CHAPTER 4
Techniques for Measuring Endocrine Disruption in Insects
4.1.3 Endocrine Disruption and Insects
4.1.4 Development of Insecticides Targeting the Endocrine System
4.1 INTRODUCTION
This chapter explores the possibilities for studying endocrine disruption in insects by considering available mechanistic in silico, in vitro, and in vivo approaches, on one hand, and apical tests that yield information on adverse population-relevant effects, on the other. The unique position of the insects among the invertebrates is highlighted as well as their love-hate relationship with humans, which initiated the gathering of knowledge on their endocrine system.
4.1.1 Insects
Global biodiversity is dominated by the class Insecta, the largest group within the subphylum Hexapoda, which also contains the Collembola (springtails), Protura, and Diplura (for more taxonomic information, see the Tree of Life project at www.tolweb.org/Hexapoda). This chapter mainly considers the insects and the springtails, but for convenience they are considered together under the term “insects.”
It is estimated that, worldwide, between 6 and 10 million species of insects exist [1]. Many insect species are used by or considered useful to humans for various reasons, including honey production, silk production, controlling other pests, providing pollination services, use as a protein-rich food source, or use as fish bait (e.g., honeybees, bumblebees, butterflies and other pollinators, beneficial predatory insects, silkworms). However, even more species are regarded as pests, because they destroy or damage plants, harvests, wood in buildings and forests; may transfer diseases; or are parasites themselves (e.g., termites, fire ants, mosquitos, fleas, lice, bark beetles, flies, aphids, butterflies, moths, locusts).
Considering their overwhelming numbers and great diversity (hence their evolutionary success) and multifaceted relationship with humans [2], insects are a fascinating organism group for closer study.
4.1.2 Insect Hormones
In insects, as in other multicellular organisms, the processes of reproduction, growth, and development (including molting and metamorphosis) are under endocrine control. More specifically, there are three important groups of hormones—the ecdysteroids, the juvenile hormones (JHs), and the neuropeptides—all of which are transported through the insect via body fluid or hemolymph. A brief description of the three most important insect hormone groups [2] is provided next.
Ecdysone, the most prominent member of the ecdysteroids (i.e., steroids that promote molting), is released from the prothoracic glands into the hemolymph and is later converted to the active form 20-hydroxyecdysone. Ecdysteroids are also produced by the ovaries of adult female insects and may be involved in ovarian maturation or be packed in the eggs to be metabolized later during embryo cuticle formation. Comprehensive information on all aspects of ecdysone biochemistry and functions can be found in Smagghe [3].
The JHs are sesquiterpenoid compounds, which are produced by the corpora allata. They have two major roles: control of metamorphosis and regulation of reproductive development. JHs maintain larval characteristics; thus, adult development requires a molt in the absence of JH (otherwise the molt leads to the next larval stage). In the adult female insect, JH stimulates yolk deposition in eggs and affects pheromone production.
Finally, the neurohormones are a group of (mostly) peptide molecules originating mainly from the brain. These small molecule messengers are the master regulators of various aspects of insect development, homeostasis, metabolism, and reproduction, including the secretion of JHs and ecdysteroids. The vital and coordinating roles of the neuropeptides were not acknowledged until relatively recently, when techniques became available to study them. For more details on insect endocrinology, see LeBlanc et al. [4] and De Loof [5] and references therein.
4.1.3 Endocrine Disruption and Insects
In this chapter, the World Health Organization/International Programme on Chemical Safety (WHO/IPCS) [6] definition for what constitutes an endocrine disruptor is taken as a point of reference: “An endocrine disrupter is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations.” Considering that the protection goal for ecotoxicological risk assessments is the maintenance of populations and not the individual (as in toxicology), an endocrine disruptor is understood to be a substance that invokes adverse population relevant effects in intact organisms through an endocrine mechanism [7]. This definition explicitly excludes secondary effects, for example, those following primary effects on other targets, such as the liver. While for mammalian systems or, more generally for vertebrates, endocrine mechanisms are reasonably well understood, for invertebrates, this is not the case. Interestingly enough, within the group of invertebrates, most information on endocrine regulation of various processes is available for insects. On one hand, this is due to the long-standing culturing of useful insect species (silkworms, fruit flies, and honeybees) requiring detailed information on their biology and endocrinology; on the other hand, it is due to the development of third-generation insecticides to control harmful insect pests by interfering with their hormone system.
The silkworm (Bombyx mori, Lepidoptera) was domesticated from the species B. mandarina and has been cultured for at least 5,000 years in China [8]. The silkworm is completely dependent on humans, does not occur in the wild, and the adults cannot fly [9]. The silkworm has been widely used as a model species to study insect biology, including the endocrine system and pheromones. Also, the full silkworm genome has been published [10] (see http://silkbase.ab.a.u-tokyo.ac.jp). Despite its economic importance and comprehensive knowledge on its biology, no chronic toxicity testing protocol for silkworms is available. For regulatory purposes, acute silkworm studies are required in Asia, but these studies hardly provide information on endocrine-mediated processes and are thus not considered here.
Another factor that contributed to the knowledge on insect endocrine systems, is the extensive use of the fruit fly (Drosophila melanogaster, Diptera) in genetic research [11]. For instance, the fruit fly genome was published eight years before that of the silkworm [12] (see http://flybase.org). As a spin-off of the genetic research, some toxicity studies were conducted with D. melanogaster, but for some reason the species did not gain so much popularity as to develop a standardized toxicity test with it. However, genotoxicity assays have been developed using D. melanogaster. The classical D. melanogaster assay for mutagen testing is the sex-linked recessive lethal (SLRL) assay comprising two generations [13]. A more recent test is the somatic mutation and recombination test (SMART), also known as the wing spot assay, that applies one generation [14]. This short (i.e., about 14 days) in vivo test is based on the loss of heterozygosity in normal genes and the corresponding expression of recessive markers, namely multiple wing hairs and flare-3, in the wing blade of the adult flies. Despite the appropriate length of these testing protocols and the fact that these cover at least one generation, the end points are focused on detecting genotoxic activity of chemicals and thus the protocols are not suitable for studying endocrine-mediated effects. Nevertheless, the SLRL and SMART protocols could be used as a basis to develop a fruit fly life cycle test.
Due to the long-standing use and culturing of honeybees (Apis mellifera, Hymenoptera), the knowledge on insect physiology has been further enhanced. However, the honeybee is a social insect living in large colonies with only a single reproductively active female, and its biology is heavily dominated by this reproductive strategy, including specific hormonal processes controlling colony life. Therefore, the information on honeybees may have relevance for other social insects, but is limited for extrapolation to insects with other reproductive strategies. After D. melanogaster and the mosquito Anopheles gambiae, the honeybee was the third insect to have its genome fully sequenced and published [15] (see http://hymenopteragenome.org/beebase). Furthermore, due to its use in agriculture for pollination services and its potential exposure to plant protection products, several protocols have been designed for toxicity testing on the honeybee. These will be dealt with later in this chapter.
4.1.4 Development of Insecticides Targeting the Endocrine System
Ideally, targets for insecticides are specific to avoid broad side-effects on nontarget organisms. Yet the spectrum of target pests should not be too narrow from a commercial point of view. Besides the main target for insecticides, the nervous system, which is targeted by important insecticide chemistries such as the organophosphates, carbamates, pyrethroids, and neonicotinoids, the insect hormone system has been a target for the development of so-called third-generation insecticides.
The main targets for insecticide development were the JHs and the molting or ecdysteroid hormones [16]. These hormones regulate developmental processes such as molting, pupation, and metamorphosis in the juvenile insect and influence reproduction in the adult insect (see also Section 4.1.2). For ecdysone/20-hydroxyecdysone, it was also hypothesized that they could fulfill the role of sex hormones [17]. In discussing the processes of development, molting, and metamorphosis, it is noteworthy to mention that there are two distinct types of insect development: hemi-metabolism and holo-metabolism. Hemi-metabolic insects do not go through metamorphosis; the juvenile is called a nymph and resembles the adult (examples are grasshoppers, stink bugs, and dragonflies). Holo-metabolic insects go through a full metamorphosis; the larval stage is maggot-like and does not resemble the adult insect (examples are beetles, butterflies, flies, bees, ants). Obviously, interference with developmental and reproductive endocrine-mediated processes in insects offers good opportunities to control an insect pest. The aim of insecticide research would thus be to find agonists (mimics) or antagonists (inhibitors or blockers). As such, these compounds are designed endocrine disruptors for insects and may serve as reference compounds against which the effects of substances with an unknown mode of action can be compared.
The Insecticide Resistance Action Committee (IRAC) [18] has developed a system to describe and group substances based on their insecticidal mode of action. The insecticides interfering with endocrine processes in insects are part of the larger group of insect growth regulators (IGRs). However, not all IGRs interfere with endocrine processes. For instance, the benzoylureas (IRAC Group 15) inhibit the biosynthesis of chitin and thus lead to deadly molting events, but this is a recognized nonendocrine mode of action [4]. The focus of this chapter is on the IRAC groups 7 and 18, which are the juvenile hormone mimics, or juvenoids (e.g., fenoxycarb, pyriproxifen, epofenonane, methoprene, triprene, kinoprene, and hydroprene) and the ecdysone receptor agonists, or diacylhydrazines (e.g., halofenozide, chromafenozide, tebufenozide, and methoxyfenozide), respectively. The diacylhydrazines are highly lepidopteran specific, with considerably lower activity in other insect groups [19]. However, halofenozide is also used to control Coleoptera larvae. Interestingly, antagonists for JHs or the ecdysone receptor are not available commercially as insecticides [20]. However, antagonistic activity at the ecdysteroid receptor has been identified for several plant-derived compounds [21]. Also, (synthetic) anti-JH compounds, such as precocene, have been identified [4,22].
A special position has the natural plant compound azadirachtin (derived from the neem tree Azadirachta indica), which has multiple modes of action, including effects on reproduction and on ecdysteroid titers in the hemolymph, ultimately affecting the molting process [23] (see also http://neemfoundation.org). In a more general context, the codevelopment of plants and herbivorous insects leads to plants defending themselves by secondary plant compounds such as phyto-ecdysteroids. It has been shown that these compounds can be quite potent [24]. Insects on the other hand have no capacity for de novo sterol synthesis and are dependent on dietary uptake of sterol (e.g., phytosterols such as sitosterol), which is converted by insects into the required cholesterol [25]. Hence, there is an interesting relationship between plants and insects considering their needs [2]. A comprehensive overview of all known ecdysteroids, their molecular structure, and their biological activity is provided on http://ecdybase.org.
One of the advantages of targeting insect hormones is that JHs and ecdysteroids do not occur in vertebrates, so that side effects of insecticides derived from these hormones on mammals, birds, and fish are either absent or of a very mild nature. However, as JHs and ecdysteroids are also found in other groups of arthropods (see, e.g., Chapter 5 in this volume) and also in another group of Ecdysozoa (the nematodes [26]), these organisms might be affected by these compounds as well. Consequently, such potentially sensitive groups should be given due consideration in an environmental risk assessment.
Finally, a very different group of insect hormones-the pheromones-have also been considered as insecticides. However, the commercialized compounds are mostly directly derived from the target insects themselves and are as such not exogenous. It is merely the confusion of one of the sexes that prevents the formation of mating pairs and thereby prevents the oviposition of fertile eggs (mating disruption).
4.2 METHODS
This section explores the available methods that inform on endocrine mechanisms and effects in insects. The methods discussed range from mechanistic in silico to in vivo apical life cycle testing.
4.2.1 Mechanistic Assays
Mechanistic assays comprise an important part of the argumentation chain as they can provide information on the endocrine mechanism underlying the adverse effect that may be observed in apical tests (described in Section 4.2.2 and in Table 4.1). As such, mechanistic assays provide one of the key elements of the WHO/IPCS [6] definition that needs to be fulfilled for defining an endocrine disruptor. Only when the mechanism is causally related to the observed adverse effects an endocrine disruptor can be identified. However, in the reverse situation, a substance cannot be classified as a nonendocrine disruptor when the mechanism for the adverse effects is not elucidated. Mechanisms can be identified by in silico methods (i.e., by modeling) or calculating the binding affinity of a molecule for a certain target site (e.g., a receptor or enzyme). Further, in vitro methods (i.e., using cell lines or [parts of] organs of organisms) or in vivo methods (i.e., using whole animals in a specific and simple setup) can provide mechanistic information for single endocrine mechanisms.
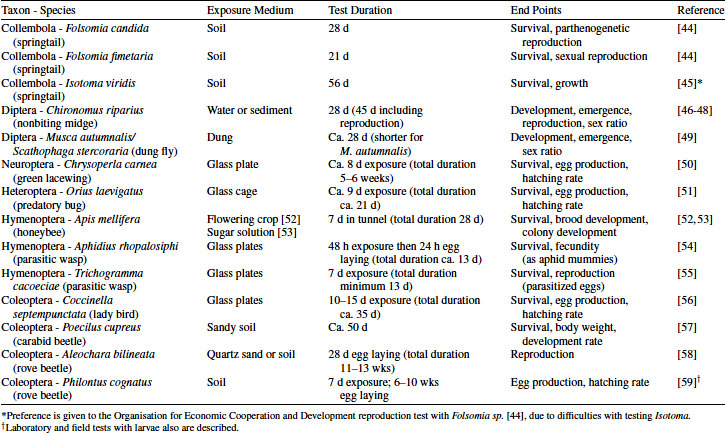
Table 4.1 Available test protocols for apical insect tests covering endocrine-mediated processes such as development and reproduction
In silico methods have been developed for both JH and ecdysone [27]. Usually these methods are based on some experimental data and molecular structures of the natural hormones and their receptors. The model can be a simple (quantitative) structure activity relation ([Q]SAR), but more sophisticated 3-D modeling of molecular structures in the binding pocket of a receptor is possible. All these elements were used to analyze inhibitors of the juvenile hormone epoxide hydrolase of the tobacco hornworm, Manduca sexta [28]. Similarly, ecdysone agonists were studied by means of a reporter gene assay and 3-D (Q)SAR analysis [29]. Finally, it is possible to conduct virtual screening for insect molting hormone receptor ligands [30]. This list of in silico methods is not exhaustive but merely illustrates that this field of insect physiology has progressed significantly and may help in selecting compounds for further testing in in vitro or in vivo systems.
The next level is the in vitro test. A test in which many compounds have been examined for ecdysteroid or anti-ecdysteroid activity is the D. melanogaster BII permanent cell line assay [24,31,32]. Soin et al. [19] developed a new D. melanogaster-based screening test for (anti-)ecdysone activity of chemicals that was found to be less prone to false positives than the BII cell line assay. Similar screening tests based on the activation of an ecdysone-responsive reporter gene exist for Lepidoptera (B. mori) [33] and Coleoptera (Anthonomus grandis) [34]. Hence, through these assays, differences between dipteran, coleopteran, and lepidopteran ecdysone activity can be studied. In Japan, the ecdysone receptor of the crustacean Daphnia magna was cloned and demonstrated great similarity with the ecdysone receptor of Drosophila [35]. This Daphnia magna-based assay is likely going to be used in screening chemicals for potential endocrine activity. Therefore, as an in vitro screening method, the D. magna assay may yield relevant information for insects.
Finally, in vivo mechanistic tests provide the most relevant information as they are derived from a system where toxicokinet factors, such as absorption, distribution, metabolism and excretion (ADME) are active, which are typically missing in in vitro systems. The oldest available assay (originally developed in 1935) for identifying ecdysone activity is the pupariation assay using third instar blowfly (Calliphora sp.) larvae [4,36]. By ligating the fly larvae at a specific age, its abdominal part will not further develop (darken) unless exposed to ecdysone. By injecting a minute amount of ecdysone (or possibly an ecdysone mimic or also hemolymph of a normally developing pupa), the abdominal part of the ligated larva will start developing. Ecdysone antagonistic activity could be studied by injecting the anterior part of the larva and studying the inhibition of pupariation. Instead of injecting, a brief immersion of either larval part is also possible. Another in vivo assay that reacts quite specific on JH activity is the production of male brood in the crustacean D. magna, which normally only produces female brood [37]. Although this test is conducted with arthropod crustaceans, the tested JH analog compounds were targeted to interfere with the insect endocrine system. The final test described here is the honeybee (Apis mellifera) larval test [38, 39]. Confusingly the test is called an in vitro method, which is probably related to the fact that bees are living in a colony with one queen and from a biological perspective the colony can be regarded as a meta-organism. In any case, from the data presented on the JH mimic fenoxycarb, typical inhibitory effects on emergence of honeybees were observed (developmental halt), while there were no effects on the larvae [39]. To use the results of the assay as mechanistic proof, more data on JH mimics need to be generated to study if the effect pattern is unique.
4.2.2 Apical Assays
Apical assays are in vivo test methods that typically address effects on insect survival, growth, development, or reproduction. These ecotoxicological tests are commonly conducted as part of plant protection product and biocide regulatory data requirements for registration purposes. Apical tests usually progress through tiers, starting with simple short-term acute tests, then long-term chronic tests, progressing to full life cycle tests and then multigeneration or population testing. While lower-tier testing usually is confined to the laboratory, higher-tier testing often is conducted under semifield or field conditions. This progress ensures the increase of realism in the test and a more ecologically relevant characterization of toxicity. To investigate endocrine disruption in invertebrates, a life cycle test is still considered the gold standard as it covers all (endocrine-mediated) processes in an organism’s life [40]. By yielding information on adverse population-relevant effects, apical tests provide another key element of the WHO/IPCS [6] definition that need to be fulfilled for defining an endocrine disruptor.
For insects, standardized chronic test protocols are available mainly for terrestrial species, namely for springtails, nontarget arthropods (NTAs, mostly beneficials, some of which are insects, but also include mites and spiders [41,42]), honeybees, and dung flies (to cover a relevant exposure route for veterinary pharmaceuticals). The only aquatic insects represented with standardized chronic tests are the chironomids, or nonbiting midges [43]. Species of these taxonomic groups are represented: Coleoptera, Collembola, Diptera, Heteroptera, Hymenoptera, and Neuroptera. For some species, several chronic tests are available, which may differ in length, studied end points, or tested exposure scenario. Apart from sexually reproducing species with sex ratios of about 1:1, there are tests with parthenogenetically reproducing species (Folsomia candida) and with social insects with only a single egg-laying female (Apis mellifera). Some of the presented Hymenopteran species are parasitic and have a fascinating biology with metamorphosis within other insects (e.g., aphids). Table 4.1 summarizes the available tests.
4.3 DISCUSSION
4.3.1 Mechanism and Effect
In the end, the difficulty with establishing endocrine disruption for any substance is to establish the link between an adverse population-relevant effect and the disruption of an endocrine mechanism causing that effect. This is not specific for insects but an overarching difficulty in the field of endocrine disruption. To deal with adverse effects in a risk assessment is common practice and as such is not particularly difficult. It is usually problematic to identify the mechanism responsible for the effect, as often this type of information is not required (in a regulatory context) or simply is lacking. In the case of insects, some methods to establish mechanistic information are available, ranging from in silico, via in vitro tests, to in vivo assays (see Section 4.2.1). Furthermore, due to the development of insecticides targeting the insect endocrine system, reference chemicals are available that invoke effect patterns typical for a certain mode of action (e.g., JH agonist or ecdysteroid agonist). Alternately, by simultaneously applying the test chemical of interest with a reference compound-say, a suspected JH antagonist with a JH mimic, such as fenoxycarb-one can study whether effects are indeed negated, thereby providing further evidence for a particular mode of action.
Clearly the possibility of differentiation between chemicals depends on the specificity of the end point. For example, mortality might be related to endocrine disruption when it is caused by a lethal molting event. However, since mortality is such a high-level end point, which integrates all kinds of toxic responses on a whole organism level, it will respond to most chemical stressors when the exposure concentrations are high enough. To differentiate between possible endocrine and nonendocrine causes for mortality, detailed information on when exactly during the test the mortality occurred (larval, pupal, or adult stage) and if molting or metamorphosis events including eclosion were successful.
Similarly, for the developmental parameters, it would be helpful to document the duration of the larval stages or alternately the molting frequency, which may give hints about JH activity.
4.3.2 Exposure Considerations
In the conduct of a risk assessment, the exposure is equally important as the toxicity (or hazard). Therefore, it makes sense to briefly consider some peculiarities regarding exposure of insects during the different phases of their life cycle. There may exist large differences between the exposure of adult and larval stages of holo-metabolic insects in particular. The differences may be due to preferential food type and/or to the environment in which the adult, respectively the larvae live. Also, it is the main purpose of the larvae to eat and gather energy for metamorphosis among other energetically costly processes, while the adults need to find a mating partner and a suitable habitat to lay their eggs. In some species, the adults use up the energy stored during the larval phase and take up little or no food themselves. Other species have clear energy demands in both larval and adult stages, and both practice an active food uptake (e.g., dragonflies).
A significant number of insects have adults confined to the terrestrial environment while their larvae reside in the water phase. Typical examples are damselflies and dragonflies (Odonata), caddisflies (Trichoptera), stoneflies (Plecoptera), mayflies (Ephemeroptera), but also Diptera like chironomids [2]. Often this difference in living environment is accompanied by a difference in food type (e.g., aquatic chironomid larvae feed on detritus while terrestrial adults require little food and feed on nectar or aphid dew). Hence, the exposure situation of these different life stages within one species can be radically different. Similarly for terrestrial holo-metabolic insects, the larvae may be living in and feeding on organic soil particles, rotten wood, or even dead meat, while the adults are free flying and live in the soil litter layer or on foliage.
Even when larvae and adults live in the same environment, their food type can be quite different. Examples of this scenario are some species of Lepidoptera of which the larvae (caterpillars) are herbivores feeding on leaves while the adult butterfly feeds on flower nectar. Even more contrasting are the preferential food types of lacewings such as Chrysoperla carnea, where the carnivorous juvenile feeds on aphids, while the herbivorous adult feeds on pollen, nectar, and honeydew [60].
Another aspect is the potential exposure duration of the different life stages. In some species the larval stage and in others the adult stage is the longer life phase. Further, in temperate regions, the stage overwintering may be the larvae (e.g., fourth instar chironomid larvae in the water) while in other species it is the adult (e.g., lacewings or lady bird beetles). Obviously, the inherent sensitivity of the various life stages (egg, larva, pupa, adult) may differ, depending on size, permeability of shell, cuticle, or exoskeleton, and physiology. An example of size-dependent sensitivity is the decreasing sensitivity of chironomid larvae with increasing size (i.e., instar) [61].
For hemi-metabolous insects, the differences in exposure between the life stages are expected to be much less pronounced, due to the similarity between the adult and the nymphs.
As a final note on exposure, insects may transfer endocrine-active chemicals up the food chain (e.g., to birds or bats), as was demonstrated in a study on aerial invertebrates at a sewage treatment works [62].
4.4 CONCLUSION
Insects have a very prominent position in global ecosystems, due not only to their numerical dominance and enormous diversity, but also to their contribution of species that fulfill key roles in these systems. In the past decade, significant progress has been made on understanding the endocrine systems that influence insect growth, development, behavior, and reproduction. Combining mechanistic information and knowledge on the effect patterns caused by exposure to insecticides with a known endocrine mode of action with available apical testing protocols provides excellent opportunities for progressing endocrine disrupter research in insects. It was noted that freshwater insects are underrepresented when it comes to available apical standard testing protocols, as there is only one method available using chironomids, while all others employ terrestrial species. Considering all of the available information and testing methods, insects have a unique position within the invertebrates.
4.5 ACKNOWLEDGMENTS
Thanks are due to my colleagues Nicole Hanewald for providing honeybee and non-target arthropod testing protocols and to Juergen Langewald and two anonymous reviewers for constructive comments.
REFERENCES
1. Chapman, A. D. (2006). Numbers of Living Species in Australia and the World. Australian Biological Resources Study, Canberra, Australia. 60 pp.
2. Gullan, P. J., Cranston, P. S. (2000). The Insects: An Outline of Entomology, 2nd ed. Blackwell Science, Oxford, UK.
3. Smagghe, G. (ed.). (2009). Ecdysone: Structure and Functions. Springer, Berlin, Germany.
4. LeBlanc, G. A., Campbell, P. M., den Besten, P., Brown, R. P., Chang, E. S., Coats, J. R., deFur, P. L., Dhadialla, T., Edwards, J., Riddiford, L. M., Simpson, M. G., Snell, T. W., Thorndyke, M., Matsumura, F. (1999). The endocrinology of invertebrates. In: deFur, P. L., Crane, M., Ingersoll, C. G., Tattersfield, L. J. (eds.), Endocrine Disruption in Invertebrates: Endocrinology, Testing, and Assessment. SETAC, Pensacola, FL, pp. 23–106.
5. De Loof, A. (2008). Ecdysteroids, juvenile hormone and insect neuropeptides: Recent successes and remaining major challenges. General and Comparative Endocrinology 155: 3–13.
6. World Health Organization/International Programme on Chemical Safety (2002). Global Assessment of the State-of-the-Science of Endocrine Disruptors. WHO, Geneva, Switzerland.
7. Bars, R., Broeckaert, F., Fegert, I., Gross, M., Hallmark, N., Kedwards, T., Lewis, D., O’Hagan, S., Panter, G. H., Weltje, L., Weyers, A., Wheeler, J. R., Galay-Burgos, M. (2011). Science based guidance for the assessment of endocrine disrupting properties of chemicals. Regulatory Toxicology and Pharmacology 59: 37–46.
8. Barber, E. J. W. (1992). Prehistoric Textiles: The Development of Cloth in the Neolithic and Bronze Ages with Special Reference to the Aegean. Princeton University Press, Princeton, NJ.
9. Kanzaki, R. (1998). Coordination of wing motion and walking suggests common control of zigzag motor program in a male silkworm moth. Journal of Comparative Physiology A, 182: 267–276.
10. International Silkworm Genome Consortium (2008). The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochemistry and Molecular Biology 38: 1036–1045.
11. Toivonen, J. M., Partridge, L. (2009). Endocrine regulation of aging and reproduction in Drosophila. Molecular and Cellular Endocrinology 299: 39–50.
12. Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F., et al. (2000). The genome sequence of Drosophila melanogaster. Science 287: 2185–2195.
13. U.S. Environmental Protection Agency. (1998). OPPTS Harmonized Test Guidelines, Series 870 Health Effects Test Guidelines, 870.5275, Sex-linked Recessive Lethal Test in Drosophila melanogaster. EPA, Office of Prevention, Pesticides and Toxic Substances, Washington, DC.
14. Andrade, H. H. R., Reguly, M. L., Lehmann, M. (2004). Wing somatic mutation and recombination test (SMART). In: Henderson, D. S. (ed.), Drosophila Cytogenetics Protocols, Humana Press, Totowa, NJ, pp. 389–412.
15. Honey Bee Genome Sequencing Consortium. (2006). Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443: 931–949.
16. Soin, T., Verslycke, T., Janssen, C., Smagghe, G. (2009). Ecdysteroids and their importance in endocrine disruption research. In: Smagghe, G. (ed.), Ecdysone: Structure and functions. Springer, Berlin, Germany, pp. 539–549.
17. De Loof, A., Huybrechts, R. (1998). “Insects do not have sex hormones”: A myth? General and Comparative Endocrinology 111: 245–260.
18. Insecticide Resistance Action Committee. (2009). Mode of action classification poster Version 6.3. Available at www.irac-online.org.
19. Soin, T., Swevers, L., Kotzia, G., Iatrou, K., Janssen, C. R., Rougé, P., Harada, T., Nakagawa, Y., Smagghe, G. (2010). Comparison of the activity of non-steroidal ecdysone agonists between dipteran and lepidopteran insects, using cell-based EcR reporter assays. Pest Management Science 66: 1215–1229.
20. Soin, T., Smagghe, G. (2007). Endocrine disruption in aquatic insects: A review. Ecotoxicology 16: 83–93.
21. Dinan, L., Whiting, P., Girault, J.-P., Lafont, R., Dhadialla, T. S., Cress, D. E., Mugat, B., Antoniewski, C., Lepesant, J.-A. (1997). Cucurbitacins are insect steroid hormone antagonists acting at the ecdysteroid receptor. Biochemical Journal 327: 643–650.
22. Furuta, K., Fujita, N., Ibushi, T., Shiotsuki, T., Yamada, N., Kuwano, E. (2010). Synthesis and anti-juvenile hormone activity of ethyl 4-[(6-substituted 2,2-dimethyl-2H-chromen-7-yl)methoxy]benzoates. Journal of Pesticide Science 35: 405–411.
23. Mordue (Luntz), A. J., Nisbet, A. J. (2000). Azadirachtin from the neem tree Azadirachta indica: Its action against insects. Anais da Sociedade Entomológica do Brasil 29: 615–632.
24. Dinan, L., Savchenko, T., Whiting, P., Sarker, S. D. (1999). Plant natural products as insect steroid receptor agonists and antagonists. Journal of Pest Science 55: 331–335.
25. Ikekawa, N., Morisaki, M., Fujimoto, Y. (1993). Sterol metabolism in insects: Dealkylation of phytosterol to cholesterol. Accounts of Chemical Research 26: 139–146.
26. Höss, S., Weltje, L. (2007). Endocrine disruption in nematodes: Effects and mechanisms. Ecotoxicology 16: 15–28.
27. Nakagawa, Y., Hormann, R. E., Smagghe, G. (2009). SAR and QSAR studies for in vivo and in vitro activities of ecdysone agonists. In: Smagghe, G. (ed.), Ecdysone: Structure and Functions. Springer, Berlin, Germany, pp. 475–509.
28. Garriga, M., Caballero, J. (2011). Insights into the structure of urea-like compounds as inhibitors of the juvenile hormone epoxide hydrolase (JHEH) of the tobacco hornworm Manduca sexta: Analysis of the binding modes and structure–activity relationships of the inhibitors by docking and CoMFA calculations. Chemosphere 82: 1604–1613.
29. Wheelock, C. E., Nakagawa, Y., Harada, T., Oikawa, N., Akamatsu, M., Smagghe, G., Stefanou, D., Iatrou, K., Swevers, L. (2006). High-throughput screening of ecdysone agonists using a reporter gene assay followed by 3-D QSAR analysis of the molting hormonal activity. Bioorganic & Medicinal Chemistry Letters 14: 1143–1159.
30. Harada, T., Nakagawa, Y., Ogura, T., Yamada, Y., Ohe, T., Miyagawa, H. (2011). Virtual screening for ligands of the insect molting hormone receptor. Journal of Chemical Information and Modeling 51: 296–305.
31. Clément, C. Y., Bradbrook, D. A., Lafont, R., Dinan, L. (1993). Assessment of a microplate-based bioassay for the detection of ecdysteroid-like or antiecdysteroid activities. Insect Biochemistry and Molecular Biology 23: 187–193.
32. Dinan, L., Bourne, P., Whiting, P., Dhadialla, T. S., Hutchinson, T. H. (2001). Screening of environmental contaminants for ecdysteroid agonist and antagonist activity using the Drosophila melanogaster BII cell in vitro assay. Environmental Toxicology and Chemistry 20: 2038–2046.
33. Swevers, L., Kravariti, L., Ciolfi, S., Xenou-Kokoletsi, M., Ragoussis, N., Smagghe, G., Nakagawa, Y., Mazomenos, B., Iatrou, K. (2004). A cell-based high-throughput screening system for detecting ecdysteroid agonists and antagonists in plant extracts and libraries of synthetic compounds. FASEB Journal 18: 134–136.
34. Soin, T., Iga, M., Swevers, L., Rougé, P., Janssen, C. R., Smagghe, G. (2009). Towards Coleoptera-specific high-throughput screening systems for compounds with ecdysone activity: Development of EcR reporter assays using weevil (Anthonomus grandis)-derived cell lines and in silico analysis of ligand binding to A. grandis EcR ligand-binding pocket. Insect Biochemistry and Molecular Biology 39: 523–534.
35. Kato, Y., Kobayashi, K., Oda, S., Tatarazako, N., Watanabe, H., Iguchi, T. (2007). Cloning and characterization of the ecdysone receptor and ultraspiracle protein from the water flea Daphnia magna. Journal of Endocrinology 193: 183–194.
36. Fraenkel, G., Zdarek, J. (1970). The evaluation of the “Calliphora test” as an assay for ecdysone. Biological Bulletin 139: 138–150.
37. Tatarazako, N., Oda, S., Watanabe, H., Morita, M., Iguchi, T. (2003). Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere 53: 827–833.
38. Aupinel, P., Fortini, D., Dufour, H., Tasei, J.-N., Michaud, B., Odoux, J.-F., Pham-Delègue, M.-H. (2005). Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bulletin of Insectology 58: 107–111.
39. Aupinel, P., Fortini, D., Dufour, H., Michaud, B., Marolleau, F., Tasei, J. N., Odoux, J. F. (2007). Toxicity of dimethoate and fenoxycarb to honey bee brood (Apis mellifera), using a new in vitro standardized feeding method. Pest Management Science 63: 1090–1094.
40. Ingersoll, C. G., Hutchinson, T., Crane, M., Dodson, S., DeWitt, T., Gies, A., Huet, M.-C., McKenney, C. L. Jr., Oberdörster, E., Pascoe, D., Versteeg, D. J., Warwick, O. (1999). Laboratory toxicity tests for evaluating potential effects of endocrine-disrupting compounds. In: deFur, P. L., Crane, M., Ingersoll, C. G., Tattersfield, L. J. (eds.), Endocrine Disruption in Invertebrates: Endocrinology, Testing, and Assessment. SETAC, Pensacola, FL, pp. 107–197.
41. Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.). (2000). Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium. 158 pp.
42. Candolfi, M. P., Barrett, K. L., Campbell, P., Forster, R., Grandy, N., Huet, M.-C., Lewis, G., Oomen, P. A., Schmuck, R., Vogt, H. (2001). Guidance document on regulatory testing and risk assessment procedures for plant protection products with non-target arthropods. Report of the SETAC/ESCORT2 Workshop. Wageningen, the Netherlands, SETAC Europe, Brussels, Belgium.
43. Taenzler, V., Bruns, E., Dorgerloh, M., Pfeifle, V., Weltje, L. (2007). Chironomids: Suitable test organisms for risk assessment investigations on the potential endocrine disrupting properties of pesticides. Ecotoxicology 16: 221–230.
44. Organisation for Economic Cooperation and Development. (2009). OECD guidelines for the testing of chemicals. Collembolan Reproduction Test in Soil. No. 232. OECD, Paris, France.
45. Wiles, J. A., Krogh, P. H. (1998). Tests with the Collembolans Isotoma viridis, Folsomia candida and Folsomia fimetaria. In: Løkke, H., van Gestel, C. A. M. (eds.), Handbook of Soil Invertebrate Toxicity Tests. John Wiley & Sons, Chichester, UK, pp. 131–156.
46. Organisation for Economic Cooperation and Development. (2004). OECD guidelines for the testing of chemicals. Sediment-Water Chironomid Toxicity Test Using Spiked Sediment. No. 218. OECD, Paris, France.
47. Organisation for Economic Cooperation and Development. (2004). OECD guidelines for the testing of chemicals. Sediment-Water Chironomid Toxicity Test Using Spiked Water. No. 219. OECD, Paris, France.
48. Organisation for Economic Cooperation and Development. (2010). OECD guidelines for the testing of chemicals. Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment. No. 233. OECD, Paris, France.
49. Organisation for Economic Cooperation and Development. (2008). OECD guidelines for the testing of chemicals. Determination of developmental toxicity of a test chemical to Dipteran dung flies (Scathophaga stercoraria L. (Scathophagidae), Musca autumnalis De Geer (Muscidae)). No. 228. OECD, Paris, France.
50. Vogt, H., Bigler, F., Brown, K., Candolfi, M. P., Kemmeter, F., Kühner, Ch., Moll, M., Travis, A., Ufer, A., Viñuela, E., Waldburger, M., Waltersdorfer, A. (2000). Laboratory method to test effects of plant protection products on larvae of Chrysoperla carnea (Neuroptera: Chrysopidae). In: Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.), Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium, pp. 27–44.
51. Bakker, F. M., Aldershof, S. A., van de Veire, M., Candolfi, M. P., Izquierdo, J. I., Kleiner, R., Neumann, Chr., Nienstedt, K. M., Walker, H. (2000). A laboratory test for evaluating the effects of plant protection products on the predatory bug, Orius laevigatus (Fieber) (Heteroptera: Anthocoridae). In: Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.), Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium, pp. 57–70.
52. Organisation for Economic Cooperation and Development. (2007). Guidance document on the honeybee (Apis mellifera L.) brood test under semi-field conditions. OECD Environment, Health and Safety Publications, Series on Testing and Assessment No. 75, ENV/JM/MONO 22.
53. Oomen, P. A., de Ruijter, A., van der Steen, J. (1992). Method for honeybee brood feeding tests with insect growth–regulating insecticides. Bulletin OEPP/EPPO Bulletin 22: 613–616.
54. Mead-Briggs, M. A, Brown, K., Candolfi, M. P., Coulson, M. J. M., Miles, M., Moll, M., Nienstedt, K., Schuld, M., Ufer, A., McIndoe, E. (2000). A laboratory test for evaluating the effects of plant protection products on the parasitic wasp, Aphidius rhopalosiphi (DeStephani-Perez) (Hymenoptera: Braconidae). In: Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.), Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium, pp. 13–25.
55. Hassan, S. A., Halsall, N., Gray, A. P., Kuehner, C., Moll, M., Bakker, F. M., Roembke, J., Yousef, A., Nasr, F., Abdelgader, H. (2000). A laboratory method to evaluate the side effects of plant protection products on Trichogramma cacoeciae Marchal (Hymenoptera: Trichogrammatidae). In: Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.), Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium, pp. 107–117.
56. Schmuck, R., Candolfi, M. P., Kleiner, R., Mead-Briggs, M., Moll, M., Kemmeter, F., Jans, D., Waltersdorfer, A., Wilhelmy, H. (2000). A laboratory test system for assessing effects of plant protection products on the plant dwelling insect Coccinella septempunctata L. (Coleoptera: Coccinellidae). In: Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.), Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium, pp. 45–56.
57. Heimbach, U., Baier, B., Barth, M., Blümel, S., Geuijen, I., Jäckel, B., Maus, C., Nienstedt, K. M., Schmitzer, S., Stäbler, P., Ufer, A., Winkelmann, G. (2002). First ring test results of a laboratory method to evaluate effects of plant protection products on larvae of Poecilus cupreus (Coleoptera: Carabidae). Pesticides and beneficial organisms. IOBC/WPRS Bulletin 25: 19–26.
58. Grimm, C., Reber, B., Barth, M., Candolfi, M. P., Drexler, A., Maus, C., Moreth, L., Ufer, A., Waltersdorfer, A. (2000). A test for evaluating the chronic effects of plant protection products on the rove beetle Aleochara bilineata Gyll. (Coleoptera: Staphylinidae) under laboratory and extended laboratory conditions. In: Candolfi, M. P., Blümel, S., Forster, R., Bakker, F. M., Grimm, C., Hassan, S. A., Heimbach, U., Mead-Briggs, M. A., Reber, B., Schmuck, R., Vogt, H. (eds.), Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods. IOBC, BART and EPPO Joint Initiative. IOBC/WPRS, Gent, Belgium, pp. 1–12.
59. Metge, K., Heimbach, U. (1998). Tests on the Staphylinid Philonthus cognatus Steph. 1832. In: Løkke, H., van Gestel, C. A. M. (eds.), Handbook of Soil Invertebrate Toxicity Tests. John Wiley & Sons, Chichester, UK, pp. 157–179.
60. Sattar, M. (2010). Investigations on Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) as a biological control agent against cotton pests in Pakistan. PhD dissertation, Sindh Agriculture University Tandojam, Pakistan.
61. Larrain, A., Riveros, A., Bay-Schmith, E., Roa, R. (1997). Evaluation of three larval instars of the midge Chironomus petiolatus as bioassay tools using a computationally intensive statistical algorithm. Archives of Environmental Contamination and Toxicology 33: 407–414.
62. Park, K. J., Müller, C. T., Markman, S., Swinscow-Hall, O., Pascoe, D., Buchanan, K. L. (2009). Detection of endocrine disrupting chemicals in aerial invertebrates at sewage treatment works. Chemosphere 77: 1459–1464.