Figure 1. Single-cell and tubular design of an NBB battery and electrode. β”-alumina is at the same time the electrolyte and the physical separation between the electrodes [2].

Electrochemical systems based on a solid electrolyte membrane of beta-alumina are capable of reversibly storing and releasing electrical energy with high efficiency. Properties of ceramics like beta-alumina are highly dependent on their microstructure and powder surface characteristic which are modified by dopants addition. As beta-alumina needs dopants to phase stabilization, it is submitted to surface segregation, which modifies its surface energy, particle size and consequently phase transformation temperature. The aim of this study is to evaluate the effects of dopants such as Mg on the thermodynamic properties of beta-alumina synthesized by Pechini’s method and its influence on the electronic properties. The phases stabilities were modified by dopants addition changing the transformation temperature of the hexagonal and rhombohedric beta-aluminas. Dense areas were observed in the material calcined at 1250°C. Temperature changes and early densification may be evidences of the surface energy and particle size influence in the whole systems stabilities.
The concern over the use of fossil fuels and environmental preservation has influenced the search for new energy sources that are at the same time powerful, renewable, clean and environmentally friendly.
Recently, energy sources such as the wind and the sun emerge as powerful alternatives despite their intermittence. During the interruption, the energy provision must continue so an alternative to provide constant energy supply is to storage the surplus energy produced at the peaks of production using batteries. These batteries are chemical systems mat are able to store energy and release when needed.
The most used batteries for this purpose are based in a ceramic electrolyte. Although ceramics are not known as good conductors, the ionic diffusion normally occurs in all solids, including ceramics. However, diffusion mechanism normally takes place through vacances and has to overcome high energy barriers, which results in low ionic mobility. Some solids, on the other hand, are known as “ionic superconductors” and presents high ionic conductivity and major field of applications [1].
A ceramic electrolyte consists in a thin membrane that allows charge transportation between the electrodes. β”-alumina is the solid electrolyte used in Na-S batteries, where the charge transportation is made via Na+ cations diffusion. Due to the type of electrolyte used, these electrochemical devices are often referred as Na-beta batteries (NBBs) [2].
Over the last decades has been a lot of progress in the studies about NBBs, but yet the material’s performance and production costs are still holding back the advances [2].
Figure 1. Single-cell and tubular design of an NBB battery and electrode. β”-alumina is at the same time the electrolyte and the physical separation between the electrodes [2].

β”-alumina is a mix of aluminum and sodium oxides, resulting in a non-stoichiometric alumina doped with sodium presenting particular atomic structure.
Figure 2. Beta-alumina crystal structure [3].

Close-packed layers containing aluminum (tetrahedral and octahedral interstices) and oxygen atoms in a spinel-like structure are alternated with loosely packed layers where the mobile sodium cations are free to move under an electric field.
β”-alumina (rhombohedral) is the preferred polymorph since its unit cell is 50% large than β-alumina (hexagonal), and therefore, it presents a higher conductivity. Nevertheless, it is unstable above 1450°C, being frequently doped with magnesium (Mg) to promote stability at high temperatures, otherwise, it is irreversibly transformed into β-alumina.
Properties of ceramics, such as beta-alumina, are highly dependent on their microstructure and powder surface characteristics, as chemical groups and surrounding interactions [4]. It is known that when dopants are added, they can follow different paths: (a) form a solid solution in the crystal bulk (b) nucleate as a second phase or (c) spontaneously migrate to the particle interface and segregate as an attempt to lower the surface energy. However, ions only segregates if the surface energy decrease is greater than the bulk energy decrease in the case of a solid solution formation [5].
Synthesis and sinterization processes are treated exclusively as kinetics phenomena and little or none of thermodynamics are discussed, especially when there are non-extensive solid solutions and the occurrence of surface segregation causing the, so called, surface effects. These effects includes changes in the material surface energy, and therefore, in the phase stability [5].
From this point of view, the determination of surface’s physical-chemical behavior can help to explain the doped and undoped materials properties and its differences. In addition, sinterization can also be subjected to this study.
Since dopants are needed to β”-alumina phase stabilization, this study was aimed at investigating the role of surface segregation in the materials properties, which modifies the surface energy.
β”-alumina powders were prepared via resin synthesis, according to the Pechini’s method [6]. Blended in the desired concentration of each cation, the polymeric precursor was calcined first at 450°C, then at 650°C for 15 hours to particle size stabilization. The powders were then heated to 1250°C to obtain the β”-alumina phase and characterized by: Thermal analysis, X-ray Diffraction, Granulometry analysis, Specific surface area determined by gas adsorption, Scanning electron microscopy and Fourier Transform Infrared Spectroscopy.
The Mg doped β”-alumina was prepared with 10:2:1 molar ratio of Al, Na and Mg, according to the chemical formula Na2MgAl10O17. Thermal analysis was performed in order to determine the phase transformation temperature, Figure 3.
Figure 3. Mg-β”-alumina precursor powder TG and DTA analysis.
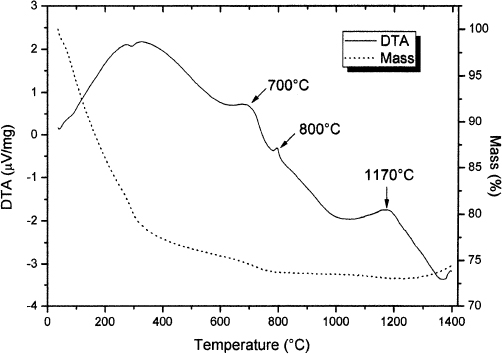
Mass loss and signal until 450°C shows the evaporation and loss of adsorbed and chemical bonded water. Above this temperature the mass loss continues, probably due to the elimination of carbon remaining from the resin pyrolysis. There are three remarkable exothermic peaks, at 700, 800 and 1170°C. X-ray diffraction was used to determine these transformations.
The final powder is composed by Mg-doped β”-alumina (rhombohedral) as major product among with β-alumina (hexagonal) and sodium aluminate. Phases proportions are shown in Table 1.
Table 1. Mg-β”-alumina powder composition and phases proportions.
| Name | Chemical Formula | Mass (%m) |
| β”-alumina | MgNa2Al10O17 | 68,2 |
| β-alumina | NaAl11O17 | 29,2 |
| Sodium Aluminate | NaAlO2 | 2,6 |
As shown in Figure 4, β”-alumina phase transformation only occurs above 1100°C. Intermediates appears about 700°C and, as the temperature increases, they are transformed into the final powder containing Mg-doped β”-alumina, β-alumina and sodium aluminate. β and β”-alumina are always produced together, which does not affect the final powder electrical properties.
Figure 4. X-ray diffraction patterns of powders calcined at various temperatures.

The Infrared spectrum, Figure 5, shows the particles surface evolution since it has been prepared. As the powder spends more time interacting with the atmosphere, it adsorbs more water, – CO groups and carbonates. Water presence (liquid and dissociated -OH groups) are clearly notice from 3687 to 2850 cm−1 and at 1640 cm−1; and CO groups and carbonates from 2500 to 2125 cm−1 and 1430 cm−1. And finally, the oxygen-metal bonds vibrations are observed below 1000 cm−1.
Figure 5. Infrared Spectrum.

The groups adsorption is an attempt to stabilize the powder surface by reducing this area energy. The change is clearly noticed, especially by the appearance of the 3600 cm−1 signal from the O-H bond in the surface.
Particle agglomeration is another way to reduce surface energy by eliminating high energy solid-gas interfaces and creating new solid-solid interfaces (lower energy). Final powder particle size distribution (Figure 6) is mostly around 63 μm, but is possible to observe by Scanning electron microscopy (Figure 7) that a great number of agglomerates are present and hexagonal (highlighted) and needle-like particles are much smaller. Particle size distribution is representing the agglomerates distribution, not particles.
Figure 6. Particle size distribution.

Figure 7. Scanning Electron Microscopy of calcined final powder.

Specific surface area decreases from 45.4 m2/g to 4.0 m2/g after calcination. As temperature increases from 650 to 1250°C, agglomerates are formed simultaneously with particle growth, decreasing specific surface area.
Powder density 3,23 g/cm3 was determinate by Helium picnometry. Using the values of specific surface area and density is possible to extrapolate and calculate the particle size considering all particles spherical, equals to 0,464 μm.
Scanning Electron Microscopy images showed powder partial sinterization at temperatures lower than 1650°C, as reported by the literature as sinterization temperature, Figure 8.
Figure 8. Scanning Electron Microscopy showing partial sinterization.

Partial sinterization at lower temperatures can be an evidence of high surface energy, as the decrease of surface area can decrease the Gibbs free energy.
A decrease or enhancement in the additive’s concentrations may affect the phase stability and powder characteristics. Two samples, one containing 0.5 and the other containing 1.5 moles of magnesium, were prepared to compare the phase formation and surface area.
The sample containing 0.5 molar ratio of Mg did not provide the Mg-β”-alumina as a the major component, although it was present in the final powder. In the other hand, 1.5 magnesium molar ratio has extrapolated Mg concentration’s for β”-alumina, which has caused the formation of spinel (MgAl2O4) among Mg-β”-alumina and β-alumina phases. Table 2 presents specific surface area comparison.
Table 2. Mg-dopped samples specific surface area.
| Mg molar ratio | 650°C | 1250°C |
| 0.5 | 61.1 m2/g | 33.9 m2/g |
| 1.0 | 45.4 m2/g | 4.0 m2/g |
| 1.5 | 54.7 m2/g | 18.6 m2/g |
Surface area analysis of these powders cannot be conclusive in determining if the Mg ions are segregating in the surface because different phases have different characteristic surface areas. Surface areas can only be compared to predict surface segregation if the same phases (and in the same proportion) are present.
An example is the high specific area presented by the 1.5 Mg-dopped sample, which would be an evidence of surface energy decrease, leading to less particle growth and high specific surface area. However, it is known that spinel presents high surface area and this enhancement could be only due to the spinel phase appearance.
Mg-β”-alumina was successfully produced by Pechini’s method. The particles’ surfaces presented groups that can change this area energy and influence in the phase stability depending on the additive’s concentration.
A full model based on the surface energy may explain phase stability and particle size (specific surface area) in order to improve this material sinterization to present great performance as an ionic electrolyte.
[1] A. J. MOULSON, J.M.H. Electroceramics: Materials, properties, applications. 2003.
[2] LU, X.; XIA, G.; LEMMON, J.P.; YANG, Z. Advanced materials for sodium-beta alumina batteries: Status, challenges and perspectives. Journal of Power Sources, v. 195, n. 9, p. 2431–2442, 2010.
[3] OJAMÄE, L, Sodium beta-alumina. In: Sodium beta-alumina. http://www.ifm.liu.se/compchem/former/nba.html.
[4] GOUVĚA, D.; PEREIRA, G.J.; GENGEMBRE, L; STEIL, M.C.; ROUSSEL, P.; RUBBENS, A.; HIDALGO, P.; CASTRO, R.H.R. Quantification of mgo surface excess on the sno2 nanoparticles and relationship with nanostability and growth. Applied Surface Science, v. 257, n. 9, p. 4219–4226, 2011.
[5] MARCOS, P.J.B. Efeitos de superfície na síntese e establlização de materials cerâmicos à base de zro2 sintetizados pelo método pechini. São Paulo, 2006. 133 p. Tese (Tese), Universidade de São Paulo.
[6] PECHINI, M.P., 1967. Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. In: Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. United States of America.