CHAPTER 12
Application of the OECD Conceptual Framework for Assessing the Human Health and Ecological Effects of Endocrine Disrupters
12.2 OVERVIEW OF THE OECD REVISED CF
12.3 APPLICATION OF THE KLIMISCH CRITERIA TO THE EE2 AND VIN CASE STUDIES
12.4 CASE STUDY: DATA EXAMPLES FOR 17α-ETHYNYLESTRADIOL
12.4.1 EE2 Case Study: Level 1 Information
12.4.2 EE2 Case Study: Level 2 Information
12.4.3 EE2 Case Study: Level 3 Information
12.4.4 EE2 Case Study: Level 4 Information
12.4.5 EE2 Case Study: Level 5 Information
12.5 CASE STUDY: DATA EXAMPLES FOR VINCLOZOLIN
12.5.1 VIN Case Study: Level 1 Information
12.5.2 VIN Case Study: Level 2 Information
12.5.3 VIN Case Study: Level 3 Information
12.5.4 VIN Case Study: Level 4 Information
12.5.5 VIN Case Study: Level 5 Information
12.1 INTRODUCTION
While scientific concern about reproductive health impacts of certain chemicals or pharmaceuticals goes back several decades in terms of human health [1,2] and wild-life populations [3,4], it was not until the 1990s that wider awareness of this issue grew beyond the scientific community. In the 1990s, for example, the United States passed the Food Quality Protection Act (1996) and amended the Safe Drinking Water Act (1996), which mandated the U.S. Environmental Protection Agency (EPA) to develop the Endocrine Disruptor Screening Program (EDSP) [5]. Similarly, the Japanese government established in 1998 the Strategic Program on Environmental Endocrine Disruptors [6], with similar initiatives taking place in many European countries and through the European Union Framework Research Programmes. In the same period, the Organisation for Economic Cooperation and Development (OECD) took the initiative at the twenty-fifth Joint Meeting of the OECD Environmental Health and Safety Program (November 1996) to establish a special activity on Endocrine Disrupter Testing and Assessment (EDTA). This initiative was made at the request of OECD member countries and international industry to ensure that testing and assessment approaches would not differ substantially among countries. The focus of the EDTA activity was agreed to: (1) provide information on and coordinate national and regional activities concerning endocrine disruption assessment; (2) develop new, and revise existing, OECD test guidelines to detect endocrine disrupters; and (3) harmonize hazard and risk assessment approaches for endocrine disrupters [7].
Building on major cooperative efforts by OECD member countries and international industry since 1996, today a number of new OECD test guidelines have been successfully developed and validated for mammals, amphibians, and fish (see other chapters for further details). Reflecting the priority given to the reproductive and developmental processes studied in many published papers, the OECD test guidelines have prioritized an assessment of androgenic, estrogenic, steroidogenic disruption, and thyroid-related end points in various model species (see other chapters for further details on the endocrine end points used in specific assays and test guidelines). At the same time, the OECD is developing and refining a conceptual framework (CF) to provide guidance concerning the newly adopted test guidelines or those that might need to be developed in the future and what types of information they are provide with respect to endocrine disrupter assessment. Originally devised at the sixth OECD EDTA meeting in 2002, the CF is intended to apply to both new and existing substances in different chemical sectors, such as pharmaceuticals, industrial chemicals, and pesticides. The CF was updated in 2011 to take into account the progress made in validating new in vitro, mammalian, and (ecological) nonmammalian test guidelines over the past seven years (see Table 12.1). An OECD guidance document on the standardized test guidelines for evaluating chemicals for endocrine disruption has recently been developed to assist with interpretation and use of the assays in the CF [8]. Since the underlying scientific principles of using data from different in vivo animal models or in vitro assays are similar across national and international jurisdictions, this chapter focuses on the OECD CF to illustrate its structure and utility.
Table 12.1 2011 OECD Revised Conceptual Framework for Testing and Assessment of Endocrine Disrupters
| Mammalian and Nonmammalian Toxicology | ||
| Level 1
Existing data and nontest information |
Physical and chemical properties (e.g., MW reactivity, volatility, biodegradability)
All available (eco)toxicological data from standardized or nonstandardized tests Read across, chemical categories, (Q)SARs and other in silico predictions, and ADME model predictions |
|
| Level 2
In vitro assays providing data about selected endocrine mechanism(s)/pathways(s) (mammalian and nonmammalian methods) |
Estrogen or androgen receptor binding affinity
Estrogen receptor transcriptional activation (OECD TG 455) Androgen or thyroid transcriptional activation (If/when TGs are available) Steroidogenesis in vitro (OECD TG 456) MCF-7 cell proliferation assays (ER ant/agonist) Other assays as appropriate |
|
| Mammalian Toxicology | Nonmammalian Toxicology | |
| Level 3
In vivo assays providing data about selected endocrine mechanism(s)/pathway(s)1 |
Uterotrophic assay (OECD TG 440)
Hershberger assay (OECD TG 441) |
Xenopus embryo thyroid signaling assay (when/if TG is available)
Amphibian metamorphosis assay (OECD TG 231) Fish Reproductive Screening Assay (OECD TG 229) Fish Screening Assay (OECD TG 230) Androgenized female stickleback screen (GD 140) |
| Level 4
In vivo assays providing data on adverse effects on endocrine-relevant end points2 |
Repeated dose 28-day study (OECD TG 407)
Repeated dose 90-day study (OECD TG 408) First-generation assay (OECD TG 415) Male pubertal assay (see GD 150)3 Female pubertal assay (see GD 150)3 Intact adult male endocrine screening assay (see GD 150) Prenatal developmental toxicity study (OECD TG 414) |
Fish sexual development test (Draft TG 234)
Fish Reproduction Partial Life Cycle Test (when/if TG is Available) Larval Amphibian Growth and Development Assay (when TG is available) Avian Reproduction Assay (TG 206) Mollusc Partial Life Cycle Assays (when TG is available)4 |
| Chronic toxicity and carcinogenicity studies (OECD TG 451-3)
Reproductive screening test (OECD TG 421 if enhanced) Combined 28-day/reproductive screening assay (OECD TG 422 if enhanced) Developmental neurotoxicity (TG 426) |
Chironomid Toxicity Test (TG 218–219)4 | |
| Level 5
In vivo assays providing more comprehensive data on adverse effects on endocrine relevant end points over more extensive parts of the life cycle of the organism2 |
Extended one-generation reproductive Toxicity Study (OECD TG 443)
Two -generation assay (OECD TG 416 most recent update) |
FLCTT (Fish Life Cycle Toxicity Test) (when TG is available)
Medaka Multigeneration Test (MMGT) (when TG is available) Avian two-generation reproductive toxicity assay (when TG is available) Mysid Life Cycle Toxicity Test (when TG is available)4 Copepod Reproduction and Development Test (when TG is available)4 Sediment Water Chironomid Life Cycle Toxicity Test (TG 233)4 Mollusc Full Life Cycle Assays (when TG is available)4 Daphnia Reproduction Test (with male induction) (OECD TG 211)4 Daphnia Multigeneration Assay (if TG is available)4 |
| Guidance notes for Table 12.1: (1) Entering at all levels and exiting at all levels is possible and depends on the nature of existing information and needs for testing and assessment. (2) The assessment of each chemical should be based on a case-by-case basis, taking into account all available information, bearing in mind the function of the framework levels. Explanatory notes for Table 12.1: 1Some assays may also provide some evidence of adverse effects. 2Effects can be sensitive to more than one mechanism and may be due to non-ED mechanisms. 3Depending on the guideline/protocol used, the fact that a substance may interact with a hormone system in these assays does not necessarily mean that when the substance is used, it will cause adverse effects in humans or ecological systems. 4At present, the available invertebrate assays solely involve apical end points that are able to respond to some EDs and some non-EDs (those in Level 4 are partial life cycle tests, while those in Level 5 are full or multiple life cycle tests). 5The new EOGRT study (OECD TG 443) is preferable for detecting endocrine disruption because it provides an evaluation of a number of endocrine end points in the juvenile and adult F1, which are not included in the second-generation study (OECD TG 416) adopted in 2001.1 |
||
12.2 OVERVIEW OF THE OECD REVISED CF
As agreed initially in 2002 and in the 2011 update, the OECD CF agreed is not a testing scheme but rather represents a pragmatic hierarchy in which the various tests that can contribute information for the detection of the hazards of endocrine disruption are placed. The tool box was originally organized into five levels, each corresponding to a different level of biological complexity for both human health (mammalian) and ecological (nonmammalian) areas. The OECD CF is also not an endocrine disrupter testing strategy. Importantly, any test at any level can be conducted as questions arise, so that it is not necessary to follow the CF in a rigid or linear manner. Instead, the CF should be used flexibly depending on the nature of existing information and the needs for testing and assessment. Entering is possible at all five levels and depends on the nature of existing information and needs for testing and assessment. Also, the assessment of each chemical should be on a case-by-case basis, taking into account all available information, bearing in mind the function of the framework levels. Briefly, examples of information that can be collated at the different levels of the CF are:
In order to illustrate the principles that are used here to underpin the application of the OECD CF for analysis of assay data, two case studies are presented in this chapter. We have used two well-studied endocrine-disrupting chemicals with different modes of action, from two difference usage classes: the pharmaceutical 17α-ethynylestradiol (EE2) and the agrochemical vinclozolin (VIN). EE2 is an estrogen receptor (ER) agonist while VIN is an androgen receptor (AR) antagonist. Importantly, given the huge amount of information available on the endocrine activity of these chemicals in diverse animal models, only examples of data can be included within the scope of this chapter. Therefore, the examples given should not be used per se to make conclusions about the overall hazard or risk assessment profile of either EE2 or VIN to human health or to the environment. Furthermore, consideration is largely restricted to studies with mammals and aquatic organisms.
12.3 APPLICATION OF THE KLIMISCH CRITERIA TO THE EE2 AND VIN CASE STUDIES
For the purposes of this chapter, the authors adopted the recommendations of Klimisch et al. [9] for reviewing (eco)toxicology data from various sources. Such an approach is often helpful so that different reviewers consistently assess data with a common approach to reliability, relevance, and adequacy (e.g., provision of data on measured test concentration in fish studies as opposed to only nominal concentrations, which are less reliable). A summary of the approach used in the current exercise is given in Table 12.2. This approach to rating studies according to their reliability enables a reviewer to focus the assessment on key studies and improves clarity of the assessment and conclusions. In the future, it is hoped that some of the uncertainties that can arise in published papers on (eco)toxicology experiments will be reduced through the adoption of improved animal usage documentation under the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines [10].
Table 12.2 Klimisch checklist as a guide for study reviews [9]
| K | Methods | Data/Information |
| 1 | Guideline study (OECD, ISO etc.) | In Vivo
Test animals (species, strain, sex, age) Purity/composition/origin of the test substance Number of animals evaluated Scope of the investigations per animal and methods description Description of the changes/lesions observed Control group or historical control data of the laboratory Description of the test conditions Description of the route and doses of administration Dose/concentration relationship In Vitro Description of the test system and test method in details Purity/composition/origin of the test substance Dose/concentration differentiated according to the toxicity of test substance on the test system; information on volatility Data on secondary effects that may influence a result (e.g. solubility, impurities, pH shifts, etc.) Appropriate –ve/+ve controls as integral parts of the test References on adequacy of method given/generally known |
| Comparable to guideline study | ||
| Procedure according to national standards (e.g., ASTM, DIN, EPA etc.) | ||
| 2 | Well-documented and meets basic scientific principles | |
| Basic data given: comparable to guidelines/standards | ||
| Comparable to guideline study with acceptable restrictions | ||
| 3 | Method not validated | |
| Documentation insufficient for assessment | ||
| Does not meet important criteria of current methods | ||
| Relevant method deficiencies—unsuitable test system | ||
| 4 | Only short abstract available | |
| Only secondary literature (review, tables, books, etc.). | ||
| K = Klimisch score | ||
12.4 CASE STUDY: DATA EXAMPLES FOR 17α-ETHYNYLESTRADIOL
In a human health context, EE2 has been intensively studied in the process of drug development using preclinical animal models and through wider medical and family planning studies. Hence the in vitro (see Table 12.3) and in vivo mammalian (see Table 12.4) data cited in this chapter are a tiny fraction of the data published; nonetheless, they illustrate how such studies can be evaluated in the context of the OECD CF. Likewise, since a number of studies showed the dramatic impacts of EE2 on fish reproduction at very low concentrations (nanogram per liter levels), this has led to a relatively large number of ecotoxicology studies that are beyond the scope of this chapter. For example, an August 2011 search on Google Scholar using the terms “fish and ethynylestradiol” gave >5,800 hits while “environment and ethynylestradiol” gave 15,000 hits; hence, in this instance, the reader is referred to Caldwell et al. [11] for a review of EE2 and its impact on aquatic species. Most important, our emphasis is on illustrating the principles of the OECD CF for nonmammalian species (see Table 12.5) rather than a definitive review of all the published data.
Table 12.3 2011 OECD Revised Conceptual Framework: Level 1 and 2 data examples for 17α-ethynylestradiol (EE2)
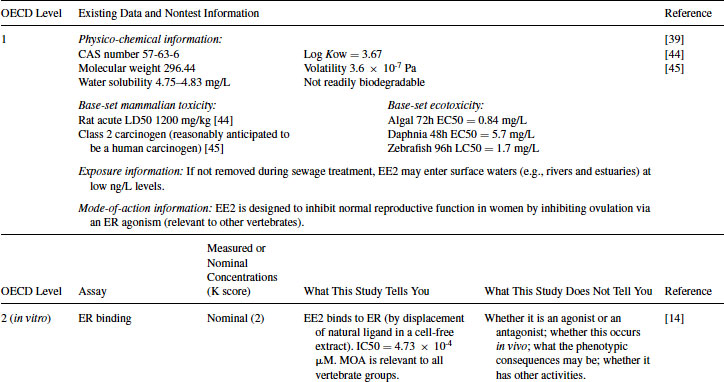
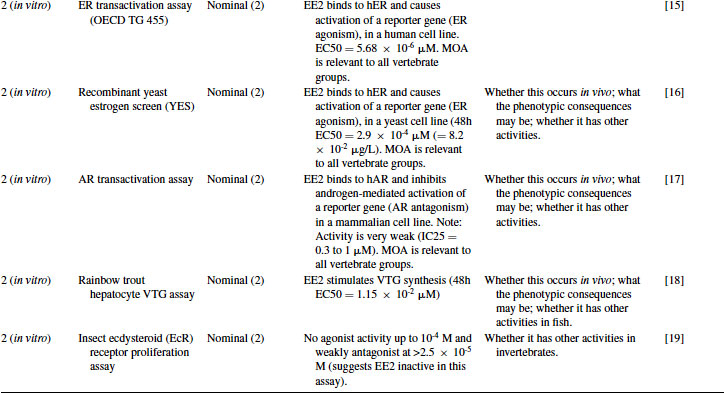
Table 12.4 2011 OECD Revised Conceptual Framework: Levels 3 to 5 data examples on mammalian toxicology studies with EE2
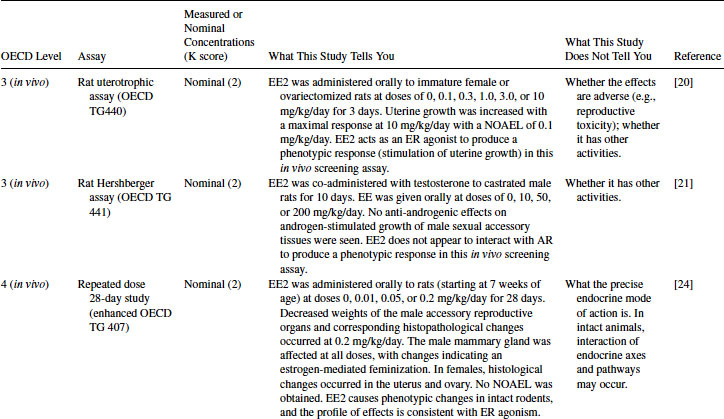
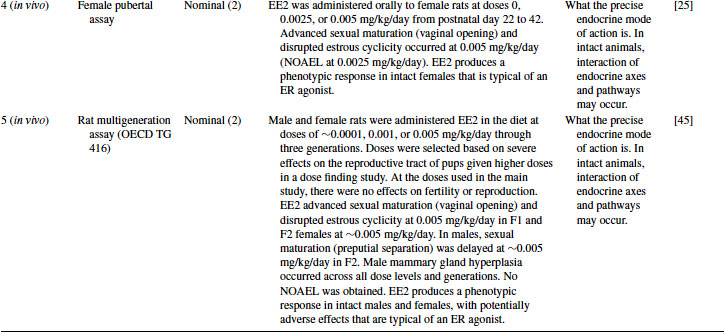
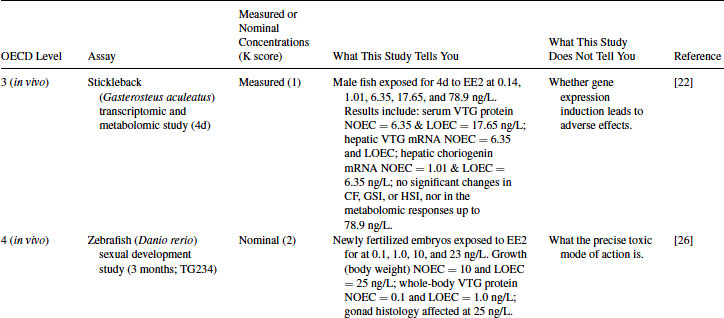
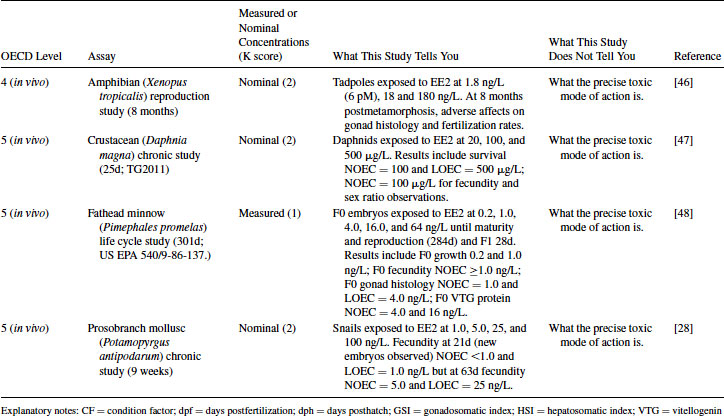
Table 12.5 2011 OECD Revised Conceptual Framework: Levels 3 to 5 data examples on nonmammalian toxicology studies with EE2
12.4.1 EE2 Case Study: Level 1 Information
EE2 (CAS number 57-63-6) is a semisynthetic alkylated oestradiol with a 17α-ethinyl substitution. It has high estrogenic potency in mammals when administered orally and is often used as the oestrogenic component in oral contraceptives (http://drugbank.ca/drugs/DB00977). The physico-chemical properties of EE2 and exposure information are shown in Table 12.3. Aside from the longer-term studies summarized elsewhere in this chapter, human pharmaceuticals traditionally have been tested for their acute toxicity in rodents and other preclinical models. The U.S. Food and Drug Administration [12] also requires such compounds to be tested in acute ecotoxicity studies using algae, crustaceans, and fish. These data, together with other available information are summarized in Table 12.3.
12.4.2 EE2 Case Study: Level 2 Information
EE2 has been tested in a wide variety of in vitro assays developed for drug discovery purposes by the pharmaceutical industry, including the recombinant hER yeast-based assay developed by Glaxo and then given to university researchers in the mid-1990s to aid ecotoxicology research [13]. It has also been tested in more recently developed assays, including OECD Test Guideline (TG) 455 (ER transactivation assay for estrogen agonists). As shown in Table 12.3, EE2 binds to the ER in a competitive binding assay [14] with a high degree of potency, similar to that of the natural estrogen estradiol. This assay, however, cannot distinguish between agonists and antagonists. Transactivation assays are therefore necessary to make this distinction, and EE2 acted as an agonist in both a mammalian hER cell line (OECD TG 455) [15] and the yeast hER cell line [16] with a high degree of potency. In contrast, although EE2 had some activity in an AR transactivation assay [17], this was considered to be very weak compared to anti-androgens such as R1881. ER agonism is therefore the most important mode of action of EE2. Given the highly conserved nature of ER, this is relevant across vertebrate taxa. Importantly, however, such studies do not provide information on whether there would be any expressed endocrine-mediated activity in biological tissues. These in vitro assays also do not take into account adsorption and metabolism of the chemical in whole animals (considered in Levels 3–5). As another example, EE2 has been tested by Pelissero et al. [18] and shown to dramatically induce the production of vitellogenin (VTG) in fish hepatocytes in vitro. Finally, evidence of the absence of in vitro activity in a given assay can be informative, as demonstrated by the use of an arthropod cell line [19], which showed EE2 to be almost inactive against the ecdysteroid receptor (see Table 12.3). Although this last assay is not a test guideline and is not relevant to vertebrate hormone systems, it provides data on the potential of a chemical to interact with an arthropod receptor and provides information complementary to the OECD in vivo chronic arthropod test guidelines (e.g., using chironomids or daphnids). All examples cited here have been rated at Klimisch code 2, since the biological aspects of the work are clearly described, but the precise chemical concentrations used were not verified by analytical chemistry (a typical approach within in vitro toxicology).
12.4.3 EE2 Case Study: Level 3 Information
EE2 has been tested in the rat uterotrophic assay for estrogen agonists. In the example given in Table 12.4, it was positive in both immature and ovariectomized rat assays as uterine weight was significantly increased [20]. The positive result in this assay corresponds well with the positive results obtained in the Level 2 ER-based in vitro assays. A no-observable-adverse-effect level (NOAEL) of 0.1 mg/kg/day was achieved when EE2 was orally administered (the most relevant route), although assays at this level are designed to be in vivo screening assays, providing only a positive or negative answer. The result confirms that EE2 acts as an ER agonist in vivo, but the uterotrophic assay cannot provide definitive information about whether the effects are adverse. The uterotrophic assay has been designed to be sensitive. The ovariectomized assay does not possess an intact hypothalamus-pituitary-gonad (HPG) axis, and the assay will largely detect only estrogenic and anti-estrogenic chemicals. Adverse effects on reproduction and development are not predicted. Likewise, if other modes of action occur, they are unlikely to be detected in this assay.
EE2 was also tested in the Hershberger assay for androgen antagonists. It was negative in this assay [21]. This indicates that the weak activity observed in the AR transactivation assay (Level 2) does not result in a positive response in vivo. Given the evidence that EE2 is estrogenic in an in vitro fish hepatocyte assay [18], together with many other published works giving similar evidence, a good example of an in vivo fish assay providing data about selected endocrine mechanisms is the use of molecular tools to assess the mode of action (MOA) of EE2 in male sticklebacks [22] (see Table 12.5). This four-day study showed induction of VTG (both as serum protein and hepatic mRNA), together with hepatic choriogenin mRNA induction at concentrations as low as 1.01 ng/L. Molecular toxicology studies such as these, supported by analytical verification of the test chemical, are very powerful in elucidating different MOAs of EE2 in fish. However, molecular and protein biomarker responses (biological markers of exposure) do not in themselves represent a toxic effect for individual fish nor do they show evidence of an adverse effect of the chemical on the health of the laboratory fish population. Applied in a field situation, biomarkers such as VTG can be very useful for tracking spatial and temporal trends in exposure, but many would argue they should not be used directly to derive predicted no effect concentrations for fish populations because there is not necessarily a direct causal link between the induction of such markers and adverse impacts on higher levels of organization [23].
12.4.4 EE2 Case Study: Level 4 Information
The Level 4 assays on EE2 provide information on the activity of EE2 in apical assays (i.e., studies using intact animals with functional endocrine axes and with end points that can be affected by multiple modes of action). These assays may also provide limited information on adverse effects, but depending on the regulatory context in which they are used, they may also be considered as screening assays. Two examples are shown for EE2 in Table 12.4, the enhanced 28-day screening assay (OECD TG 407) [24] and the female pubertal assay [25].
Both assays gave positive results with EE2, although the dose range in TG 407 was 40 times higher than the female pubertal assay. Decreased weights of reproductive organs weights and histopathological changes occurred in males at 0.2 mg/kg/day in the TG 407. The most sensitive end point in the TG 407 assay was histopathological changes in the male mammary gland at doses from 0.01 mg/kg/day. Changes in the uterus and ovary were apparent at doses from 0.01 mg/kg/day in the TG 407 assay; in the female pubertal assay disruption of estrous cyclicity occurred at 0.005 mg/kg/day. The female pubertal assay includes the end point of vaginal opening (typically occurring in control rats around postnatal day 33), and this was advanced by EE2 [24]. In the TG 407, assay animals are already seven weeks old at the start of the assay and therefore have already achieved this landmark.
EE2 produced responses in both studies that are typical of an estrogen agonist and confirm that the mode of action demonstrated in the Level 2 and 3 assays results in phenotypic responses in intact animals. The precise mode of action could not be discerned from these studies alone but is supported by the lower-level data. The severity of the effects on apical end points is indicative of a possible adverse effect on reproductive organs in both sexes. Although a NOAEL was not achieved in TG 407, where effects occurred at the lowest dose of 0.01 mg/kg/day, a NOAEL could be derived from the female pubertal assay, where no effects were observed at 0.0025 mg/kg/day and this may provide information for risk assessment [24].
In terms of nonmammalian species, the weight of evidence from Levels 2 and 3 (together with the likelihood of EE2 being released into surface waters mentioned in Level 1) justifies moving into chronic exposure studies with fish (e.g., using fathead minnows, medaka, sticklebacks, or zebrafish). An example of such a study is the assessment of sexual development in zebrafish using the OECD TG 234 (Table 12.5), showing adverse effects in terms of gonadal pathology and growth [26]. In general, in ecotoxicology, significant impacts on survival, development, growth, and reproduction end points are used in environmental (ecological) hazard assessment for deriving predicted no effect concentrations (PNECs) or environmental quality standard (EQS) values [23]. These population-relevant end points do not in themselves, however, indicate what the likely MOA is, nor do they quantify the possible severity of EE2 effects over the entire life cycle of fish (see Level 5). The concomitant measurement of VTG in this zebrafish partial life cycle study is also useful in providing a common basis for interspecies comparisons between laboratory and field studies.
12.4.5 EE2 Case Study: Level 5 Information
At Level 5, a three-generation rat reproduction study on EE2 is available [27] (Table 12.4). This type of study should give a comprehensive assessment of effects on endocrine end points, fertility, reproduction, and development. In the NTP study [27], EE2 had no effects on fertility or reproduction. This lack of effect was probably due to the low doses employed in the study (∼ 0.0001, 0.001 or 0.005 mg/kg/day) as a result of severe effects on the reproductive organs occurring at higher doses in the dose-finding study. EE2 affected sexual development: advanced sexual maturation (vaginal opening) and disrupted estrous cyclicity at 0.005 mg/kg/day in F1 and F2 females at ∼0.005 mg/kg/day. In males, sexual maturation (preputial separation) was delayed at ∼0.005 mg/kg/day in F2 pups. Male mammary gland hyperplasia occurred across all dose levels and generations, and therefore no NOAEL was obtained [27].
The effects seen in this study are typical of those of an ER agonist and are consistent with the effects observed in the Level 4 studies. The effects on development and the severity of the effects seen in the dose-finding study indicate a likely adverse effect on fertility, reproduction, and development. As with the Level 4 studies, the precise MOA could not be discerned from these studies alone, but it is supported by the data at Levels 2 and 3. In this study, the effects on the male mammary gland were observed at doses as low as 0.0001 mg/kg/day; therefore, the NOAEL of 0.0025 mg/kg/day suggested by the Level 4 studies is no longer justified for risk assessment [27].
In conclusion, the weight of evidence from studies at Levels 2 to 5 in the CF demonstrates that EE2 is an ER agonist in vitro and in vivo. It does not appear to act as an AR antagonist in vivo. Studies in intact animals and reproduction studies demonstrate that it has adverse effects on reproductive organs, postnatal development, and probably fertility and reproduction in mammalian species. These effects result from its MOA as an estrogen agonist. Given similarities in ER across species, it is likely that similar adverse effects will occur in other vertebrates.
As shown in Table 12.5, the OECD CF usefully categorizes the importance of full life cycle studies with fish for chemicals with exposure profiles (Level 1), intrinsic estrogenic activity in vitro (Level 2) and in vivo (Level 3), together with evidence of significant adverse impacts on population-relevant end points in a fish partial life cycle study. In this instance, the 12-week growthNOEC (no observed effect concentration; NOEC) for the zebrafish sexual development test was 10 ng/L (nominal concentration), whereas the 284-day growthNOEC for the fathead minnow full life cycle test was 0.2 ng/L (measured concentration), which illustrates the importance of such Level 5 studies for chemicals like EE2. However, molluscs in addition to fish are known to be highly sensitive to the population-level impacts of EE2 [28], even though the precise MOA for such effects in molluscs is poorly understood in comparison to fish. Interestingly, while crustacean (Daphnia magna) life cycle tests have been far more widely used than mollusc or fish life cycle tests for many years, in the case of EE2 it is clear that they are far less sensitive than molluscs or fish (Table 12.5) [11]. This is also suggested via the weight of evidence presented within the OECD CF since in Level 2 studies collated for EE2, arthropod (crustacean and insect) tissues appear to be far less sensitive to EE2 than fish tissues.
12.5 CASE STUDY: DATA EXAMPLES FOR VINCLOZOLIN
Vinclozolin (3-(3-5-dichlorophenyl)-5-methyl-5-vilyl-1, 3-oxazolidine-2,4-dione) is a nonsystemic fungicide used to control various blights and rots caused by fungal pathogens in a wide variety of crops (e.g., canola, kiwi, lettuce, and raspberries) and can also be applied to ornamentals and turf. However, concerns over toxicity in mammals have led EPA to conclude that principal toxic effects induced by VIN and/or its metabolites (termed M1 and M2) are related to its anti-androgenic activity and its ability to act as a competitive antagonist at the AR. VIN exerts its effects most dramatically during the developmental stages of animals, ultimately resulting in reproductive effects; it also interferes with lipid metabolism and/or storage [29].
12.5.1 VIN Case Study: Level 1 Information
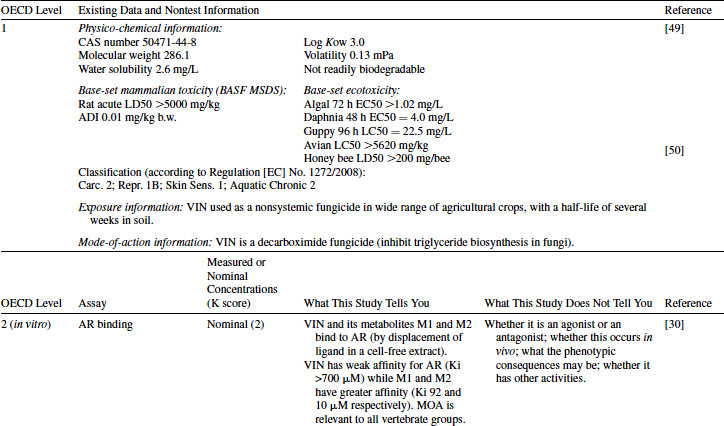
The physico-chemical properties of VIN, together with exposure information, are shown in Table 12.6. A comprehensive suite of mammalian toxicology and ecotoxicology studies are typically required by authorities in Europe, Japan, and North America to support the registration of this agrochemical active ingredient. This includes information on absorption, distribution, metabolism, and excretion. VIN is metabolized to two endocrine-active metabolites: M1 and M2 [30]. Examples of data provided by industry sources are summarized in Table 12.6.
Table 12.6 2011 OECD Revised Conceptual Framework: Level 1 and 2 data examples for vinclozolin
12.5.2 VIN Case Study: Level 2 Information
Since the early 1990s, VIN has been the focus of a large number of in vitro endocrine disruptor-related studies. A Google Scholar search in August 2011 for “vinclozolin endocrine” generated over 2,700 hits, so, as with EE2, the examples cited in this case study (Table 12.6) are simply selected to illustrate the use of the OECD CF for compounds with complex MOAs.
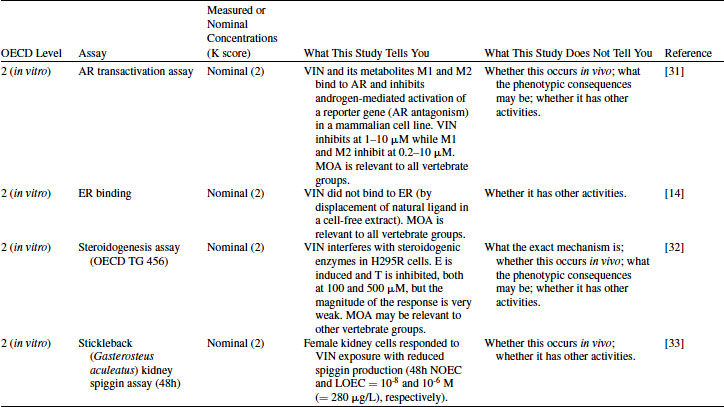
VIN has been tested in a rat AR competitive binding assay and found to be positive [30]. However, the binding of VIN itself to AR is very weak (Ki >700 μM) while its two metabolites have much higher binding affinity: M1 has Ki = 92 and M2 has Ki = 10 μM [30]. This example illustrates the importance of having knowledge about the metabolism of the chemical under consideration; without this, the interaction of VIN with AR would be greatly underestimated. As with the EE2 example, a positive result in this assay cannot distinguish between agonism and antagonism, nor can the in vivo consequences of the AR interaction be accurately predicted. The AR transactivation assay provides the knowledge that VIN is an AR antagonist [31]. Similarly to the AR binding assay, VIN itself has weak activity while metabolites M1 and M2 are more potent. M1 and M2 inhibit androgen-mediated activation of the reporter gene at lower doses than VIN, and the magnitude of change is greater. VIN has also been tested in an ER binding assay but was negative [14]. VIN was positive in the steroidogenesis assay (OECD TG 456) using the human cell line H295R [32]. Estradiol is increased and testosterone is inhibited, although the magnitude of the change was small. This may indicate that VIN has some ability to interfere with steroidogenesis, although it is also possible that the H295R assay possesses some endogenous AR. M1 and M2 were not tested in the steroidogenesis assay, and the metabolizing ability of these cells is expected to be limited. The results from these four assays indicate that AR antagonism is the primary mode of action for VIN. The assays shown in Table 12.6 are based on mammalian receptors/systems, but the high degree of conservation of AR across species makes this mode highly relevant across taxa. Steroidogenesis metabolic pathways may be less well conserved across vertebrate species, but the positive result in this assay may still provide an alert.
In terms of Level 2 studies with nonmammalian systems, Jolly et al. [33] used a novel in vitro assay that utilizes cultures of primed female stickleback kidney cells for the screening of potential androgenic and anti-androgenic chemicals. Stickleback kidney cells are natural targets for steroid hormones and are able to produce a protein, spiggin, in response to androgenic stimulation by dihydrotestosterone (DHT). VIN significantly inhibited DHT-induced spiggin production in a concentration-dependent manner in this elegant in vitro system (Table 12.6). This suggests that VIN has a similar intrinsic endocrine MOA in fish as in mammals; however, as with fish hepatocytes, such an assay shows intrinsic activity at the tissue level but does not take into account absorption and metabolism in whole animals (considered in Levels 3 to 5).
12.5.3 VIN Case Study: Level 3 Information
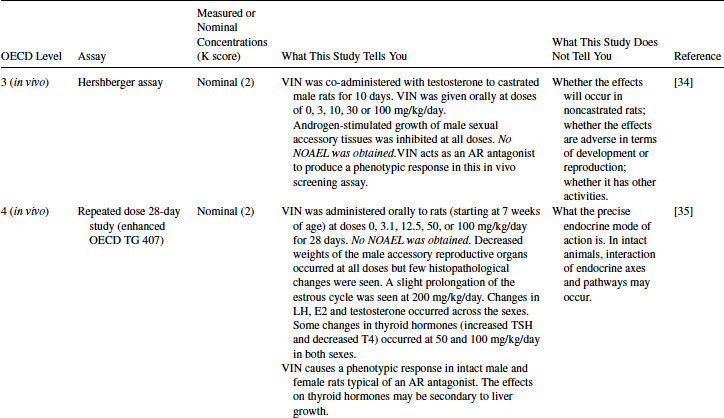
VIN has been tested in the Level 3 Hershberger assay for estrogen antagonists (see Table 12.7). It was positive in the castrated rat assay where testosterone-stimulated growth of male sexual accessory tissues was inhibited at all doses (0, 3, to 100 mg/kg/day) [34]. This result is in concordance with the Level 2 AR binding and transactivation assays. As the Hershberger is an in vivo assay, the metabolism of VIN to its more active metabolites is enabled. VIN is therefore confirmed to be an AR antagonist in vivo, but, as with the example of EE2 in the uterotrophic assay, this assay cannot provide definitive information about whether the effects are likely to be adverse. The castrated rat provides a sensitive assay but does not have an intact HPG axis. It also has limited ability to detect other modes of action.
Table 12.7 2011 OECD Revised Conceptual Framework: Levels 3 to 5 data examples on mammalian toxicology studies with VIN
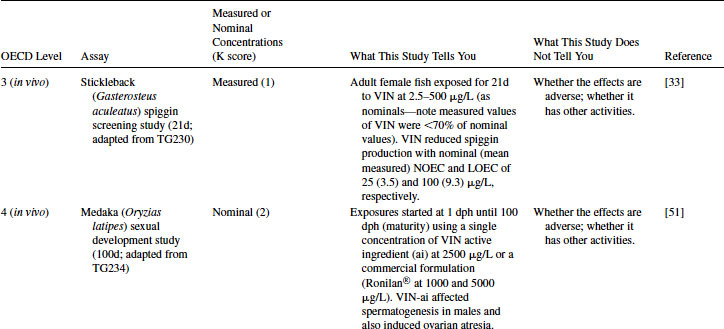
A Level 3 in vivo fish assay using the stickleback was also used by Jolly et al. [33] to further assess the androgenic and anti-androgenic activities of various chemicals (Table 12.8). As with the in vitro work just described, the basis for this study is that stickleback kidney cells are able to produce spiggin in response to androgenic stimulation by DHT. Again, VIN significantly inhibited DHT-induced spiggin production in a concentration-dependent manner in this in vivo system after 21 days at 9.3 μg/L (see Table 12.8). As with the VTG end point used in the EE2 case study, these protein biomarker-focused experiments, supported by analytical verification of the test chemical, are very powerful in elucidating different MOAs of VIN in fish. However, protein biomarker responses do not directly address a toxic effect for individual fish nor do they show evidence of an adverse effect of the chemical on the health of the laboratory fish population [23].
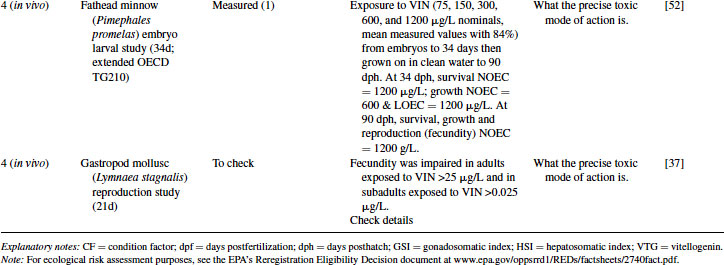
Table 12.8 2011 OECD Revised Conceptual Framework: Levels 3 to 5 data examples on nonmammalian toxicology studies with VIN
12.5.4 VIN Case Study: Level 4 Information
As described in the example of EE2, the Level 4 assays on VIN provide information on the effect of VIN in intact animals. These assays also give some information on possible hazard and risk. The examples given for VIN in Table 12.7 are the 28-day repeated dose study (enhanced OECD TG 407) [35] and the male pubertal assay [36]. Both assays gave positive results, with effects on endocrine end points typical of that expected for an AR antagonist. Decreased weights of the male accessory reproductive organs occurred in both assays. The pubertal assay also showed delayed sexual maturation (preputial separation), and luteinizing hormone was increased—a typical response of the HPG axis to inhibition of testosterone production by an AR antagonist [36]. The TG 407 assay uses both sexes and while females were not as affected as males by VIN, some changes in the estrous cycle and serum hormones were seen [35]. Although these Level 4 assays cannot unequivocally discern mechanism, when the results are supported by the Level 2 and 3 assays, it is clear the effects seen are due to AR antagonism.
Changes in thyroid hormones (T4) and TSH were observed in TG 407 [35], although not in the male pubertal assay. This could possibly be indicative that VIN affects thyroid function; however, no changes in thyroid weight or histopathology were observed. VIN increased liver weight, and it is likely that a consequence of this is increased hepatic metabolism of T4 and a subsequent rise in TSH via feedback control mechanisms. It is therefore concluded that VIN does not have a primary effect on thyroid systems.
The severity of the changes seen in the male reproductive organs indicates a strong possibility of adverse effects on reproduction and development. Both assays used similar doses (3 or 10 mg/kg/day to 100 mg/kg/day), and neither could give a NOAEL, as effects were seen at all doses [35,36].
As with EE2 for nonmammalian species, the weight of evidence from Levels 2 and 3 (together with the likelihood of VIN being released into surface waters mentioned in Level 1) justifies moving into chronic exposure studies with fish. Two examples are given in Table 12.8, including a medaka 100-day sexual development test (but only using a single exposure concentration of 2500 μg/L and a nominal concentration) and a 34-day fathead minnow embryo-larval test giving a growthNOEC = 600 μg/L based on measured concentrations. Invertebrate partial life cycle tests on VIN have also been reported, for example, using a 21-day partial life cycle test with the freshwater gastropod mollusc Lymnaea stagnalis [37]. A significant impairment was observed in male fertility at concentrations exceeding 0.025 μg/L. Furthermore, fecundity was impaired in adult L. stagnalis exposed to concentrations exceeding 25 μg/L. However, these studies are not designed to elucidate the MOA behind such adverse effects at the population level.
12.5.5 VIN Case Study: Level 5 Information
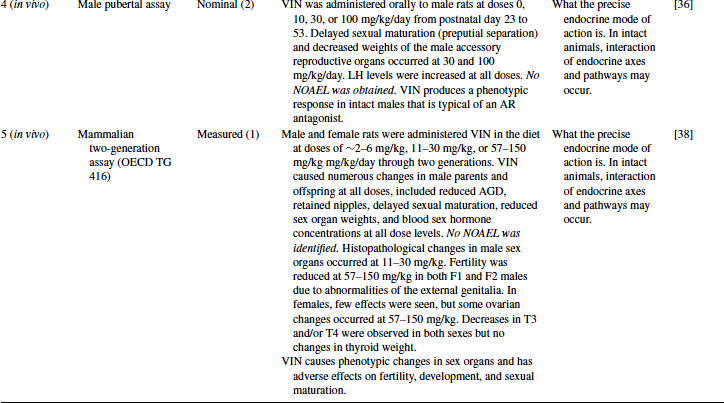
A two-generation study on VIN in the rat confirms the suggestion of adverse effects on reproduction and development provided by the Level 4 assays[38]. Severe effects such as reduced anogenital distance (AGD), retained nipples and major histopathological changes in sex organs were noted in male pups. When these animals were bred, fertility was reduced by half in high-dose males. The authors suggested that fertility was reduced because abnormalities of the male genitalia meant that many animals were unable to mate. Females were less adversely affected, but histopathological changes in ovaries were noted [38].
Decreases in the thyroid hormones T3 and T4 were also noted in this study, but, as in the TG 407 study, there were no effects on the thyroid itself. The changes are therefore likely to be due to liver growth and enzyme induction. The CYP marker enzyme benzoxyresorufin O-dealkylase was determined in the livers of F1 pups and found to be induced up to eightfold.
The effects on AGD, male reproductive organs, delayed sexual development, and nipple retention in males are typical of a strong AR antagonist. Although this assay in isolation cannot elucidate the precise endocrine mode of action, when combined with the data from Levels 2, 3, and 4, it is clear that this is the primary, and possibly the sole, endocrine mode of action. Level 5 assays provide information for use in both hazard and risk assessment. Evaluation of hazard is clear in this example, but no NOAEL was obtained in the study, reducing its usefulness for risk assessment.
In conclusion, the weight of evidence from studies at Levels 2 to 5 in the CF demonstrates that VIN is an AR antagonist in vitro and in vivo. This is the major, if not the sole, MOA. Studies in intact animals and reproduction studies show that it has adverse effects on reproductive organs, pre- and postnatal development, fertility, and reproduction in mammalian species. These effects result from its MOA as an androgen antagonist. Given similarities in AR across species, it is likely that adverse effects will occur in other vertebrates although the phenotype of changes may differ.
While effects in fish and invertebrate partial life cycle studies have been described for VIN (see Table 12.8), to our knowledge no full life cycle or multigeneration studies have been published in fish or aquatic invertebrates for this chemical. Selection of suitable organisms should reflect the MOA information for VIN as it may be used to guide the selection of priority test species for environmental risk assessment purposes [39,40]. An OECD TG for a fish full life cycle remains a priority (see Chapter 7), and it is expected that OECD fish and invertebrate life cycle test guidelines will both play a larger role in endocrine disrupter testing and assessment in the future [41–43].
12.6 CONCLUSIONS
The EE2 and VIN case studies included in this chapter have illustrated the utility of the 2011 OECD CF for organizing both in vitro and in vivo data in a rational and consistent manner for the assessment of available evidence on the potential for an endocrine disruption. Clearly, only a limited number of study examples could be included within the scope of this chapter, and more work should be done with a wider range of chemicals in order to critically evaluate and strengthen as necessary the CF.
At this stage, however, a number of positive conclusions can be drawn:
- The 2011 revised OECD CF usefully captures a diverse range of experimental studies in different in vitro and in vivo test systems (including both research methods and OECD TG studies).
- Linked to the Klimisch criteria, the OECD CF provides a logical process for critically evaluating studies that show both positive and negative results (namely, highlighting the benefits of evidence of absence of endocrine activity over absence of evidence of endocrine activity).
- The OECD CF works well for the two case study chemicals (EE2 and VIN) with different MOAs, allowing the differentiation between studies showing intrinsic endocrine activity in vitro (Level 2) versus expressed adverse effects in vivo (Levels 4 and 5).
- The OECD CF offers a unique opportunity to consider side-by-side data from mammalian and nonmammalian studies and the possibility to consider the relevance of available evidence across a range of taxonomic groups.
- The OECD CF helps by gathering all relevant information in one place (from relevant physical-chemical properties, metabolism, fate properties, in vitro mechanistic studies to relevant animal studies) to build a case around a chemical investigated.
- For Level 3 of the OECD CF, the approach also works well for the two case study chemicals (EE2 and VIN) in allowing the differentiation between studies showing intrinsic endocrine activity in vivo versus expressed adverse effects in vivo (Levels 4 and 5).
- Levels 4 and 5 of the OECD CF both provide valuable quantitative adverse effects (e.g., LOEC) and no-effect (NOAEL and NOEC) data on human health- or wildlife population–relevant end points to be used in risk assessment. The evidence available for EE2 underlines the greater sensitivity of full life cycle (Level 5) type tests versus partial life cycle tests (Level 4), which needs to be considered during chemical risk assessments.
REFERENCES
1. Herbst, A. L., Ulfelder, H., Poskanzer, D. C. (1971). Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine 284: 878–881.
2. Gill, W. B., Schumacher, G. F. B., Bibbo, M., Straus, F. H., Schoenberg, H. W. (1979). Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. Journal of Urology 122: 36–39.
3. Cooke, A. S. (1973). Shell thinning in avian eggs by environmental pollutants. Journal of Environment and Pollution 4: 85–152.
4. Bryan, G. W., Gibbs, P. E., Hummerstone, L. G., Burt, G. R. (1986). The decline of the gastropod Nucella lapillus around south-west England: evidence for the effects from tributyltin from antifouling paints. Journal of the Marine Biological Association of the United Kingdom 66: 611–640.
5. Fenner-Crisp, P., Maciorowski, A. F., Timm, G. E. (2000). The endocrine disruptor screening program developed by the U.S. Environmental Protection Agency. Ecotoxicology 9: 85–91.
6. Japanese Ministry of Environment. (1998). Japanese Ministry of Environment Strategic Program on Environmental Endocrine Disruptors (SPEED ’98). Available at: www.env.go.jp/en/chemi/ed/speed98/sp98.html.
7. Huet, M. C. (2000). OECD activity on endocrine disrupters test guidelines development. Ecotoxicology 9: 77–84.
8. Organisation for Economic Cooperation and Development. (2011). Draft Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. Available at: www.oecd.org/document/12/0,3343,en_2649_34377_1898188_1_1_1_1,00.html.
9. Klimisch, H.-J., Andreae, M., Tillmann, U. (1997). A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regulatory Toxicology and Pharmacology 25: 1–5.
10. Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology 8(6): e1000412. doi:10.1371/journal.pbio.1000412.
11. Caldwell, D. J., Mastrocco, F., Hutchinson, T. H., Länge, R., Heijerick, D., Janssen, C., Anderson, P. D., Sumpter, J. P. (2008). Derivation of an aquatic predicted no-effect concentration for the synthetic hormone, 17 alpha-ethinylestradiol. Environmental Science & Technology 42: 7046–7054.
12. Food and Drug Administration Center for Drug Evaluation and Research. (1998). Guidance for Industry-Environmental Assessment of Human Drugs and Biologics Applications, Revision 1. FDA-CDER, Rockville, MD.
13. Routledge, E. J., Sumpter, J. P. (1996). Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environmental Toxicology and Chemistry 15: 241–248.
14. Blair, R. M., Fang, H., Branham, W. S., Hass, B. S., Dial, S. L., Moland, C. L., Tong, W., Shi, L., Perkins, R., Sheehan, D. M. (2000). The estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicological Sciences 54: 138–153.
15. Organisation for Economic Cooperation and Development. (2006). Pre-validation and interlaboratory validation for the stably transfected transactivation assay to detect estrogenic activity. Available at http://www.oecd.org/chemicalsafety/testingofchemicals/37504278.pdf.
16. Folmar, L. C., Hemmer, M. J., Denslow, N. D., Kroll, K., Chen, J., Cheek, A., Richman, H., Meredith, H., Grau, E. G. (2002). A comparison of the estrogenic potencies of estradiol, ethinylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquatic Toxicology 60: 101–110.
17. Vinggaard, A. M., Niemela, J., Wedebye, E. B., Jensen, G. E. (2008). Screening of 397 chemicals and development of a quantitative structure-activity relationship model for androgen receptor antagonism. Chemical Research in Toxicology 21: 813–823.
18. Pelissero, C., Flouriot, G., Foucher, J. L., Bennetau, B., Dunogues, J., Le Gac, F., Sumpter, J. P. (1993). Vitellogenin synthesis in cultured hepatocytes; an in vitro test for the estrogenic potency of chemicals. Journal of Steroid Biochemistry and Molecular Biology 44: 263–272.
19. Dinan, L., Bourne, P., Whiting, P., Dhadialla, T. S., Hutchinson, T. H. (2001). Screening of environmental contaminants for ecdysteroid agonist and antagonist activity using the Drosophila melanogaster B(II) cell in vitro assay. Environmental Toxicology and Chemistry 20: 2038–2046.
20. Kanno, J., Onyon, L., Haseman, J., Fenner-Crisp, P., Ashby, J., Owens, W. (2001). The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: Phase 1. Environmental Health Perspectives 109: 785–794.
21. Yamasaki, K., Takeyoshi, M., Sawaki, M., Imatanaka, N., Shinoda, K., Takatsuki, M. (2003). Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology 183: 93–115.
22. Katsiadaki, I., Williams, T. D., Ball, J. S., Bean, T. P., Sanders, M. B., Wu, H., Santos, E. M., Brown, M. M., Baker, P., Ortega, F., Falciani, F., Craft, J. A., Tyler, C. R., Viant, M. R., Chipman, J. K. (2010). Hepatic transcriptomic and metabolomic responses in the stickleback (Gasterosteus aculeatus) exposed to ethinyl-estradiol. Aquatic Toxicology 97: 174–187.
23. Hutchinson, T. H., Ankley, G. T., Segner, H., Tyler, C. R. (2006). Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environmental Health Perspectives 114: 106–114.
24. Andrews, P., Freyberger, A., Hartmann, E., Eiben, R., Loof, I., Schmidt, U., Temerowski, M., Folkerts, A., Stahl, B., Kayser, M. (2002). Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD Test Guideline no. 407). Archives of Toxicology 76:194–202.
25. U.S. Environmental Protection Agency. (2007). Integrated Summary Report for Validation of a Test Method for Assessment of Pubertal Development and Thyroid Function in Juvenile Female Rats as a Potential Screen in the Endocrine Disruptor Screening Program Tier-1 Battery. Available at: www.epa.gov/scipoly/oscpendo/pubs/female_isr_v4.1c.pdf/.
26. Van den Belt, K., Verheyen R., Witters, H. (2003). Effects of 17α-ethynylestradiol in a partial life-cycle test with zebrafish (Danio rerio): Effects on growth, gonads and female reproductive success. Science of the Total Environment 309: 127–137.
27. National Toxicology Program, Department of Health and Human Services. (2011). Report on Carcinogens, 12th ed. Available at: http://ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/EstrogensSteroidal.pdf.
28. Jobling, S., Casey, D., Rodgers-Gray, T., Oehlmann, J., Schulte-Oehlmann, U., Pawlowski, S., Braunbeck, T., Turner, A. P., Tyler, C. R. (2003). Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquatic Toxicology 65: 205–220.
29. U.S. Environmental Protection Agency. (2000). Reregistration eligibility decision (RED)—Vinclozolin. Reference EPA 738-R-00–023. Available at: www.epa.gov/oppsrrd1/REDs/2740red.pdf.
30. Kelce, W. R., Monosson, E., Gamcsik, M. P., Laws, S. C., Gray, L. E. Jr. (1994). Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicology and Applied Pharmacology 126: 276–285.
31. Wilson, V. S., Bobseine, K., Lambright, C. R., Gray, L. E. Jr. (2002). A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicological Sciences 66: 69–81.
32. Hecker, M., Newsted, J. L., Murphy, M. B., Higley, E. B., Jones, P. D., Wu, R., Giesy, J. P. (2006). Human adrenocarcinoma (H295R) cells for rapid in vitro determination of effects on steroidogenesis: Hormone production. Toxicology and Applied Pharmacology 217: 114–124.
33. Jolly, C., Katsiadaki, I., Morris, S., Le Belle, N., Dufour, S., Mayer, I., Pottinger, T., Scott, A. P. (2009). Detection of the anti-androgenic effect of endocrine disrupting environmental contaminants using in vivo and in vitro assays in the three-spined stickleback. Aquatic Toxicology 92: 228–239.
34. Yamasaki, K., Sawaki, M., Ohta, R., Okuda, H., Katayama, S., Yamada, T., Ohta, T., Kosaka, T., Owens, W. (2003). OECD validation of the Hershberger assay in Japan: Phase 2 dose response of methyltestosterone, vinclozolin, and p,p′-DDE. Environmental Health Perspectives 111: 1912–1919.
35. Shin, J. H., Moon, H. J., Kim, T. S., Kang, I. H., Ki, H. Y., Choi, K. S., Han, S. Y. (2006). Repeated 28-day oral toxicity study of vinclozolin in rats based on the draft protocol for the “Enhanced OECD Test Guideline No. 407” to detect endocrine effects. Archives of Toxicology 80: 547–554.
36. Monosson, E., Kelce, W. R., Lambright, C., Ostby, J., Gray, L. E. Jr. (1999). Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicology & Industrial Health 15: 65–79.
37. Ducrot, V., Teixeira-Alves, M., Lopes, C., Delignette-Muller, M. L., Charles, S., Lagadic, L. (2010). Development of partial life-cycle experiments to assess the effects of endocrine disruptors on the freshwater gastropod Lymnaea stagnalis: A case-study with vinclozolin. Ecotoxicology 19: 1312–1321.
38. Matsuura, I., Saitoh, T., Ashina, M., Wako, Y., Iwata, H., Toyota, N., Ishizuka, Y., Namiki, M., Hoshino, N., Tsuchitani, M. (2005). Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using vinclozolin. Journal of Toxicological Sciences 30 (Spec. no.): 163–188.
39. European Centre for Ecotoxicology and Toxicology of Chemicals. (2007). Intelligent testing strategies in ecotoxicology: Mode of action approach for specifically acting chemicals. ECETOC Technical Report 102. 145 pp.
40. Bars, R., Broeckaert, F., Fegert I., Gross M., Hallmark N., Kedwards, T., Lewis, R., O’Hagan, S., Panter, G. H., Weltje, L., Weyers, A., Wheeler, J. R., Galay-Burgos, M. (2011). Science based guidance for the assessment of endocrine disrupting properties of chemicals. Regulatory Toxicology and Pharmacology 59: 37–46.
41. Hutchinson, T. H. (2007). Small is useful in endocrine disrupter assessment—Four key recommendations for aquatic invertebrate research. Ecotoxicology 16: 231–238.
42. Gourmelon, A., Ahtiainen, J. (2007). Developing test guidelines on invertebrate development and reproduction for the assessment of chemicals, including potential endocrine active substances—The OECD perspective. Ecotoxicology 16: 161–167.
43. Matthiessen, P. (2008). An assessment of endocrine disruption in mollusks and the potential for developing internationally standardized mollusk life cycle test guidelines. Integrated Environmental Assessment and Management 4: 274–284.
44. Sciencelab. (2011). Ethinyl Estradiol Material Safety Data Sheet. Available at: www.sciencelab.com/msds.php?msdsId=9923943.
45. National Toxicology Program. (2007). TR0547—NTP Technical report on the multigenerational reproductive toxicology study of ethinyl estradiol (CAS NO. 57–63–6) in Sprague-Dawley rats (feed studies). Available at: http://ntp.niehs.nih.gov/files/547_board_web.pdf.
46. Gyllenhammer, I., Holm, L., Eklund, R., Berg, C. (2009). Reproductive toxicity in Xenopus tropicalis after developmental exposure to environmental concentrations of ethynylestradiol. Aquatic Toxicology 91: 171–178.
47. Goto T., Hiromi, J. (2003). Toxicity of 17alpha-ethynylestradiol and norethindrone, constituents of an oral contraceptive pill to the swimming and reproduction of cladoceran Daphnia magna, with special reference to their synergetic effect. Marine Pollution Bulletin 47: 139–142.
48. Länge, R., Hutchinson, T. H., Croudace, C. P., Siegmund, F., Schweinfurth, H., Hampe, P., Panter, G. H., Sumpter, J. P. (2001). Effects of the synthetic estrogen 17 alpha-ethinylestradiol on the life-cycle of the fathead minnow. Environmental Toxicology and Chemistry 20: 1216–1227.
49. Tomlin, C. D. S. (ed.). (2003). The Pesticide Manual—A World Compendium, 13th ed. British Crop Protection Council, Alton, UK. 1344 pp.
50. ESIS. (2011). European Chemical Substances Information System. Available at: http://esis.jrc.ec.europa.eu/index.php?PGM=cla.
51. Kiparissis, Y., Metcalfe, T. L., Balch, G. C., Metcalfe, C. D. (2003). Effects of the antiandrogens, vinclozolin and cyproterone acetate on gonadal development in the Japanese medaka. Aquatic Toxicology 63: 391–403.
52. Makynen, E. A., Kahl, M. D., Jensen, K. M., Tietge, J. E., Wells, K. L., Van Der Kraak, G., Ankley, G. T. (2000). Effects of the mammalian antiandrogen vinclozolin on development and reproduction of the fathead minnow (Pimephales promelas). Aquatic Toxicology 48: 461–475.
53. Pawlowski, S., van Aerle, R., Tyler, C. R., Braunbeck, T. (2004). Effects of 17alpha-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicology and Environmental Safety 57: 330–345.
The views expressed in this chapter reflect the personal perspectives of the authors and do not reflect organizational policies in any way.