Fig. I Flow Chart

A novel citrate gel method was used to synthesize Zinc substituted Cobalt ferrite nano-particles with chemical formula Co1-xZnxFe2O4 where x=0, 0.2, 0.4, 0.6, 0.8 and 1). X-ray diffractions studies conformed the spinel formation of nano-ferrite structure and the particle size in the range of 17–24nm. TEM studies confirm the particles size of the nano-ferrites in nano-range. Electron diffraction patter of Co0.4Zn0.6Fe2O4 sample indicating small Particle size of nano crystalline sample. The broad rings suggest a small particle size. The starting materials used in the preparation of cobalt-zinc nano particles are Cobalt nitrate, Zinc nitrate, Ferric nitrate and citric acid having molar ratio of 1:3 were dissolved in de-ionized water. Citric acid acts as chelating agent and helps in the homogenous distribution of metal ions. The pH of the solution is adjusted to 7 by using ammonia solution.
Ferrites are compounds of ferric oxide Fe2O3, and a metallic oxide. Electromagnetic ferrite particles have wide range applications, such as information storage system, medical diagnostics micro wave devices1 radar, digital recordings in catalysis magnetic refrigeration, in Ferro fluid technology2, and in photo magnetism3. The magnetic nano ferrites also have extensive applications in permanent magnates, magnetic drug delivery, hyperthermia4, for cancer treatment5, and molecular imaging agents in magnetic resonance imaging6 (MRI).
The magnetic property of ferrites MFe2O4 depends on the cation configuration Here M represents Mn+2, Co+2, Ni+2, Cu+2 and Zn+2, closely packed arrangement of oxygen atoms and M2+, Fe3+ ions can occupy either tetrahedral (A) or octahedral (B) sites.32 O-atoms are observed in unit cell of a cubic system with 8 (tetrahedral) (A site) and 16 (octahedral) (B site).
Substituent’s7 stands important for determining the magnetic properties, Electric transport properties and physical properties of ferrites. Substitution may be direct replacement of Fe3+ on tetrahedral (A) or octahedral (B) sub lattice, thus redistribution of Fe3+ ions in between A- and B-sub lattices can modify the ferromagnetic spin structure. Mostly nature of the substituent decides the extent of Iron redistribution, which leads to a structural phase transition in the crystal symmetry, due to the John-Teller effect8.
Various methods have been reported in the literature to prepare ferrites with some advantages and disadvantages. Forced hydrolysis method has difficultly to prepare in large scale production of ferrites, whereas precursor method has better micro structural and compositional control. Sol-gel and Micro emulsion techniques involves use of large amounts of organic solvents. In Co-precipitation method9 PH of metal salt solution rises with the addition of base in order to precipitate the hydroxides and In Conventional method chemical in-homogeneity, coarser particle size, and introduction of impurities are the disadvantages. Concurring the disadvantages, citrate precursor method has been selected for the present study. The widely used chemical methods are Glyoxylate precursor method10, sucrose method11,12, reverse micelle technique13, hydrothermal method. In citrate precursor method citric acid is the organic precursor that acts as multidentate ligand and complexes with multivalent atoms to form chelates. High uniformity of metallic constituents and high degree of chelation of the metal ions increases with increasing the concentration of citric acid. Citrate method gives lowest value for the lattice parameter and particle size and yields more homogenous nano materials at lower processing temperatures. Thus, it presents a large superficial area and good sinterability in relation to powders obtained by other synthesis techniques14–20
The mixed Co-Zn ferrite having the chemical formula Co1-xZnxFe2O4 were synthesized by Citrate Precursor Method using Raw Materials Cobalt nitrate, Zinc nitrate, Citric acid and Iron nitrate. All raw materials ware mixed with distilled water individually, thus solution formed is mixed together except Iron nitrate, and PH is maintained at 7 by addition of ammonia, finally Iron nitrate solution is added drop by drop with continuous stirring. Solution is turned as gel on hot plate when heated at 80°C, on combustion burnt ash is formed which is calcinated at 400°C. Above mentioned Reaction procedure is shown in Flow chart I.
The structural characterization was carried out using X-Ray Diffractomerter (Rigaku) with a diffracted beam monochromatic Cu Kα, radiation (λ = 1.5405 A°), radiation source between the Bragg Angles 20° to 80° in steps of 0.04°/Sec. The 2θ vs. intensity data obtained from this experiment showed in Fig I.
Fig. I Flow Chart

The Identification of crystalline phases of the X-ray diffraction pattern was done by comparison with PDF–4 reference data from the international Centre for Diffraction data (ICDD). Confirmation of cubic spinal structure in single phase have been detected by all 1 Bragg reflections, and no impurity peak is identified. The plane (311) is the strongest reflection which reveals the spinal phase. The peaks indexed to (220), (311), (400), (511) and (440) plains are allowed planes of a cubic spinal structure in single phase21
Using the high intensity 311 peak Calculation of crystallite size was done. Scherrer Formula22 is used, while taking the instrumental brooding into account23.
(1) 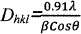
Where Dhkl --- is the crystalline size perpendicular to (h k l) pane,
λ --- Is the wave length of X-ray24 used
β --- (Degree) is the width of diffraction peak i.e., Full Width Half Maxima (FWHM)
θ --- The peak position.
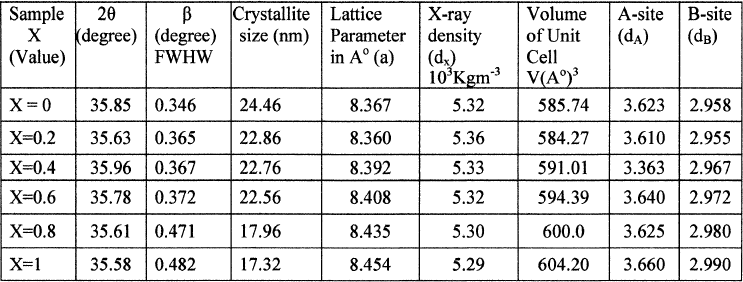
The crystallite size is in the range of 17–24nm for the different compositions, shown in table I. The crystallite size was not same for all samples even they prepared under identical conditions. This reveals different concentrations of Zn, favoring the variation of crystallite size. Lattice parameter of the samples was calculated by using the following relation
Table I: Values of Crystallite size, Lattice parameter (a), d–spacing (d), X–ray density (dx) and hopping length for A-site(dA) and B-site (dB) of Co-Zn Nano Ferrite(X=0, 0.2, 0.4, 0.6, 0.8, 1)
(2) 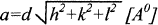
Where (h k l) --- are the Miller Indices, d --- Is inter planner spacing.
The variation of lattice parameter with composition is shown in the Fig II. From this plot it is clear that lattice parameter increases with increasing the zinc substitution in cobalt ferrites. Which is the evidence for the larger ionic radius of Zn+2 (88pm) when compare with Co+3 ion (83.8pm), so zinc can be easily incorporated and Non magnetic Zn ion occupies A site in cubic ferromagnetic spinels. Exchange in between the A and B sub lattices results change in magnetic properties from sample to sample, and also leads to form mixed Co-Zn ferrites which obeys Vegard’s law25.
Fig. II X-ray diffraction pattern of Co-Zn Nano-ferrites calcinated at 400°C.

The X-ray density (dx) was caliculated from the values of lattice parameter (a) using the formula26, shown in Table
(3) 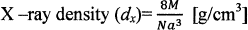
Where 8—represents the number of moleules in a unit ceil of spinel lattice, M—is the molecular weight of composition, N—is the Avogadro’s number, a is lattices parameter
X-ray density (dx) decreases with the increasing Zn substitution in Co Ferrites is shown in Fig III. Lattice parameter and molecular weight of the samples control X-ray density of ferrites thus, Zn+2 substitutions if increased X-ray density decreases.
Fig. III Graph between the lattice parameter (a) Vs Zn Concentration.

The Volume of the Unit Cell is calculated by using the following equation, which depends on the Lattice Parameter. Lattice Parameter increases with increasing Zn Substitution in Co Ferrite the Unit cell values also increases. Values are shown in Table I.
(4) 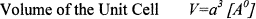
The distance between magnetic ions on B and A Sites is calculated according to the following relations27
(5) 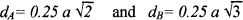
Where (a)—is the lattice parameter
The values of the hopping length for octahedral (dA) and tetrahedral (dB) sites are listed in Table I. It is clear that the distance between the magnetic ions increases as the Zn content increases.
Analytical characterization of nano particles and imaging is commonly performed by TEM to know size, morphology, shape and elemental distribution. TEM image of Co0.4Zn0.6 Fe2 O4, sample is shown in fig.4. It is clearly representing that the size of most particles lies ~20nm. XRD measurements of the sample Co0.4Zn0.6 Fe2 are closely coinciding with the particle size determined from TEM. Electron diffraction patter of Co0.4Zn0.6 Fe2 O4 sample indicating small Particle size of a nano crystalline sample. The broad rings suggest a small particle size.
Fig. IV Graph between the X-ray density Vs Zn Concentration
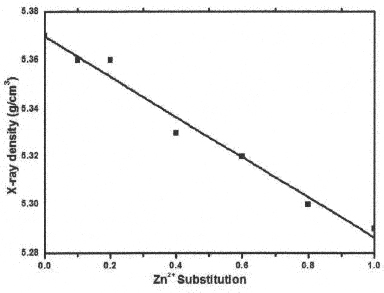
Fig V. TEM image of Co0.4Zn0.6Fe2O4, SEAD pattern of Co0.4Zn0.6 Fe2 O4.

One of the authors (D.R) is grateful to Prof. T.L.N. Swamy, Principal Nizam College for his encouragement to carry out this research work. The authors are thankful to Prof. C. Gyana Kumari, Head, Department of Chemistry, Osmania University, and Hyderabad for her encouragement in carrying out the research activities.
1S.N Doha, Role of particle size on structural and magnetic behavior of nano crystalline Cu-Ni Ferrite, Solid State Phenomena., 171, 79–91 (2011).
2GVM jacintho, A.G.Brolo, P.Corio Paulo A.Z.Suarez,and JC. Rubim,structural investigation of MFe2O4 (M=Fe,Co)magnetic fluids., J. Phys. Chem.C,113,7684(2009).
3A.K.Giri, E..M. Kirkpatrick, P. Moongkhamklang, and S.A. Majetich, and V.G. Harris, photomagnetis m and structure in cobalt ferrite nanoparticle., Appl. Phys. Letters., 80,2341(2002).
4P.Pradhan, J.Giri, G.Samanta,H.D. Sarma,K.P. Mishra, Bellarj, R.Banerjee, and D.Bahadur,Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application.,J.Biomed.Mater.Res.Part B Appl. Biomater., 81B,12(2007).
5M.Sincai,D.Ganga,D.Bica,and L.Vekas, The anticancer effect of locoregional magnetic Cobalt ferrite in dog mammary adeno carcinoma,J. Magn. Magn.Mater., 225,235–240(2001).
6JH.Lee,YM. Huh, YW Jun, JW Seo. JT Jang HT.Song, S Kim,EJ.Cho,HG.Yoon,JS.Suh, AndJ.Cheon, Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging, Nat. Med., 13, 95(2007).
7P.K.Roy,B. Bibhuti.Nayak,andJ. Bera,Study on electromagnetic properties of La substituted Ni-Cu-Zn ferrite synthesized by auto-combution method,Magn.Mater., 320, 1128(2008)
8M. C. Dimri, A. K. Verma, S. C. Kashyap, D. C. Dube and O. P. Thakur, “Structural, Dielectric and Magnetic Properties of NiCuZn Ferrite Grown by Citrate Precursor Method, Materials Science and Engineering., 133,42–48(2006).
9M.A. Ahmed,N.Okasha and SIEI-Dek,preparation and characterization of nanometric Mn ferrite via different methods,Nanotechnology., 19,065603(2008).
10C.Caizer and M.Stefanescu, Magnetic characterization of nanocrystalline Ni-Zn ferrite powder prepared by the glyoxylate precursor method,J.Phys.D.Appl.Phys.,35,3035–3040(2002).
11P.Pramanik, A Novel chemical route for the preparation of nanosized oxides, phosphates,vanadates,molybdates and tungstates using polymer precursor Bull. Mater. Sci.,22, 335,(1999).
12R. N. Das,Nano crystalline ceramics from sucrose process Mater. Lett.,47,344(2001).
13S. Gubbala,H. Nathani, K. Koizol,and R.D. K.Misra, Magnetic properties of nanocrystalline Ni-Zn,Zn-Mn,and Ni-Mn ferrites synthesized by reverse micelle technique,PhysicaB.,348,317–328(2004).
14S.Biamino, C.Badini, Combustion synthesis of lanthanum chromite starting form water solution investigation of process mechanism by DTA-TGA-MS,J.Eur.Geram.Soc.,24,3021–34(2004).
15H.Zhang, X. Jia, Z. Liu, and LiC. The low temperature preparation of nanocrystalline MgAl2O4 spinel by citrate sol-gel process, Mater Lett., 58, 1625–8(2004).
16A.Saberi,F.Golestani-Fard,H.Sarpoolaky,M.Willert-Porada,T.Gerdes,and R.Simon,Chemical synthesis of nanocrystalline magnesium aluminatespinel(MgAl2O4)via nitrate-citrate combustion, J. Alloys Compd., 462,142–6(2008).
17S.Vivekanandhan, M.Venkateshwarlu,and N. Satyanarayana,Synthesis and characterization of nanocrystalline Li Ni0.5 Co0.5 VO4 powders by citric acid assisted sol-gel combustion process, J Alloys Ciompd., 462,328–34(2008).
18ZJ.Wu, XB. Zhao, J. Tu, GS. Cao, JP.Tu,and TJ.Ahu, Synthesis of Li1+xV3O8 by citrate sol-gel route at low temperature, J. Alloys .Compd.,403,345–8(2007).
19Y.Wu, Y.He, T.Wu, T.Chen, W.Weng,and H.Wan,Influence of some parameters on the synthesis of nanosized NiO material by modified sol-gel method, Mater Lett., 61,3174–8(2007).
20S.Sahi,AR.Daud,M.and Hashim,A comparative study of nickel-zinc ferrites by sol-gel route and solid-state reaction, Mater Chem. Phys., 106,452–6(2007).
32S.A. Mazen,S.F. Mansour,and H.M.Zaki,Some physical and magntic properties of Mg-Zn ferrite,Cryst. Res. Technol., 38, 471–478 (2003).
22BD.Cullity, Elements of X-ray diffraction, Addition Wesley, Boston., 132(1959).
23ST. Mahmud, AKMA. Hossain, AKMA. Hakim. Seki,T. Kawai,and H. Tabata,influence of micro structure on the complex permeability of spinel types Ni-Zn ferrites, J. Magn. Magn. Mater., 305:269(2006)
24B. D. Cullity, Elements of X-ray diffraction, Wesley Pub.Co.Baston., 101–356(1987).
25L. Vergard, Z. Phys., 5, 17(1921).
26R.C. Kumbale. P. A. Shaikh, S.S. Kamble, Y.D. Kolekar J. Alloys Compd., 478 (2009), p. 599
27B.Viswanathan, V. R.K. Murthy Ferrite Materials Science and Technology NarosaPubl House, New Delhi (1990)