relative permittivity εr was about 17.5, and the dielectric relaxation phenomenon was measured around 300 kHz. This tendency did not depend on sintering temperatures, the addition of V2O5 and process of calcination. In the lower frequency region, the permittivity excessively added V2O5 sample was higher than no addition sample. Fig. 6 (b) shows the temperature dependence of the relative permittivity at typical frequencies. The small peak was confirmed at around 550°C. It seems that the peak was due to the occurrence of phase transition.
Figure 6. (a) The relative permittivity of Ca3(VO4)2 from CaO and V2O5 at room temperature. (b) The temperature dependence of relative permittivity at typical frequencies.

Using CaCO3 as a raw material, the samples was successfully prepared with Ca3(VO4)2 main phase, owing to introduce calcinations processes. However, there was a secondary phase of CaO. In contrast, using CaO as a raw material, Ca3(VO4)2 of the single phase was obtained, not depending on sintering temperature. Because vanadium had a possibility of lost, in the case of V2O5 was more excessive than the stoichiometric composition, samples displayed the single phase of Ca3(VO4)2 and the relative density was 98.4% with 0.25 at.% excessive adding.
From results of measurement, it is confirmed that the relative permittivity at room temperature was 17.5 and the dielectric relaxation was around 300 kHz. In addition, it was confirmed that this tendency was not depended on sintering temperature and amount of vanadium. In the case of high temperature, it presents that the maximum value of relative permittivity was 400 at 800°C and 1 kHz.
1A. M. Glass, S. C. Abrahams, A. A. Ballman, G. Loiacono, Calcium Orthovanadate A New High Temperature Ferroelectric, Ferroelectrics, 17, 579–582 (1978).
2L.H. Brixner, P.A. Flournoy, Calcium Orthovanadate Ca3(VO4)2 A New Laser Host Crystal, J. Electrochem. Soc., 112, 303–308 (1965).
3L. H. C. Andrade, D. Reyes Ardila, J. P. Andreeta, M. Siu Li, Optical properties of Nd+3 -doped Ca3(VO4)2 single crystal fiber, Opt. Mater., 22, 369–375 (2003).
4H. Zhang, M. Lv, Z. Xiu, S. Wang, G. Zhou, S. Wang, Z. Qiu, A. Zhang, Synthesis and photoluminescence properties of a new red emitting phosphor : Ca3(VO4)2:Eu3+, Mn2+, Mater. Res. Bull., 42, 1145–1152 (2007).