Figure 1. SEM images of scorodite particles (a) with and (b) without Cu prepared by DSMP method.

In order to immobilize arsenic by-products produced in the processing of nonferrous metals, large scorodite particles with nearly 20 μm diameter were synthesized from Fe(II) and As(V) aqueous solutions, with and without copper, and their morphologies and structures were analyzed using transmission electron microscopy (TEM). The results showed that scorodite particles with copper have round polyhedral shape, whereas scorodite particles without copper have octahedral shape. The compositional profile of the scorodite particles with copper analyzed using an energy-dispersive X-ray spectrometer attached to TEM showed that the elements are almost homogeneously distributed in the particles, although the particle surfaces have slightly higher concentration of iron. The dissolution characteristics of the scorodite particles prepared from the Fe(II) and As(V) aqueous solutions were investigated. It was shown that the concentration of arsenic dissolved from the particles is low regardless of copper, although it depends on the temperature and pH of the solution.
Several metalloids such as arsenic, antimony, and tellurium are produced as by-products in the processing of nonferrous metals. Among these elements, arsenic must be immobilized as a stable compound because it is toxic. Scorodite (FeAsO4·2H2O) is the most promising compound for immobilizing arsenic in water because of the low solubility of scorodite. A large number of studies on the synthesis of scorodite particles from Fe(III) and As(V) solution have been performed, in which the dissolution characteristics of the scorodite particles were investigated at approximately room temperature1–7. The results showed that the solubility of arsenic dissolved from crystalline scorodite particles is lower than that of the amorphous ferric arsenate under a given pH3. It was also shown that arsenic in the crystalline scorodite particles becomes stable with increasing Fe/As molar ratio in aqueous solution. In addition, arsenic in small crystalline scorodite particles is less stable compared with the large scorodite particles. Therefore, it is considered that the large particles of crystalline iron-rich scorodite particles are adequate for long-term storage of arsenic.
Large crystalline scorodite particles of approximately 20 μm diameter were coprecipitated by injecting oxygen gas or air into a hot aqueous solution containing Fe(II) and As(V) ions8–13. The size of these scorodite particles is much larger than that of the crystalline scorodite particles synthesized from a solution containing Fe(III) and As(V) ions. The Fe/As molar ratio in the scorodite particles can be controlled by adjusting the amounts of Fe(II) and As(V) ions8. These large scorodite particles can be obtained in the presence of zinc or copper ions under atmospheric conditions10. Thus, large scorodite particles synthesized from Fe(II) and As(V) ions are promising agents for immobilizing arsenic in aqueous solutions containing high concentrations of arsenic.
So far, the morphology of scorodite particles synthesized from Fe(II) and As(V) ions have been observed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM)14. Morphological characteristics such as particle size and solution conditions (e.g., type of solvent and pH) affect the arsenic solubility of these scorodite particles. In order to utilize large scorodite particles for storing arsenic, it is necessary to characterize their morphology in relation to their dissolution characteristics in aqueous solution. The objective of this study was to investigate the morphology and dissolution characteristics of large scorodite particles containing copper produced from Fe(II) and As(V) ions in aqueous solutions under different conditions, because solutions obtained from the processing of nonferrous metals often contain copper. The results on the dissolution characteristics were compared with the morphology of the scorodite particles by SEM and TEM.
Scorodite particles were produced by the Dowa Metals and Mining scorodite process (DSMP), reported in a previous paper8–10. Some essential points are given here. An aqueous solution containing 50 g/L As(V) and 55.85 g/L Fe(II) was prepared by adding Fe(II) sulfate hexahydrate to As(V) solution. To prepare scorodite particles with copper, a 20 g/L Cu(II) solution was added to the solution mentioned above. The solutions were heated up to 368 K with stirring, after which oxygen gas was injected into the solution at 95 °C for 5 h. During this process, 0.2-μm sized solid particles were coprecipitated in the suspension. These particles were washed and dried after separating them from the suspension by filtration. The arsenic and iron concentrations of the scorodite particles were determined using inductively coupled plasma atomic emission spectrometry (ICP-AES). The concentrations of arsenic, iron, and copper, other than oxygen and hydrogen, were estimated to be approximately 47, 51, and 2 mol%, respectively. The arsenic concentration of scorodite is nearly equal to that of iron, as shown in its chemical formula (FeAsO4·2H2O); however, the scorodite synthesized in the present study was slightly rich in iron.
For performing dissolution experiments on scorodite particles, aqueous solutions with pH values ranging from 1 to 8 were prepared by adding NaOH and HCl solutions to an aqueous solution. Scorodite particles (10 g) were transferred to 100 mL of the aqueous solution in a bottle, which was then stored at a given temperature for 12 h. The bottle was sealed and shaken at an amplitude of 40 mm and a frequency of 200 cycles/min for 6 h at 25 or 45 °C. A syringe filter with 0.2-μm sized pores was used for separating the scorodite particles from the solution. The pH value of the filtered solution was measured at room temperature, and the concentrations of arsenic and iron in solution after dissolution experiments were determined using ICP-AES.
The morphology and microstructure of the scorodite particles were measured using field emission SEM and TEM. For TEM observation, thin samples were prepared from scorodite particles using a focused ion beam (FIB) apparatus with gallium ions. The compositional profile of elements at a local region of the sample was analyzed using an energy-dispersive X-ray spectrometer attached to a transmission electron microscope.
Figures 1(a) and (b) show the SEM images of scorodite particles with and without copper, respectively. Scorodite particles with copper had round-like polyhedral shape, whereas those without copper had octahedral shape14. The size of the largest particles in both cases was approximately 20 μm, although a few microsized particles were also observed. These results imply that copper modifies the shape of scorodite particles during particle growth, as divalent copper is different from trivalent iron.
Figure 1. SEM images of scorodite particles (a) with and (b) without Cu prepared by DSMP method.

The cross section of a polyhedral scorodite particle containing copper was observed using TEM. Figures 2 (a), (b), and (c) show a bright-field image, an electron-diffraction pattern, and an index assignment of the diffraction pattern, respectively. A layer was observed surrounding the sample, because it was covered by carbon deposits to hold the thin sample. Although there are bent contours in the image, these results show that the particle is a single crystal and that the normal direction of the thin sample is almost parallel to the [001] direction.
Figure 2. Bright field image obtained by TEM (a); electron diffraction pattern (b); and index assignment of diffraction pattern (c).

The concentrations of arsenic, iron, and copper in the particle were analyzed using TEM-EDS. Figure 4 shows the concentrations of these elements versus distance from near the surface to the interior of the particle at an interval of 500 nm. The concentrations of these three elements were almost similar to each other (100 nm to 2.5 μm). Based on the amounts of iron, arsenic, and copper present in the scorodite particles, the relative concentration of arsenic was estimated to be approximately 46 at%, which is comparable to that determined using ICP-AES. Notably, the iron concentration in the near-surface layer was higher than that in the interior. This difference may be attributed to the synthetic conditions of the scorodite particles fabricated in the present study, as the concentration of iron in aqueous solution is higher than that of arsenic. This tendency becomes significant in the final stage of the synthesis, in which most of the arsenic is consumed and iron is retained in aqueous solution. This condition reflects the concentration in the near-surface layer.
Figure 3. Compositional profiles of Fe, As, and Cu in a scorodite particle containing Cu.
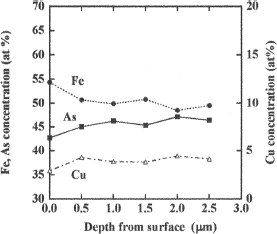
Figure 4. Solubility of As from scorodite with and without Cu at different pH values at 25 and 45 °C.

Figure 4 depicts the pH dependence of the solubility of arsenic dissolved from scorodite particles, with copper and without copper, at 25 and 45 °C. The broken lines in Figure 4 indicate the detection limit of the ICP-AES analysis. The pH values were measured at room temperature after dissolution experiments. The solubility of arsenic from the scorodite particles fabricated in this study was comparable to the previous data in a wide range of pH values14. The solubility at 45 °C decreases as pH increases from 1 to 4, and increases as pH increases from 4 to 8. Hence, the arsenic solubility curve at 45 °C has a minimum value at approximately pH 4. The arsenic solubility decreases on reducing the temperature, and the minimum in the arsenic solubility curve shifts toward slightly higher pH (approximately pH 5) with decreasing temperature. The arsenic solubility at pH 3–5 depends on temperature, and the concentration of arsenic dissolved from the scorodite particles in aqueous solution at pH 5 at 45 °C is higher than the value obtained at 25 °C.
Figure 5 shows the pH dependence of the solubility of iron dissolved from the scorodite particles in aqueous solution at 25 and 45 °C. The aqueous solutions were identical to those used for measurements of the arsenic solubility shown in Figure 4. Although a few solubility data for pH values near 6 may be lower than the detection limit of the present ICP-AES analysis, the pH dependence of the iron solubility may reveal a minimum value near pH 6. In contrast to the arsenic solubility, the iron solubility does not seem to strongly depend on temperature. In addition, Figure 6 shows the solubility of copper from the scorodite particles with copper at various pH values at 25 and 45 °C. This result indicates that the copper solubility decreases with increasing pH, and that the solubility at 45 °C is higher than that at 25 °C, although several solubility data are lower than the detection limit of ICP-AES.
Figure 5. Solubility of Fe from scorodite with and without Cu at different pH values at 25 and 45 °C.

Figure 6. Solubility of Cu from scorodite with Cu at different pH values at 25 and 45 °C.
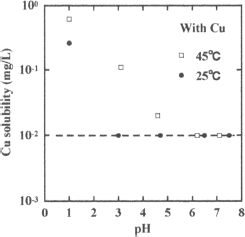
Figure 7 shows the molar ratios of arsenic to iron dissolved from the two types of scorodite particles as a function of pH at 25 and 45 °C. Concentrations of arsenic or iron below the detection limit in Figures 5 and 6 are excluded. The solid line in Figure 7 corresponds to the As/Fe molar ratio obtained from the total chemical composition of the two types of scorodite particles. As shown in Figure 8, the As/Fe ratio obtained from the dissolution experiments do not appear to be consistent with the composition ratio of scorodite. In particular, the dissolution characteristics of arsenic and iron in solution at pH <3 clearly differ from those at pH >5. The As/Fe values at 45 °C decrease on increasing pH from 1 to 3, whereas they increase on increasing pH from 5 to 8, irrespective of the addition of copper. The present results imply that the nonhomogeneous dissolution of arsenic and iron ions from the scorodite particles takes place, though the pH dependences of large scorodite particles synthesized in the present method are fundamentally similar.
Figure 7. Solubility (As/Fe molar ratio) of scorodite particles with and without Cu at different pH values at (a) 25 °C and (c) 45 °C. Figures in expanded scale are shown in (b) and (d), respectively, where the average composition ratios are shown.

Large scorodite particles, with and without copper, were synthesized from Fe(II) and As(V) ions in aqueous solution, and their morphologies were observed using TEM. The dissolution experiments were carried out using ICP-AES.
The TEM observation showed that the scorodite particles with copper had round-like polyhedral shape, and the scorodite particles without copper exhibited octahedral shape. TEM-EDS analysis showed that the concentration of elements was almost similar in the particles, although an iron-rich layer was formed on the particle surfaces, which might have resulted from the higher concentration of iron in solution. The morphology and compositional profile of scorodite help in reducing the solubility of arsenic.
The solubility of arsenic from scorodite particles was low at a wide pH range, and it decreased with decreasing temperature. The minimum in the arsenic solubility curve shifts from approximately pH 4 to 5 on decreasing the temperature from 45 to 25 °C. The concentration of dissolved arsenic at 25 °C is lower than that at 45 °C in aqueous solution at pH 5. The As/Fe molar ratio in aqueous solution depended on pH values. These dissolution characteristics indicate nonhomogeneous dissolution of the scorodite particles. Consequently, conditions of aqueous solutions such as pH and temperature are important for the long-term storage of arsenic as scorodite.
The authors express their sincere thanks to Dr. T. Fujita for valuable discussions.
1J. E. Dutrizac and J. L. Jambor, The synthesis of crystalline scorodite, FeAsO4 · 2H2O Hydrometallurgy, 19, 377–84 (1988).
2E. Krause and V. A. Ettel, Solubility and stability of scorodite, FeAsOo’2HrO:New data and further discussion, Am. Mineralogist, 73, 850–54 (1988).
3E. Krause and V. A. Ettel, Solubilities and stabilities of ferric arsenate compounds, Hydrometallurgy, 22, 311–37 (1989).
4N. J. Welham, K. A. Malatt, and S. Vukcevic, The stability of iron phases presently used for disposal from metallurgical systems—A review, Miner. Eng., 13, 911–31 (2000).
5D. Langmuir, J. Mahoney, and J. Rowson, Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4 2H2O) and their application to arsenic behavior in buried mine tailings, Geochim. Cosmochim. Acta, 70, 2942–56 (2006).
6M. C. Bluteau and G. P. Demopoulos, The incongruent dissolution of scorodite — Solubility, kinetics and mechanism, Hydrometallugy, 87, 167–77 (2007).
7M. A. Gomez, L. Becze, J. N. Cutler, and G. P. Demopoulos, Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3–As2O5–H2SO4 solutions, Hydrometallurgy, 107, 74–90 (2011).
8T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part I, Hydrometallurgy, 90, 92–02 (2008).
9T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part II. Effect of temperature and air, Hydrometallurgy, 90, 85–91 (2008).
10T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Effects of zinc, copper and sodium ions on ferric arsenate precipitation in a novel atmospheric scorodite process, Hydrometallurgy, 93, 30–38 (2008).
11T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Effect of pH on atmospheric scorodite synthesis by oxidation of ferrous ions: Physical properties and stability of the scorodite, Hydrometallurgy, 96, 189–98 (2009).
12T. Fujita, R. Taguchi, M. Abumiya, M. Matsumoto, E. Shibata, and T. Nakamura, Immobilization of Arsenic from Novel Synthesized Scorodite—Analysis on Solubility and Stability, Mater. Trans., 50, 321–31 (2009).
13K. Shinoda, T. Tanno, T. Fujita, and S. Suzuki, Coprecipitation of Large Scorodite Particles from Aqueous Fe(II) and As(V) Solution by Oxygen Injection, Mater. Trans., 50, 1196–01 (2009).
14S. Fujieda, K. Shinoda, T. Inanaga, M. Abumiya, and S. Suzuki, Dissolution Characteristics and Morphology of Large-sized Scorodite Particles Synthesized from Fe(II) and As(V) in Aqueous Solution, High Temp. Mater. Processes, 31, 451–58 (2012).