6. THE AFRICAN BOTTLENECK
MOST OF US are familiar with the basic outlines of the human evolutionary story. Our distant ancestors were a group of apelike creatures who started walking upright millions of years ago in Africa, eventually developing bigger brains and scattering throughout the world to become the humans of today. But there’s another story that has received less attention. Advances in genetics have given us a sharper understanding of what happened between the “walking upright” and the “buying the latest tablet computer” chapters of the tale.
Written into our genomes is the signature left behind by an event when the early human population dwindled to such a small size that our ancient ancestors living in Africa may have come close to extinction. Population geneticists call events like these bottlenecks. They’re periods when the diversity of a species becomes so constrained that evidence of genetic culling is obvious even thousands of generations later. Sometimes the shrinking of a population is the result of mass deaths, and indeed, there is evidence that humans may have been fleeing a natural disaster when we walked out of Africa roughly 70 thousand years ago. But our species probably experienced multiple genetic bottlenecks beginning as far back as 2 million years. And those earlier bottlenecks were caused by a force far more powerful than mass death: the process of evolution itself.
In fact, the African bottlenecks are an example of the paradoxical nature of human survival. They provide evidence that humans nearly died out many times, but also tell a story about how we evolved to survive in places very far away from our evolutionary home in Africa.
The Fundamental Mystery of Human Evolution
Given our enormous, globe-spanning population size, humans have remarkably low genetic diversity—much lower than other mammal species. All 6 billion of us are descended from a group of people who numbered in the mere tens of thousands. When population geneticists describe this peculiar situation, they talk about the difference between humanity’s actual population size and our “effective population size.” An effective population size is a subgroup of the actual population that reasonably represents the genetic diversity of the whole. Put another way, humanity is like a giant dance party full of billions of diverse people. But population geneticists, elite party animals that they are, have managed to find the one ideal VIP area that contains a small group of people who very roughly capture the diversity of the party as a whole. In theory, that room contains the party’s effective population size. If they all started randomly having sex with each other, their children might loosely reproduce the diversity and genetic drift of our actual, billions-strong population.
The weird part is that compared with our actual population size, the human effective population in that VIP area is very low. In fact, today’s human effective population size is estimated at about 10,000 people. As a point of comparison, the common house mouse is estimated to have an effective population size of 160,000. How could there be so many of us, and so little genetic diversity?
This is one of the fundamental mysteries of human evolution, and is the subject of great debate among scientists. There are a few compelling theories, which we’ll discuss shortly, but there is one point that nearly all evolutionary biologists will agree on. We are descended from a group of proto-humans who were fairly diverse 2 million years ago, but whose diversity crashed and passed through a bottleneck while Homo sapiens evolved. That crash limited our gene pool, creating the small effective population size we have today. Does some kind of terrible disaster lurk in the human past? An event that could have winnowed our population down to a small group of survivors, who became our ancestors? That’s definitely one possibility. Evolutionary biologist Richard Dawkins has popularized the idea that the population crash came in the wake of the Toba catastrophe, a supervolcano that rocked Indonesia 80,000 years ago. It’s possible this enormous blast cooled the African climate for many years, destroying local food sources and starving everybody to death before sending fearful bands of Homo sapiens running out of Africa.
But, as John Hawks, an anthropologist at the University of Wisconsin, Madison, put it to me, a careful examination of the genetic evidence doesn’t reveal anything as dramatic as a single megavolcanic wipeout. Instead of some Hollywood special-effects extravaganza, human history was more like a perilous immigration story. To understand how immigration can turn a vast population into a tiny one, we need to travel back a few million years to the place and time where we evolved.
The Human Diaspora
Humanity’s first great revolution, according to the anthropologist Ian Tattersall of the American Museum of Natural History, was when it learned to walk upright, more than 5 million years ago. At the time, we were part of a hominin group called Australopithecus that shared a very recent common ancestor with apes. Australopithecines hailed from the temperate, lush East African coast. They were short—about the size of an eight-year-old child—and covered in a light layer of fur. They may have started walking on their hind legs because it helped them hunt and find the fruits that dominated their diets. Whatever the reason, walking upright was unique to Australopithecus. Her fellow primates continued to prefer a four-legged gait, as they do today.
Over the next few million years, Australopithecus walked from the tip of what is now South Africa all the way up to where Chad and Sudan are today. Our ancestors also grew larger skulls, anticipating a trend that has continued throughout human evolution. By about 2 million years ago, Australopithecus was evolving into a very human-looking hominin called Homo ergaster (sometimes called Homo erectus). Similar in height to humans today, a couple of H. ergaster individuals could put on jeans and T‑shirts and blend in fairly well on a typical city street—as long as they wore hats to hide their slightly prominent brows and sloping foreheads. Another thing that would make our H. ergasters feel perfectly comfortable loping down Market Street is the way so many in the crowd around them would be clutching small, hand-sized tools. Our tools may contain microchips whose components are the products of advanced chemical processing, but the typical smartphone’s size and heft are comparable to the carefully crafted hand axes that anthropologists have identified as a key component of H. ergaster’s tool kit. H. ergaster wouldn’t need anyone to explain the meat slowly cooking over low flames in kebab stands, either: There’s evidence that their species had mastered fire 1.5 million years ago.
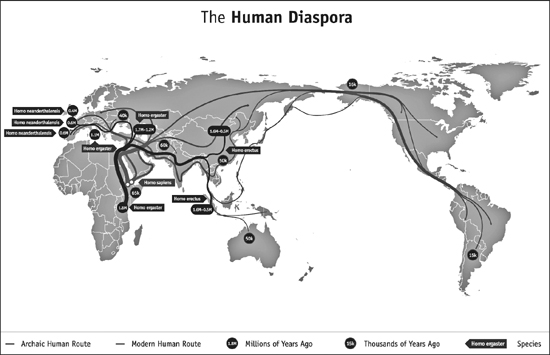
In this map, you can see the different waves of human expansion out of Africa, starting over one million years ago and continuing up into the Homo sapiens diaspora about 100,000 years ago. (illustration credit ill.6)
(Click here to see a larger image.)
There are many ways to tell the story of what happened to H. ergaster and her children, who eventually built those smart phones and invented the tasty perfection that is a kebab. H. ergaster was one of many bipedal, tool-using hominids roaming southern and eastern Africa who had evolved from Australopithecus. The fossil record from this time is fairly sparse, so we can’t be sure how many groups there were, what kinds of relationships they formed with each other, or even (in some cases) which ones evolved into what. But each group had its own unique collection of genes, some of which still survive today in Homo sapiens. And those are the groups whose paths we’re going to follow.
This path is both a physical and a genetic one. A visitor to the American Museum of Natural History in New York can track its progress in fossils. Glass-enclosed panoramas offer glimpses of what we know about how H. ergaster and her progeny created hand axes by striking one stone against another until enough pieces had flaked off that only a sharp blade was left. Reconstructed early human skeletons stand near sparse fossils and tools, a reminder that our ideas about these people come, literally, from mere fragments of their bodies and cultures. Ian Tattersall has spent most of his career poring over those fragments, trying to reconstruct the tangled root structure of humanity’s evolutionary tree.
One thing we know for sure is that early humans were wanderers. Not only did they spread across Africa, but they actually crossed out of it many times, starting about 2 million years ago. Anthropologists can track the journeys taken by H. ergaster and her progeny by tracing the likely paths between what remains of these peoples’ campsites and villages, often identifying the group who lived there based on the kinds of tools they used.
Tattersall believes there were at least three major radiations, or population dispersals, out of Africa. Despite the popularity of Dawkins’s Toba volcano theory, Tattersall believes there was “no environmental reason” for these immigrations. Instead, they were all spurred by evolutionary developments that allowed humans to master their environments. “The first radiation seems to have coincided with a change in body structure,” he mused. Members of H. ergaster had a more modern skeletal structure featuring longer legs than their hominid cohorts, which meant they could walk quickly and efficiently over a variety of terrains. Tattersall explained that there were environmental changes in Africa during this time, but not enough to suggest that humans fled environmental destruction to greener pastures. Instead they were simply well suited to explore “unfamiliar environments, ones very unlike their ancestral environments,” he said. H. ergaster’s rolling gait was an adaptation that allowed the species to continue adapting, by spreading into new lands where other hominids literally could not tread.
As early humans walked into new regions, they separated into different, smaller bands. Each of these bands continued to evolve in ways that suited the environments where they eventually settled. We’re going to focus on four major players in this evolutionary family drama: our early ancestor H. ergaster and three siblings she spawned—Homo erectus, Homo neanderthalensis, and Homo sapiens.
H. erectus was likely the evolutionary product of that first exodus out of Africa that Tattersall described. About 1.8 million years ago, H. erectus crossed out of Africa through what is today Egypt and spread from there all the way across Asia. These hominins soon found themselves in a very different environment from their siblings back in Africa; the winds were cold and snowy, and the steppes were full of completely unfamiliar wildlife. Over the millennia, H. erectus’s skull shape changed and so did her tool sets. We can actually track how our ancestors’ tools changed more easily than how their bodies did because stone preserves better than bone. Scientists have reconstructed the spread of H. erectus by unearthing caches of tools whose shapes are quite distinct from what other groups used. From what we can piece together, it seems that H. erectus founded cultures and communities that lasted for hundreds of thousands of years, and spread throughout China and down into Java.
Over the next million years, other groups of humans followed in H. erectus’s footsteps, walking through Egypt to take their siblings’ route out of Africa. But as the Stanford paleoanthropologist Richard Klein told me, these journeys probably weren’t distinct waves of migration. Walking in small groups, these humans were slowly expanding the boundaries of the hominin neighborhood.
Fossil remains in Europe suggest that about 500,000 to 600,000 years ago, some of H. ergaster’s progeny, on emerging from Africa, decided to go left instead of right, wandering into the western and central parts of the Eurasian continent. These Europeans evolved into H. neanderthalensis. They often set up homes in generously sized cave systems, and there’s evidence that some groups lived for dozens of generations in the same caves, scattered throughout Italy, Spain, England, Russia, and Slovenia, among other countries. Neanderthals evolved a thicker brow and more barrel-chested body to cope with the colder climate. We’ll talk more about them in the next chapter.
Back in Africa, H. ergaster was busy, too, establishing home bases all over the coasts of the continent, reaching from southern Africa all the way up to regions that are today Algeria and Morocco. By 200,000 years ago, H. ergaster’s skeletal shape was indistinguishable from that of modern humans. A species we would recognize as H. sapiens had emerged. And that’s when human beings made their next evolutionary leap—one that perfectly complemented our ability to walk upright into new domains.
How We Evolved to Tell Stories
“When Homo sapiens came along there was something totally radical about it,” Tattersall enthused. “For a hundred thousand years, Homo sapiens behaved in basically the same ways its ancestors had. But suddenly something happened that started a different pattern.” Put simply, humans started to use the giant brains they’d evolved to fit inside their gradually enlarging craniums. What changed? Tattersall said there are no easy answers, but evolution often works in jumps and starts like that. For example, birds evolved feathers millions of years before they started flying, and animals had limbs long before they started walking. “We had a big brain with symbolic potential before we used it for symbolic thought,” he concluded. In what anthropologists call a cultural explosion over the past 100,000 years, humans developed complex symbolic communication, from language and art to fashion and complex tools. Instead of looking at the world as a place to avoid danger and score food, humans disassembled it into mental symbols that allowed us to imagine new worlds, or new versions of the world we lived in.
Humans’ new facility with symbols allowed us to learn about the world around us from other humans rather than starting from scratch with direct observations each time we went to a new place. Like walking, symbolic thought is an adaptation that leads to more adaptations. Modern humans could venture into new territory, discover its resources and perils, then tell other bands of humans about it. They might even pass along designs for tools that helped us gain access to foods specific to a certain area, like crushers for nuts or scoops for tubers. Aided by our new capacity for imagination, those bands of humans could familiarize themselves with alien regions before ever visiting them. For the first time in history, people could figure out how to adapt to a place before arriving there—just by hearing stories from their comrades. Symbolic thought is what allowed us to thrive in environments far from warm, coastal Africa, where we began. It was the perfect evolutionary development for a species whose body propelled us easily into new places. Indeed, one might argue that the farther we wandered, the more we evolved our skills as storytellers.
Let’s go back, for a moment, to that first radiation out of Africa, nearly 2 million years ago when H. ergaster, with her small but effective tool kit, crossed into the Sinai Peninsula. At roughly the same time, we find evidence of humanity’s first genetic bottleneck. And yet, as Tattersall and many others have pointed out, there is no evidence of a giant disaster thinning the population, leaving the survivors to flee across the Middle East and Asia. The bottleneck is clearly a sign of a population crash, but what caused it? As I said earlier, the effective population size for H. sapiens is estimated at roughly 10,000 individuals; but the University of Utah geneticist Chad Huff recently argued that soon after H. ergaster left, our effective population size was about 18,500. It’s likely this bottleneck is actually a record of human groups growing smaller as they thinned out across the Eurasian continent, meeting adversity every step of the way. At the same time, according to anthropologist John Hawks, the bottleneck is a mark of evolutionary changes that could only happen to a population that was always on the move.
It started with that first trek out of Africa, which split humanity into several groups. As Hawks explained in a paper he published with colleagues in 2000, one cause for a genetic bottleneck can be speciation, or the process of one species splitting into two or more genetically distinct groups. We’ve already touched on how H. ergaster evolved into at least three sibling groups, but that’s a dramatic oversimplification. For example, H. ergaster likely evolved into a group called Homo heidelbergensis in Africa, which then speciated into H. sapiens and another group that speciated into Neanderthals and their close relatives the Denisovans later on. There are many complexities in the lineage of H. erectus, too, especially once the group reached Asia. Evolution is a messy process, with many byways and dead ends. By the time H. ergaster reached the Sinai, the group would have undergone at least one speciation event—the one that led to early H. erectus. That means only a subset of H. ergaster genes survived in H. erectus, and a subset of its genes survived in the H. ergaster groups who stayed behind. If these groups remained small, and there’s ample reason to believe that they did, you now have two isolated gene pools that are less diverse than the original one. That’s how speciation creates a genetic bottleneck.
But even without speciation events, humans’ habit of walking all over the place would have caused a bottleneck. The very act of wandering far from home, into many dangers, can shrink both the population and the gene pool over the course of generations. Population geneticists call this process the founder effect. To see how the founder effect works, let’s follow a band of H. erectus passing through lands edging the Mediterranean Sea and finding its way into India. Remember, this isn’t one long trek. Maybe the coast of today’s state of Gujarat appeals to a few members of H. erectus, and so a band decides to settle down for a while in that region. This settlement is called a founder group, and it has less diversity than the group it came from simply because it has fewer members. In the next generation, a new group splits off from the Gujaratis and heads south along the coast. Generally we assume that each time a group left for untouched lands, it left a group behind. So each new group becomes a founder population in its own right, and has less genetic diversity than the group back in Gujarat—even if you factor in some intermarriage between different founder groups. Multiple founder events in a row would have had the odd effect of increasing humanity’s population while decreasing human genetic diversity. Now, consider the fact that our H. erectus explorers in India are a microcosm of the way all humans spread across the Earth. After hundreds of generations of wandering, humans managed to increase their populations gradually while retaining the low diversity caused by genetic bottlenecks.
Back in Africa, early humans were also speciating and wandering, forming new bands, each of whose genetic diversity was lower than the last. But when a small band of hominins called H. sapiens evolved, about 200,000 years ago, something strange happened. Tattersall believes that humans underwent some kind of genetic change that spurred a cultural shift. Suddenly, between 100,000 and 50,000 years ago, the fossil record is full of sculpture, shell jewelry, complex tools made from multiple kinds of material, ochre-and-carbon cave paintings, and elaborate burial sites. Possibly, as Randall White, an anthropologist at New York University, suggests in his book Prehistoric Art, humans were using jewelry and clothing to proclaim allegiance with particular groups. H. sapiens wasn’t just interacting with the world. They were using symbols to mediate their relationship with it. But why the sudden shift from a hominin with the capacity for cultural expression to a hominin who actively created culture?
It could be that one small group of H. sapiens developed a genetic mutation that led to experiments with cultural expression. Then, the capacity to do it spread via mating between groups because storytelling and symbolic thought were invaluable survival skills for a species that regularly encountered unfamiliar environments. Using language and stories, one group could explain to another how to hunt the local animals and which plants were safe to eat. Armed with this information, humans could conquer territory more quickly. Any group that could do this would have a higher chance of surviving relocation time and again. The more those groups survived, the more able they were to pass along any genetic predisposition for symbolic communication.
Perhaps H. sapiens’ knack for symbolic culture was also a result of sexual selection, in which certain genes spread because their bearers are more attractive to the opposite sex. Put simply, these attractive people get laid more often, and therefore have more chances to spread their genes to the next generation. In his book The Mating Mind, evolutionary psychologist Geoffrey Miller argues that among ancient humans, the most attractive people were good with language and tools. The result would be a population in which sexual selection created successively more symbol-oriented people. Two anthropologists, Gregory Cochran and Henry Harpending, amplify this point. They argue that some of the genes that spread like wildfire through the human population over the past 50,000 years are associated with cranial capacity—brain size—and language ability. “Life is a breeding experiment,” Cochran and Harpending write in their book The 10,000 Year Explosion.
Our capacity for symbolism evolved quickly, partly because our mating choices would have been shaped by our needs as creatures who evolved to survive by founding new communities. Over the past million years, humans bred themselves to be the ultimate survivors, capable of both exploring the world and adapting to it by sharing stories about what we found there.
How Can We Possibly Know All This?
A lot of the evidence we have for the routes that humans took out of Africa comes from objects and places you can see with your own eyes. Paleontologists have found our ancestors’ ancient bones, as well as their tools. To figure out the ages of these tools and skeletons, we use the same kinds of dating techniques that geologists use to discover the history of rocks. In fact, when an anthropologist talks about “dating the age of fossils,” he or she isn’t actually talking about the bones themselves—to date old bones, anthropologists carefully excavate them and take samples of the rock surrounding them. Then they pin a date on those rocks, under the assumption that the bones come from roughly the same era as the rocks or sand that covered them up. Basically, we date fossils by association, which is why you’ll often hear scientists suggesting that a particular fossil might be between 100,000 and 80,000 years old. Though we can’t pin an exact month or year on each fossil discovery, we do have ample evidence that certain humans like H. ergaster came before other humans like H. erectus in evolutionary and geological time.
Over the past decade, however, the study of ancient bones has been revolutionized by new technologies for sequencing genomes, including DNA extracted from the fossils of Neanderthals and other hominins who lived in the past 50,000 years (sadly, we don’t have the ability to sequence DNA from Australopithecus or H. ergaster bones—their DNA is too decayed). At the Max Planck Institute in Leipzig, Germany, an evolutionary geneticist named Svante Pääbo and his team have developed technology to extract nearly intact genomes from Neanderthal bones. First they grind the bones to dust and chemically amplify whatever DNA molecules they can find, then analyze this genetic material using the same kinds of sequencers that decode the DNA of living creatures today. We’ll deal with the Neanderthal genome more in the next chapter, but suffice it to say that we have pretty solid evidence about the genetic relationships between H. sapiens and its sibling species H. neanderthalensis.
A lot of the evidence for humans’ low genetic diversity has been made possible by DNA-reading technologies developed since the first human genome was sequenced, in the early 1990s. Though that first human genome took over a decade to sequence, we now have machines capable of reading the entire set of letters making up one genome in just a few hours. As a result, population geneticists are accumulating a diverse sampling of sequenced human genomes, from people all over the world. Many of these genomes are collected into data sets that scientists can feed into software that does everything from make very simple comparisons between two genomes (literally analyzing the similarities and differences between one long string of letters and another), to extremely complex simulations of how these genomes might have evolved over time.
One of the first pieces of genetic evidence for the serial-founder theory emerged when scientists had collected DNA sequences from enough people that we could start to analyze genetic diversity in different regions all over the world. Geneticists discovered a telltale pattern: People born in Africa and India tend to have much greater genetic diversity than people born elsewhere. This is precisely the kind of pattern you’d expect to see in a world population that grew out of founder groups originating in Africa. Remember, each successive founder group has less and less genetic diversity. So people descended from groups that stayed in Africa or India are from early founder groups. People in Europe, Australia, Asia, and the Americas were the result of hundreds of generations of founder effects—so we’d expect them to have less genetic diversity. When you add this genetic evidence to the physical evidence from fossils and tools left behind by people leaving Africa, you wind up with a fairly solid theory that founder effects created our genetic bottleneck.
An Eruption That Launched Humanity
Though it’s likely that the genetic bottlenecks we observe in the human population were caused mostly by founder effects and sexual selection, there is some evidence that the final human radiation out of Africa was precipitated by a catastrophe. Ancient humans had been crossing the Sinai out of Africa and into the rest of the world for over a million years, but roughly 80,000 years ago there was an extremely large migration that changed the world and every human on it. H. sapiens, a human with language, clothing, and sophisticated tools, took over Africa, then migrated beyond its borders. Certainly it’s possible that this wave of human immigrants was spurred by mass deaths in the wake of the Toba eruption. But that’s debatable.
What’s certain is another explosion that nobody denies: the one in human symbolic communication. Our capacity for culture is what allowed us to survive in the perilous lands beyond the warm, fecund West African regions where Australopithecus first stood up. We never stayed in any one place for long. We moved into new places, founding new communities. And when we evolved complex symbolic intelligence, our growing facility with tools and language made these migrations easier. We could take advantage of many kinds of environments, teaching each other about their bounties and dangers in advance.
As H. sapiens poured off the continent of our birth, we discovered lands inhabited by our sibling hominins. We had to adapt to a world that already had humans in it. What came next will take us into one of the most controversial areas of population genetics and human evolutionary history.
