
The properties of BOF (Basic Oxygen Furnace) dust are small granularity and high Fe content, which would be a good addition for BF (Blast Furnace) injection. In this research, the effects of BOF dust on coal combustion, PCI process and BF smelting are studied. The most suitable ratio of BOF dust injected into BF with coal is determined. This study provides a feasible option for the BOF dust ash recycling, which contributes to the development of recycling economy, energy conservation, and promotes the sustainable development of the iron and steel enterprises.
A large amount of iron dust would be produced in the BOF during the process of oxygen blowing and collected by dry dedusting system. The BOF dust, which is a valuable secondary resource, has the character of fine granularity and high iron content. Jingtang plant’s study suggested that it could be used to produce pellets by cold solid pellet process, through material analyses and mixture calculations[1]. Based on the analysis of chemical composition of BOF dust, Panzhihua plant used it to develop a final slag regulator, and successfully recycled 80% dust. The results were satisfactory[2]. The research and practice by Laiwu plant, 5% or less of the BOF dust were added into pelletizing shaft furnace, and the result shown that it helped reduce the amount of bentonite addition and production costs. With considerable economic and social benefits, the process is good for the development of recycling economy[3]. Using BOF dust instead of some high-priced iron ore in the iron sinter process in a study by Chengde plant showed that it could improve miscellaneous materials ratio, and achieved good result[4]. Wuhan Iron and Steel Company developed a new red iron manufacturing technology by using BOF dust, which was a new ways for BOF dust recycling[5]. However, the research of injection of BOF dust into the Blast Furnace through tuyere is less[6–9]. Based on this, the effects of BOF dust on coal combustion, PCI process and BF smelting are studied in this paper. The feasibility of BOF dust as an addition for BF injection and the appropriate adding quantity are also discussed in this paper.
The materials used in this study were taken from the ironworks. The proximate analysis and ultimate analysis of two different coal samples are given in Table I. The chemical compositions of BOF dust, coal ash and coke ash are stated in Table II. The particle size distribution of BOF dust can be seen in Figure 1.
Table I. Proximate analysis and ultimate analysis of coal samples (ω, %)

Table II. Chemical compositions of BOF dust, coal ash and coke ash (ω, %)

Figure 1. The particle size distribution of BOF dust

Table II shows that the BOF dust contains 63.6% TFe, which is good for BF smelting. The percentages of Na2O, K2O, S, which are bad for BF, are less compared with other materials.
Figure 1 shows that the particle size distribution of BOF dust is very narrow, and the maximum particle size is 5.03 μm, which is rather small compared with the pulverized coal. So, it can be injected into the BF with coal directly, not need grinding.
The aim of the experiments was to evaluate the influence of different BOF dust addition percentage on the coal combustion, PCI process and BF smelting. So in the process of experiments, the percentage of BOF dust in coal reached about 0%, 1.96%, 3.85%, 5.67%, 7.41%, 9.09%, respectively.
In order to make BOF dust and coal mixed well, some measures were taken in the samples preparation process. First, put a certain amount of BOF dust into distilled water and stir for 10min. Second, add a certain amount of pulverized coal into the water and continue to stir them for 24h. Third, dry the samples in an oven at 80°C after filtration, and then mill the dry samples for 20min. The reference coals should follow the above procedure for processing to ensure that different coal samples have the same properties.
The combustion characteristics of different samples were analyzed with a thermo-gravimetric analyzer (WCT-2C, produced by Beijing Optical Instrument Factory) in this work. Reactor diameter was 60mm, the reaction atmosphere was air, and the diameter of two crucibles was 6 mm. Approximately 10 mg of pulverized coal sample was heated at a heating rate of 20°C/min from room temperature to 1000°C. The gas flow rate was kept at 100 ml/min. One testing condition has been conducted three times.
The calorific values of different samples were measured by WZR-Al microcomputer automatic calorimeter which produced by the Changsha special Instrument Co. Ltd.
It can be seen in Table III, BOF dust mainly contains iron oxides, and the carbon is rarely. The content of coal ash, volatile matter and fixed carbon are affected when mixed with coal. Theoretical calculation has been done to determine the effect, as shown in Table III.
Table III. The effect of different ratios of BOF dust on coal proximate analysis
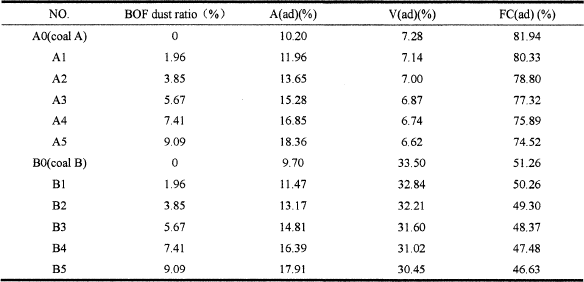
From Table III, it can be concluded that, with the increase of BOF dust ratio, the content of coal ash increases and the content of volatile matter and fixed carbon decreases. When the BOF dust ratio is 7.41%, the contents of coal ash are 16.85% and 16.39% respectively, and beyond the limits of 15% of the BF injection requirement.
In summary, from point of view of pulverized coal ash content, the appropriate ratio of BOF dust ash should be controlled less than 5.67% for coal A and 7.41% for coal B.
(A) TG curve analysis
The weight loss curve (TG) and differential weight loss curve (DTG) of anthracite coal A and bituminous coal B with different BOF dust content in the combustion process are shown in Figure 2 and Figure 3 respectively.
Figure 2. TG, DTG curves for coal A and BOF dust of different ratios

Figure 3. TG, DTG curves for coal B and BOF dust of different ratios

It can be seen from Figures 2, the TG curves of different BOF dust content samples shift to the lower temperature position from 528 to 693 °C, comparing with the coal A. It indicates that the BOF dust has no effect on the devolatilization and volatile combustion of coal A. But it has catalysis on the fixed carbon combustion.
From the Figure 3, it can be seen that all the curves are almost overlapped before 346°C, but after the temperature the curves of mixed samples begin to shift to lower temperature position. It indicates that the effect of BOF dust on the combustion of coal B is different from that of coal A. The BOF dust only has no effect on the devolatilization of coal B. The volatile combustion and fixed carbon combustion are promoted by BOF dust addition.
Both in Figure 2 and Figure 3, the DTG curves of all mixed samples shift to lower temperature position. However, the change shapes of the curves of different coals are different. It indicates that the influence mechanism of BOF dust on the coal combustion vary with the coal. The shapes of DTG curves of mixed samples are similar to that of coal A in Figure 2. With the BOF dust content increase, the distance of DTG curves shifted to the left first increases and then decreases, and the peak point of DTG curves first increases and then decreases at the same time. It indicates that, with the BOF dust content increase, its role on promoting combustion of coal A increases initially then reduces. The DTG curve shapes of mixed samples show two peaks, which are different from that of coal B in Figure 3. It indicates that the ignition way of coal B shifts from non-homogeneous phase ignition to homogeneous phase ignition[10]. However, the effects on coal B combustion vary with BOF dust content. With the increase of BOF dust content, the positive impact on coal B combustion first increases and then decreases.
(B) Ignition characters
In this study, TG-DTG technique was used to determine the ignition temperature[11]. From the comparison of ignition temperature of different samples in Figure 4, the relationship between ignition temperature and the BOF dust content can be deduced. The results show that the ignition temperatures of coal A and coal B decrease firstly and increase subsequently with the increase of BOF dust content. The alkali metals, alkaline earth metals, transition metals and their compounds can be used as catalysts to accelerate the reaction rate of coal with oxygen. Especially, there are large amounts of Fe2O3 in the BOF dust. During the coal combustion process, the Fe2O3 can be expected to supply oxygen for combustion and be reduced to FeO. When BOF dust content over a certain amount, BOF dust will cover the area where oxygen and coal contact, and the combustion of coal is suppressed[12–14].
Figure 4. Relation between ignition temperature and different blending ratio of BOF Dust
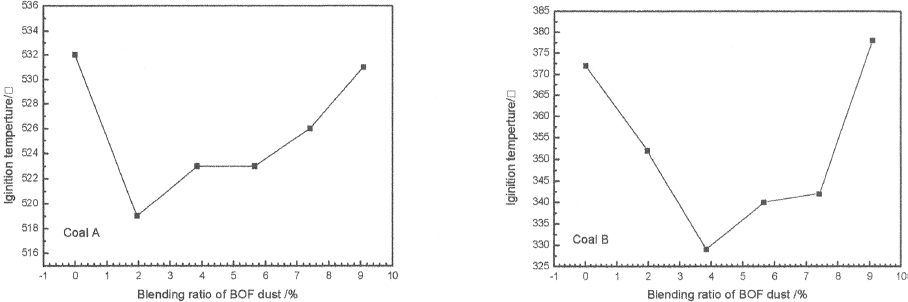
From the perspective of ignition characters, the most suitable BOF dust content for coal A is between 1.96–3.85%; and the most optimum addition content for coal B is between 3.85–5.67%.
(C) Comprehensive combustion index SN
A comprehensive combustion index SN, which can well indicate the coal combustion characteristics, is used in this research[15]. The higher the value of SN is, the better the combustion ability of coal is.
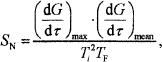
| In the formula, | (dG/dτ)max is the maximum combustion rate (mg / min); |
| (dG/dτ)mean is the average burning rate (mg / min); | |
| Tf is the burnout temperature (°C); | |
| Ti is the ignition temperature (°C). |
The comprehensive combustion indexs of the experimental samples can be seen in Table IV.
Table IV. Comprehensive combustion index of combustion for BOF dust and coal blends
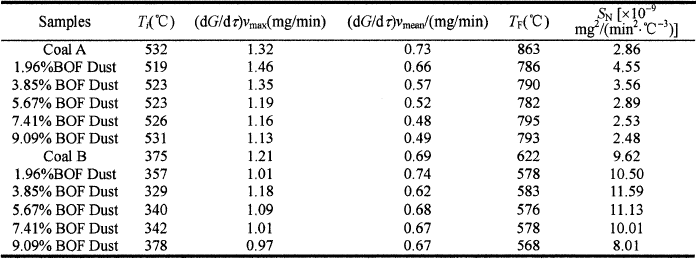
It can be concluded from the Table IV and Figure 5, with the BOF dust content increase, the comprehensive combustion index SN of coal first increases and then decreases. For coal A, when adding 1.96% BOF dust, the comprehensive combustion index SN increases from 2.86×10−9 to 4.55×10−9 mg2/(min2·°C3), and the combustion performance is the best at this situation. For coal B, when adding 3.85% BOF dust, the comprehensive combustion index SN arrives to 11.59×10−9 mg2/(min2·°C3), which is the highest value among the experiments.
Figure 5. Relation between comprehensive combustion index and BOF dust content

The appropriate addition content of BOF dust for coal determined by the comprehensive combustion index SN is consistent with that determined by the ignition characters.
The calorific value test results of different samples can be seen in Table V, and the relation between coal calorific value and BOF dust content as shown in Figure 6.
Table V. The calorific value test results of different samples
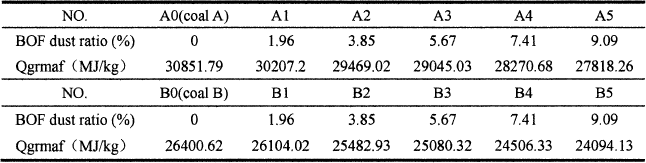
Figure 6. Relation between coal calorific value and BOF dust content

It can be concluded from the Table V and Figure 6, with the increase of BOF dust content, the calorific values of both coal A and coal B decrease. There are two reasons to lead this phenomenon. On the one hand, there are little carbon in the BOF dust, and all the BOF dust can be regard as ash. When adding into coal, the fixed carbon content of coal reduces. So the coal calorific value decreases with the increase of BOF dust content. On the other hand, there are large amounts of Fe2O3 in the BOF dust. Fe2O3 will decompose thermally and react with C during the combustion process. All of these reactions can consume heat and lead to the effective calorific value of coal decrease[16].
When the BOF dust content arrives to 9.09%, the calorific value of coal A reduces by 9.8%, and the calorific value of coal B reduces by 8.7%. The result indicates that the BOF dust has an impact on calorific value of coal. So from the perspective of calorific value, the addition amount of BOF dust should not be too much.
BOF dust mainly contains iron oxide, calcium oxide, silicon dioxide, aluminum oxide and manganese oxide, and the carbon content is relatively very little. Therefore, considering the possible change of slag basicity, sulfur content in hot metal, theoretical combustion temperature, and coke ratio inside the furnace with BOF dust injection, a mass balance and local heat balance model was developed to calculate the change of coke ratio, slag basicity and theoretical combustion temperature with BOF dust injection which provided theoretical basis for blast furnace operation. In this research, coal A was selected. The calculational results are listed in Table VI.
Table VI. The changes of coke ratio, slag basicity and theoretical combustion temperature with different blending ratio of BOF dust injection
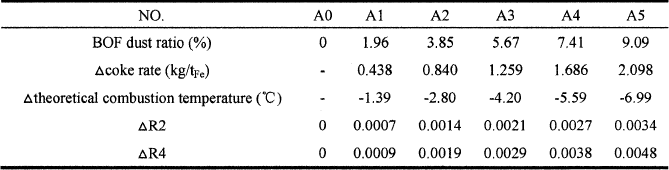
Injection of BOF dust into the Blast Furnace through tuyere has directly impacted on the basicity of the tuyere slag, and then has affected the final slag basicity. From the perspective of chemical composition of BOF dust, it can be regarded as a basic slag. The tuyere slag has been prepared according to the amount of coal and coke which are burned near the tuyere. The calculational results show that the basicity of the tuyere slag increases with the BOF dust content increase, whether has calculated through R2 or R4, as shown in Figure 7.
Figure 7. The change of tuyere slag basicity with different blending ratio of BOF dust injection

As the burden descent, different types of slags are formed at different locations in the BF. The primary salg is supposed to consist of the gangue content in burden. The bosh slag is assumed to be the product of the primary slag, all additives and fluxes and some dissolved ash from the coke consumed. The ash released in the raceway, when coal and coke are burnt, forms the tuyere slag. The tuyere slag contains the constituents in flux or ore, if they are injected. The bosh slag and tuyere slag are mixed and after some additional reduction of the mixture slag, the final slag is formed. As a general rule, the high alkalinity of bosh slag will be a serious problem in blast furnace. Injection of BOF dust with coal will increase the alkalinity of tuyere slag, and relieve the problem of bosh slag.
In a study by Kushima et al.[17] on the injection of iron ore fines into the Hirohata No.3 BF, it was found that the Si content in hot metal was decreased. If the fines containing CaO was used, the effect was greater because of a lower aSiO2 caused by increased basicity of the tuyere slag.
As shown in Figure 8, the theoretical combustion temperature decreases with the BOF dust content increase. When injection of the BOF dust with coal, BF will produce more slags and take away more heat, compared with no BOF dust injection. And the iron oxides coming from BOF dust will be reduced and consume some heat. All of these reasons cause the decrease of theoretical combustion temperature. However, the theoretical combustion temperature is only little changing. The results show that, when the content of BOF dust arrives to 9.09%, the theoretical combustion temperature only reduces by 6.99°C. And this change will not affect BF much.
Figure 8. The change of theoretical combustion temperature with different blending ratio of BOF dust injection

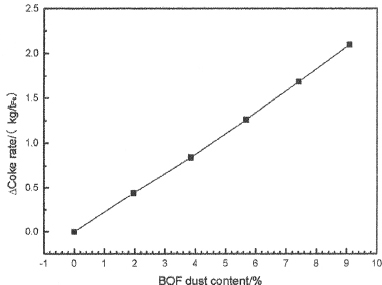
As shown in Figure 9, the coke ratio increases with the BOF dust content increase. The reason is that the more BOF dust injection, the less coal calorific value is, and the less heat provided by coal. In order to meet the heat balance, the coke ratio will be increase. The result shows that, when the content of BOF dust arrives to 9.09%, the coke ratio increases by 2.098kg/tFe. And this change also will not affect BF much.
Figure 9. The change of coke ratio with different blending ratio of BOF dust injection
Blast furnace operation practice shows that the problem of theoretical combustion temperature decrease and coke ratio increase can be solved by oxygen enrichment and improvement of blast temperature. During the range of the experiment, the influence of BOF dust injection on BF operation can be completely controlled on the production site.
According to the test result, the density of BOF dust is 0.7987kg/m3. But, compared with BOF dust, the density of coal A and coal B would be much less, with values 0.5376 kg/m3 and 0.5005 kg/m3, respectively. Therefore, the specific gravity of mixed coal is increased, and the injection pressure of pulverized coal should be increased appropriately.
The present work was supported by Natural Science Foundation of China and Baosteel under Grant (No. 51134008); National Basic Research Program of China (973 Program) (No. 2012CB720401).
[1] X.WANG, Q.M. CUI, D.L. XU, “Recycle using of Converter Dust Ash Cold Solid Pellet in Jingtang Plant,” The Eighth China Steel Annual Conference Proceedings, (2011). (in Chinese)
[2] S.H. LIU, “Study on Cyclic Utilization of Converter Dust Removal Resource for Panzhihua Steel,” Iron Steel Vanadium Titanium, 31(03)(2010), 88–92. (in Chinese)
[3] X.C. ZHENG, J. Yang, “Practice of Adding Dry Dust at Steelmaking Process into Pelletizing Shaft Furnace,” Sintering and Pelletizing, 33(03)(2008), 47–49. (in Chinese)
[4] Adding the Steel Dust Alone into Sintering Machine Achieved Good Results in Chengde Plant. GTXH, (2007). (in Chinese)
[5] B.J. HU, S.Q. WEN, “A Method of Red Iron Manufacture with Steel Dust,” Patent No.: 86108450. (in Chinese)
[6] L.S. ÖKVIST, D.Q. CANG, et al, “The Effect of BOF Slag and BF Flue Dust on Coal Combustion Efficiency,” ISIJ International, 44(09)(2004), 1501–1510.
[7] D. SENK, H.W. GUDENAU, et al, “Dust Injection in Iron and Steel Metallurgy,” ISIJ International, 46 (12)(2006), 1745–1751.
[8] H.T. MAKKONEN, J. HEINO, et al, “Optimisation of steel plant recycling in Finland: dusts, scales and sludge,” Resources, Conservation and Recycling, 35(2002), 77–84.
[9] T. SAWADA, R. NAKAJIMA, et al, “Flux Injection from the Tuyere for Low Silicon Operation,” Ironmaking Conf. Proc., (1990), 309–315.
[10] L.H. WEI, D. QI, R.D. LI, “Effects of Alkali Metal on Combustion of Pulverized Coal and Kinetic Analysis,” Journal of China Coal Society, 35(10)(2010), 1706–1711. (in Chinese)
[11] X.J. FIE, J.L. ZHANG, et al, “Kinetic Analysis and Effects of Catalysts on Combustion Characteristic of Pulverized Coal,” Iron and Steel, 47(07)(2012), 74–79. (in Chinese)
[12] X.Z. GONG, Z.C. GUO, Z. WANG, “Effect of K2CO3 and Fe2O3 on Combustion Reactivity of Pulverized coal by Thermogravimetry Analysis,” Journal of Fuel Chemistry and Technology, 37(01)(2009), 42–48. (in Chinese)
[13] Y.H. LIU, D.F. CHE, et al, “Effect of Iron Compounds on Coal Combustion Characteristics,” Journal of Xi’an Jiao tong University, 34(09)(2000), 20–24. (in Chinese)
[14] Q. WANG, X.H. WU, et al, “Combustion Reaction Kinetics Study of Huadian Oil Shale-coke,” Proceedings of the CSEE, 26(07)(2006), 27–34. (in Chinese)
[15] X.M. JIANG, J.B. LI, J.R. QIU, “Study on Combustion Characteristic of Micro-pulverized Coal,” Proceedings of the CSEE, 20(06)(2000), 71–75. (in Chinese)
[16] S.M. KANG, S. JOO, D.J. MIN, I.O. LEE, “Simultaneous Behavior of Pulverized Coal Char Combustion and Fine Iron Oxide reduction by Injecting the Mixture Coal Char and Iron Oxide,” ISIJ Int., 36(02)(1996), 156–163.
[17] K. KUSHIMA, M. NAITO, et al, “Iron ore injection into blast furnace raceway,” Ironmaking Conf. Proc., (1988), 457–466.