One of the most important and promising electrolytes for intermediate temperature solid oxide fuel cells (IT-SOFC) are those based in gadolinium-doped ceria. The electrolytes were obtained by mechanical alloying in a high-energy planetary ball mill, analyzing the relation between the milling time (0, 2, 4, 8, 16 and 20 h) and the crystallite size, lattice parameter and particle size. The effect of the concentration of gadolinium (5, 10, 15 mol %) and the use of another rare earth (praseodymium, neodymium, europium or erbium) as co-dopant also were evaluated. Two sintering methods were used to increase the green body density: the conventional route and PECS; showing a highly dependence on the density and grain size respect to the sintering route. The best results were observed with the conventional route, with high densities for samples sintered at 1723 K for 6 h. Although PECS produced smaller grain sizes due to lower sintering temperatures, the relative densities were only about 90% after a heat treatment; due to the partial reduction of ceria in low oxygen partial pressures. The ionic conductivity was measured on the CS samples, with the highest values for samples with 10% and 15% of Gd and Pr or Nd as co-dopant.
Due to the environmental problems in the actuality, the employment of green and less aggressive energies has become a necessity. One example of this are the solid oxide fuel cells (SOFC), which present higher efficiencies than more common devices thanks to the simultaneous oxidation of a fuel in an anode and the reduction of an oxidant in a cathode1. However, the principal material used as electrolyte (Yttria-stabilized zirconia YSZ) is only capable to work at high temperatures (around 1173–1273 K); being the consequences of this limitation the quickly degradation of structural materials and the high maintenance costs of the cells.
Gadolinium-doped ceria (GDC) has been one of the most promising materials to replace YSZ, working at intermediate temperatures2–4 (IT-SOFC, around 873–973 K). The high stability of the fluorite type structure of ceria allows it to replace even me 40% of its cations with dopants, creating oxygen vacancies to control the neutrality of the solution; therefore, GDC and other solid solutions based on ceria have been widely analyzed but the effects of an another rare earth as co-dopant are not well known yet5–7.
The preparation and sintering processes are as important as the material, affecting the final density, grain size, morphology and other properties of the samples. It has been shown that by mechanical alloying, the grain ad crystallite size can be decreased in ceramics; being an effective, quick and safe method to prepare the powders.
Also, the sintering route plays an important role. Pulsed electric current sintering (PECS) is a non-conventional method capable to decrease the sintering time and temperature by the flow of a current through the sample thickness. The pattern interchange periods of current with periods of no-current, densifying the material by Joule effect. The high heating rate is beneficial when the time has to be decreased, while the sintering is carrying out in a vacuum chamber. By PECS, the grain size is smaller than the one obtained by conventional sintering (CS), with cleaner grain boundaries8–11. However, there have been several attempts to obtain high densified cerium-based electrolytes, these experiments were carried out under special conditions that limited their reproducibility, by using a high-pressure (~530 MPa) modified spark plasma apparatus (HP-SPS)12 or by employing elevated heating rates (~500 °C/min)13.
The objectives of the present work were to obtain highly densified samples by means of mechanical alloying and conventional sintering or PECS in order to be used as IT-SOFC electrolytes; evaluating the effect of milling time, gadolinium concentration as a first dopant and the effect of a second dopant in the lattice parameter, crystallite size and particle size of the milling powders, and evaluating the effect of the sintering route in their final density and conductivity of the consolidated samples.
Gadolinium-rare earth co-doped ceria powders were prepared by mechanical alloying in a RETSCH PM400 high-energy planetary mill. Commercial powders of CeO2, Gd2O3, Pr2O3, Eu2O3, Er2O3 and Nd2O3 (Aldrich, 99.99%) were milled at 300 rpm, using YSZ vials and balls, with a ratio weight powder:weight balls 1:8. In order to determine the milling time, a composition of ceria with 15 mol % of gadolinium was milled for 0, 2, 4, 8, 16 and 20 h; the powder obtained was analyzed by XRD and SEM techniques. After defining the optimal milling time; different compositions, varying the mol % of gadolinium (5, 10 and 15 %) and the co-dopant (Pr, Nd, Eu or Er) in a fixed concentration of 3 mol % (as shown in Table I), were milled during 16 h.
Table I. Experimental matrix, varying the concentration of Gd as first dopant and the type of co-dopant in a 3 mol % fixed concentration.

The powders were sintered by two different routes: conventional sintering (CS) and pulsed electric current sintering (PECS). By CS, the compressibility curve was obtained in a CARVER 3925 Hydraulic Press. Then, the powders were pressed into disc-shaped pellets using 20 ton of load with a 20 mm of diameter die. The green bodies were sintered using a Carbolite HTF 1700 furnace, in air at 1723 K for 6 h, with a heating rate of 8 Kmin−1 from room temperature up to 1273 K and 4 Kmin−1 up to 1723 K for all samples.
By PECS, in a Doctor Sinter SPS-1050 system, a 20 mm of diameter graphite die was used. The samples were sintered at 1473 K and 1673 K, with a heating rate of 200 Kmin−1, pressureless and changing the holding time between zero, one and two minutes, due to the reduction of ceria at low oxygen partial pressures presents in the chamber of the equipment14–16. In this case, boron nitride (BN) was used as isolator to prevent and control the gradient of the ceria reduction through the sample’s thickness, being placed in the top and the bottom of the powder; also avoiding the carbon diffusion from the die to inside the sample. In order to remove the boron nitride, the disc-shaped electrolytes were polished using 200, 300, 600 and 1200 SiC paper.
After measuring their density by Archimedes method, it was concluded that a post heat treatment was needed in order to increase it. The samples were annealed at 1423 K in air during 24 and 48 h, using a heating rate of 4 Kmin−1.
In order to measure the electrolytes conductivity, it was necessary to place platinum electrodes (CL11–5349, Heraeus) in both surfaces, top and bottom, of one sample for each composition. The paste was place in one face of the sample, dried at 398 K for 30 min and sintered at 1123 K for 1 h; then, the procedure was repeated for the other face.
XRD patterns for milled powders, CS samples and PECS samples, were obtained in a D500 SIEMENS diffractometer with Cu Kα radiation source. The crystallite sizes were measured using Scherrer equation and the lattice parameters were calculated by Cohen method for cubic structures. Particle sizes and morphologies for all cases were observed and measured by scanning electron microscopy in a JSM-6400 JEOL microscope.
The density of the samples was measured using Archimedes method and compared against the theoretical to establish the relative density. AC impedances measurements were carried out using a NorECs Norwegian Electro Ceramics AS ProboStat conductivity cell, a TSV12/50/300 Elite vertical furnace and a SP-150 BIO-LOGIC potentiostat/galvanostat, from 673 K up to 1173 K (every 100 K), in air. The employed frequency range was from 1 MHz to 10 mHz, while the magnitude of the voltage used was limited to 50 mV peak-to-peak.
The SEM images of 15GDC samples milled at different time (0, 2, 4, 8, 16 and 20 h) shows the effect of the milling time in particle size and distribution. Fig. 3a shows the powder without being milled, with big agglomerates and no solid solution formed. As the milling time increase (2 h, 8 h and 20 h for figures b, c and d), a clearly reduction in the particle size is observed. However, for 20 h, the particle distribution is not as homogenous as that for 8 and 16 h.
Figure 1. SEM images of 15GDC milled during: (a) 0 h, (b) 2 h, (c) 8 h, and (d) 20 h.

Particle size for each milling time was measured, increasing for 4 h and then decreasing again, as shown in Figure 2. Although the smallest particle size was obtained for 20 h, the standard deviation is smaller for 16 h of milling, with particles more homogenously distributed.
Figure 2. Variation of particle size for 15GDC powder with milling time.

Crystallite size was measured from the XRD patterns of each time, decreasing in an exponential way, as observed in Figure 3. Finally, lattice parameters were calculated using Cohen method, and showing an opposite behavior than crystallite size, increasing while increasing the milling time (Figure 4). From these results, it was concluded that the powders show the best behavior for 16 h, with not only a good acceptance of gadolinium into ceria structure, but also better distribution of particle size and no bigger changes in crystallite size respect to 20 h milled powder.
Figure 3. Variation of crystallite size for 15GDC powder with milling time.
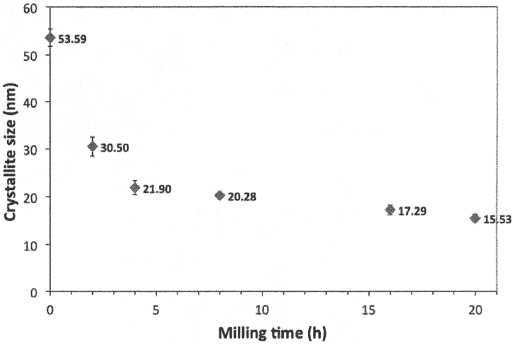
Figure 4. Variation of lattice parameter for 15GDC with milling time.

The compositions from Table I were all milled during 16 h, observing similar behavior for particle sizes, crystallite sizes and lattice parameters. Figure 5 shows the SEM images for 5GDC-Eu, 10GDC-Eu and 15GDC-Eu; as is shown, the particle size increased for 10GDC-Eu respect to 5GDC-Eu, decreasing again for 15GDC-Eu composition. Compositions with Er and Pr as co-dopants exhibit the same behavior, contrasting with Nd compositions, which particle size decrease for 10 mol % of gadolinium (Figure 6).
Figure 5. SEM images showing the particle size and distribution of (a) 5GDC-Eu, (b) 10GDC-Eu, and (c) 15GDC-Eu; all milled for 16 h.

Figure 6. Variation of particle size for different co-dopants (Pr, Nd, Eu, Er) as a function of mol % of gadolinium.

From the XRD patterns for each composition, the crystallite size was measured using Scherrer equation. As in particle size, compositions with Pr, Eu and Er show the biggest crystallite size for 10 mol % of gadolinium; in contrast, this is the lowest value for Nd composition (Figure 7).
Figure 7. Variation of crystallite size for different co-dopants (Pr, Nd, Eu, Er) as a function of mol % of gadolinium.

Lattice parameter once again was calculated using Cohen method for cubic structures. As shown in Figure 8, lattice parameters have a different respond against gadolinium concentration. In the case of Nd, the smallest lattice parameter is for 10 mol % of Gd, as same as in particle size and crystallite size; Eu also shows the same curve than before. However, for Pr and Er, the behavior is not the same. For Pr, the slope of the curve is negative, decreasing the lattice parameter while increasing the gadolinium concentration. For Er, the slope is slightly positive, almost horizontal, increasing the value while increasing the gadolinium concentration.
Figure 8. Variation of lattice parameter for different co-dopants (Pr, Nd, Eu, Er) with mol % of gadolinium.

The obtained values for particle size, crystallite size and lattice parameter of the samples, along with the standard deviation for each measurement, are also shown in Table II.
Table II. Particle size, crystallite size, lattice parameter and standard deviation for each milled sample.

After milled, 15GDC samples were sintered by PECS. 7 g of powder were placed into a graphite die and sintered at 1473 and 1673 K, with zero, one and two minutes of holding time. In this case, due to the reduction of ceria at low oxygen partial pressure and its polarization by the pass of electric current, it was necessary to use boron nitride as isolator. No pressure was applied in order to prevent the cracks of the samples for its volume changes. Figure 9 shows the XRD patterns for samples sintered by PECS at different conditions; as is shown, at 1673 K without holding time an important reduction of ceria to Ce2O3 take place, being also a contamination for the diffusion of the graphite into the sample. At 1473 K with two minutes of holding time there is also a reduction of ceria, but this did not occur with one and zero minutes of holding time. Also, an increment in the grain size from zero to one minutes can be observed.
Figure 9. XRD patterns for 15GDC samples, sintered by PECS at 1673 and 1473 K varying the holding time.

The density of the samples was measured by Archimedes method, obtained the highest value for the sample sintered at 1473 K with one minute of holding time, around 82% of relative density. However, this value is still very low for the samples to be used as electrolytes for IT-SOFC; for this reason, the samples were annealed in air at 1423 K in air during 24 and 48 h, using a heating rate of 4 Kmin−1, observing a change in their color from dark gray to pink, due to their reoxidation. Then again the density was measuring, reaching between 90 and 91% respectively. As there is no significance change between these values and the density could not be increased by other way, it was concluded that PECS was not the best sintering route for ceria-based electrolytes using normal conditions of pressure and heating rates. Other authors have been working with the sintering of ceria-based samples by PECS modifying pressure conditions or with high heating rates16–18.
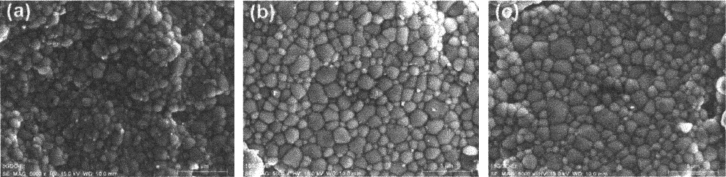
By CS, the powders were pressed into disc-shaped pellets using 20 ton loads with a 20 mm of diameter die. The green bodies were sintered in air at 1723 K for 6 h, with a heating rate of 8 K min−1 from room temperature up to 1273 K and 4 K min−1 up to 1723 K for all samples. Figure 10 shows the SEM images for Er composition; increasing the particle size for 10 mol% of gadolinium and then slightly decreasing for 15 mol% of Gd. Also, can be observed the nonexistence of open porosity in the samples, assuring a high density.
Figure 10. SEM images for Er samples sintered by CS, varying the gadolinium concentration: (a) 5 mol%, (b) 10 mol%, and (c) 15 mol%.
Particle sizes were measured from SEM images, with the highest values for 15GDC-Nd and 15GDC-Eu. Figure 11 shows the mean value for particle sizes, describing a similar behavior than particle sizes before sintering.
Figure 11. Variation of mean value of particle size for different co-dopants with gadolinium concentration after conventional sintering.

The final relative density for CS samples was also measured by Archimedes method, obtaining the highest values for 15GDC-Nd and 15GDC-Pr and the lowest values for 5GDC-Er and 10GDC-Er (Figure 12). These values can be explained by means of the ionic radius values, being smaller for Er and Eu and bigger for Nd and Pr. As can be observed, by CS was possible to obtain high densified samples, with more than 95% of relative density, which can be used as electrolytes for IT-SOFC.
Figure 12. Variation of relative density for different co-dopants with gadolinium concentration after conventional sintering.

Table III shows the measured values for grain size after CS and final density of the samples, along with the standard deviation for each parameter.
Table III. Grain size, final density and their standard deviation for each sample after CS.
Impedance measurements were carried out using a conductivity cell ProboStat, a vertical furnace and a potentiostat/galvanostat and placing two Pt electrodes, one in each surface of the samples, as described above. All samples showed the same behavior, obtaining the Nyquist diagrams for each one at different temperatures from 673 up to 1173 K. Figure 13 shows the response of a 10GDC-Nd sample, forming two semicircles at 773K, corresponding to bulk and grain boundary contributions.
Figure 13. Nyquist diagram corresponding a 10GDC-Nd sample working at 773 K.

Conductivity measurements are shown in Figure 14, for all co-dopants and gadolinium concentrations. As is observed, the electrolytes show the typical respond for an ionic conductor, with the highest values of conductivity for samples 10GDC-Pr, 15GDC-Pr and 15GDC-Nd. This shows that, with co-dopants which radius is bigger than the cerium one, the conductivity increase. All the obtained values display an error between 1 and 3% of the measured magnitude due to instrumental and human errors present during measuring. Table IV shows the measured conductivity for all samples working at 973 K.
Figure 14. Variation of ln(°T) with 1000/T for samples (a) with Nd and Eu as co-dopants, (b) with Pr and Er as co-dopants.

Table IV. Conductivity of all samples working at 973 K.

Solid solutions of GdxRE0.03Ce0.97-xO1.985-x/2 were successfully prepared by mechanical alloying in a high-energy planetary mill. The effects of milling time, gadolinium concentration and co-dopant type in crystallite size, particle size, lattice parameter and morphology have been studied. The effects of the sintering route in the same parameters, the final density obtained and ionic conductivity of the samples also have been evaluated.
In conclusion, smaller particle size and crystallite size are obtained with higher milling time, but the decreasing rate slows down after 16 h milling showing no significant difference for 20 h. Bigger crystallite size was obtained for co-dopant with bigger deviation from the cerium radius (Praseodymium, Neodymium) and the particle size was bigger for elements with smaller radius (Erbium, Europium). Lattice parameter has shown to have the same behavior than particle size for Nd and Eu.
Obtain GDC samples with high density by PECS (without applied pressure nor high heating rate) presents high complexity due to its reduction in low oxygen partial pressures. Samples sintered by PECS, using BN as isolator, showed densities around 82%, being increased with a further heat treatment up to 90%.
It was possible to obtained high densified (more than 95%) samples by conventional sintering, at 1723 K for 6 h. In both cases, CS and PECS, the samples showed a coloration from the gray milled powders to pink or brown, according to the co-dopant, due to the re-oxidation of the powders.
CS samples with 10 and 15 mol % of gadolinium showed better conductivity, obtaining high values at 973 K for Nd and Pr. According to this, the samples are good as electrolytes for IT-SOFC. The conductivity shows a dependence on the density and the particle size of the samples; being bigger for co-dopants which radii are bigger than the cerium one.
The authors would like to thank the Mechanical Engineering Department of Nagaoka University of Technology (NUT) for providing PECS facility.
This work was financially supported by Universidad Michoacana de San Nicolás de Hidalgo under Grant No. CIC-2012–2013–1.24.
1 K. Hayashi, M. Yokoo, Y. Yoshida, and H. Arai, Solid Oxide Fuel Cell Stack with High Electrical Efficiency. NTT Technical Review, 7, 1–5 (2009).
2 D. Bhattacharyya, and R. Rengaswamy, A review of Solid Oxide Fuel Cell (SOFC) Dynamic Models. Ind. Eng. Chem. Res., 48, 6068–86 (2009).
3 K. Eguchi, T. Setoguchi, T. Inoue, and H. Arai, Electrical Properties of Ceria-Based Oxides and Their Application to Solid Oxide Fuel Cells, Solid State Ionics, 52, 165–172 (1992).
4 W. Zhao, S. An, and L. Ma, Processing and Characterization of Bi2O3 and Sm2O3 Codoped CeO2 Electrolyte for Intermediate-Temperature Solid Oxide Fuel Cell. J. Am. Ceram. Soc., 94, 1496–1502 (2011).
5 T.S. Stefanik, Electrical Properties and Defect Structure of Praseodymium-Cerium Oxide Solid Solutions, Ph.D. Thesis, Department of Materials Science and Engineering, Massachusetts Institute of Technology (2003).
6 T.S. Zhang, L.B. Kong, Z.Q. Zeng, H.T. Huang, P. Hing, Z.T. Xia, and J.A. Kilner, Sintering Behavior and Ionic Conductivity of Ce0.8Gd0.2O1.9 with a Small Amount of MnO2 Doping. J. Solid State Electr., 7, 348–354 (2003).
7 R. Sen, S. Das, and K. Das, Combustion and Ball Milled Synthesis of Rare Earth Nano-Sized Ceria Powder, Materials Sciences and Applications, 2, 416–420 (2011).
8 Z.A. Munir, D.V. Quach, and M. Ohyanagi, Electric Current Activation of Sintering: A Review of the Pulsed Electric Current Sintering Process, J. Am. Ceram. Soc., 94, 1–19 (2011).
9 U. Anselmi-Tamburini, J.E. Garay, Z.A. Munir, A. Tacca, F. Maglia, and G. Spinolo, Spark Plasma Sintering and Characterization of Bulk Nanostructured Fully Stabilized Zirconia: Part I. Densification Studies, J. Mater. Res., 19, 3255–62 (2004).
10 K.A. Khor, L.G. Yu, S.H. Chan, and X.J. Chen, Densification of Plasma Sprayed YSZ Electrolytes by Spark Plasma Sintering (SPS), J. Eur. Ceram. Soc., 23, 1855–63 (2003).
11 J. Yang, Z. Wen, Z. Gu, and D. Yan, Ionic Conductivity and Microstructure of Solid Electrolyte La2Mo2O9 Prepared by Spark-Plasma Sintering, J. Eur. Ceram. Soc., 25, 3315–21 (2005).
12 U. Anselmi-Tamburini, F. Maglia, G. Chiodelli, A. Tacca, G. Spinolo, P. Riello, S. Bucella, and Z.A. Munir, Nanoscale Effects on the Ionic Conductivity of Highly Doped Bulk Nanometric Cerium Oxide, Advanced Functional Materials, 16, 2363–68 (2006).
13 T. Mori, T. Kobayashi, Y. Wang, J. Drennan, T. Nishimura, J.G. Li, and H. Kobayashi, Synthesis and Characterization of Nano-Hetero-Structured Dy doped CeO2 Solid Electrolytes Using a Combination of Spark Plasma Sintering and Conventional Sintering, J. Am. Ceram. Soc., 88, 1981–84 (2005).
14 S.R. Bishop, K.L. Duncan, and E.D. Wachsman, Defect Equilibria and Chemical Expansion in Non-Stoichiometric Undoped and Gadolinium-Doped Cerium Oxide, Electrochimica Acta, 54, 1436–14 (2009).
15 F. Giordano, A. Trovarelli, C. de Leitenburg, and M. Giona, A Model for the Temperature-Programmed Reduction of Low and High Surface Area Ceria, J. Catal., 193, 273–282 (2000).
16 R.G. Ranga, and B.G. Mishra, Structural, Redox and Catalytic Chemistry of Ceria Based Materials, Bulletin of the Catalysis Society of India, 2, 122–134 (2003).
17 X. Hao, Y. Liy, Z. Wang, J. Qiao, and K. Sun, A Novel Sintering Method to Obtain Fully Dense Gadolinia Doped Ceria by Applying a Direct Current, J. Power Sources, 210, 86–91. (2012).
18 M.G. Chourashiya, J.Y. Patil, S.H. Pawar, and L.D. Jadhav, Studies on Structural, Morphological and Electrical Properties of Ce1-xGdxO2-(x/2), Mater. Chem. Phys., 109, 39–44 (2008).