CHAPTER 13
The Prospects for Routine Testing of Chemicals for Endocrine-Disrupting Properties and Potential Ecological Impacts
13.2 ARE THERE GAPS IN THE TEST SUITE FOR EDCS?
13.3 “NEW” MODES OF ENDOCRINE-DISRUPTING ACTION
13.4 HOW SHOULD TESTS FOR EDCS BE DEPLOYED IN AN INTEGRATED FASHION?
13.5 USE OF WEIGHT OF EVIDENCE WHEN ASSESSING POSSIBLE EDCS
13.1 INTRODUCTION
It will be apparent from Chapter 3 that regulations covering the hazard and risk assessment of endocrine-disrupting chemicals (EDCs) are beginning to come into force under several jurisdictions, and it seems likely that such evaluations will become universal over the next few years. Furthermore, as an essential underpinning to these regulations, suitable in vitro and in vivo tests for EDCs are beginning to be internationally standardized, although many gaps still exist (see Section 13.2). We have also seen in Chapter 12 that it is possible to design a tool box for the testing of EDCs. These will be important in the future because their objectives are to use available resources with maximum efficiency by minimizing animal use while also minimizing the occurrence of false negatives. The issue of efficiency is crucial because of the huge numbers of new and existing chemicals that await assessment for possible endocrine-disrupting (ED) properties. If they all had to be tested with a range of EDC-responsive in vivo assays using species from several vertebrate and invertebrate taxa, the financial costs would be unmanageably high (thus holding back the development of new and possibly safer chemicals), and the ethical costs in terms of animal use would be unacceptable to many. Given the fact that, by definition, a chemical that interacts with the endocrine system can be conclusively identified as an EDC only if it causes adverse effects in vivo, how can this circle be squared?
At least a partial answer lies in achieving the complete integration of EDC screening and testing into mainstream chemicals evaluation, in order to minimize duplication of studies. Hand in hand with this integration will be the use of weight-of-evidence (WoE) techniques [1] to clarify what is known or predictable about a chemical before embarking on in vivo assays. These concepts are explored below in a little more detail.
13.2 ARE THERE GAPS IN THE TEST SUITE FOR EDCS?
To a large extent, the answer to the question of whether there are gaps in the EDC test suite depends on one’s philosophy of ecotoxicological testing and chemical assessment and on whether one is concerned merely to identify hazards or to conduct true risk assessment. Clearly, no practical testing scheme could realistically hope to identify all the environmental hazards possessed by a chemical or all the taxa likely to be affected, and toxicity testing is necessarily restricted a small group of species. Thus, most existing chemical regulation schemes rely to a large degree on acute mortality data in just four taxa (unicellular algae as surrogates for all plants; crustaceans—usually daphnids—as surrogates for all invertebrates; and fish and rodents as surrogates for all vertebrates). At higher testing tiers, routine longer-term tests may involve vascular plants, insects, fish, birds, and mammals, but the total number of taxa tested against a given chemical is nevertheless extremely small unless the substance is suspected to have special properties (if, e.g., it has been designed as a pesticide to disable certain taxa not routinely tested—e.g., molluscs; or it is known to affect biochemical targets not present in the standard test suite; or exposure in the natural environment is very widespread). Despite this limited routine coverage of end points and taxa, the existing chemical assessment systems employed by the major regulatory authorities (i.e., Australia, Canada, the European Union, Japan and the United States) miss relatively few substances that ultimately are shown to be causing a problem in the environment. A good example of a chemical that did slip through the net is the anti-inflammatory drug diclofenac, which found its way into cattle carcasses and ultimately caused renal failure in Asian vulture populations that fed on them [2]. We cannot hope to detect all such problems in advance, although methods are improving.
In the relatively new field of endocrine disrupters, the internationally standardized testing suite for non-rodents is still very small, including three fish screens (OECD Test Guidelines [TGs] 229 and 230; OECD Guidance Document (GD) 148), one amphibian screen (OECD TG 231), and one fish partial life cycle test (OECD TG 234) (www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-2-effects-on-biotic-systems_20745761;jsessionid=3atv08vuvruoj.epsilon). For rodents, there are two EDC-sensitive screens (the Hershberger Bioassay, OECD TG 441; and the Uterotrophic Assay, OECD TG 440) and several with suitable apical end points (e.g., the Extended One-Generation Reproductive Toxicity Study, OECD TG 443), but there are no standardized EDC-sensitive screens or apical tests for birds and reptiles and no EDC-sensitive screens and few apical tests for invertebrates. The question therefore arises as to how many more EDC assays are needed and what end points they should include.
The major gap in the EDC testing suite is undoubtedly the lack of any internationally standardized, mechanistic invertebrate assays. There are two partial or whole aquatic invertebrate life cycle tests (i.e., the daphnid reproduction test, OECD TG 211; and the chironomid life cycle test, OECD TG 233) that might be expected to detect important apical effects such as impaired reproduction without diagnosing modes of action, but none with end points that would clearly reveal the presence of an EDC. The same applies to a range of reproductive or developmental tests with soil invertebrates (enchytraeid worms, OECD TG 220; earthworms, OECD TG 222; mites, OECD TG 226; dipteran larvae, OECD TG 228; and collembolans, OECD TG 232). This is a particular problem for regulatory authorities, such as the European Union, that are intent on regulating EDCs by hazard rather than risk and therefore need to be sure that an ED mechanism is occurring. Furthermore, in some major invertebrate phyla (e.g., the molluscs), there are not even any standardized life cycle tests, and it is probably true to say that the serious environmental pollutant and endocrine disrupter tributyltin (TBT), derived mainly from now-banned anti-fouling paints that were first registered in the 1970s, would still not be flagged as a problem if products containing it were to be registered for the first time today [3,4].
The main reason for the dearth of EDC-sensitive diagnostic screens with invertebrates is simple: Our knowledge of invertebrate endocrinology is not yet good enough to understand how EDCs act in these important phyla. This was the case a decade ago [5] and remains largely true today. Again using the example of TBT, there are now at least five competing explanations of how this chemical exerts masculinizing effects in female molluscs, so it is not surprising that standardized screening assays for EDCs in molluscs have not yet been devised. We probably are nearer such assays in arthropods due to the research conducted by pesticide companies on chemical structures targeted at the endocrine systems of insect pests, but none has yet reached the stage of international acceptance. Our relative ignorance of invertebrate endocrinology also prevents reliable read-across from or to vertebrates, even though we know that some steroidal EDCs can produce apparent endocrine disruption in some invertebrates (e.g., molluscs [6,7]). The only solution to this difficulty is to conduct more basic research on hormone systems in several ecologically and economically important invertebrate phyla (especially echinoderms, molluscs, and annelids).
Turning to the vertebrates, the situation is a bit brighter given the broad commonality of their endocrinology. The hormones in this group have been highly conserved in evolutionary terms, so there is some scope for so-called read-across from one class to another. For example, Pickford [8] found that out of 32 chemicals active against the thyroid system in rodents, none was inactive in thyroid-sensitive amphibian assays. While this is helpful, it must of course be remembered that the consequences of a particular type of disruption may not be the same in different groups. Thus, estrogenic activity exerted by a weak estrogen receptor agonist such as nonylphenol might cause vitellogenin induction in a fish or amphibian, but that effect would not be seen in a mammal, although other impacts (e.g., reduced reproductive success) might be common across all vertebrate groups. It is also extremely hard to compare data obtained from mammalian experiments that dose by feeding or injection with fish or amphibian assays that generally expose the test organisms via the ambient water.
In consequence, there is a perceived need for more EDC-sensitive tests and/or screens that involve a wider range of vertebrates and endpoints. In OECD at present, there are programs or proposals to develop a partial fish life cycle TG including reproduction (probably with the fathead minnow, Pimephales promelas), a fish full life cycle test (FFLCT) including mechanistic end points, a medaka multigeneration test (MMGT) with the Japanese medaka (Oryzias latipes) also with mechanistic end points, a partial life cycle amphibian TG with the clawed frog (Xenopus laevis), and a two-generation avian TG with the Japanese quail (Coturnix japonica), all of which are expected to include a suite of apical end points as well as mechanistic end points diagnostic of endocrine activity. However, at present, there are no international plans to develop mechanistic in vivo EDC screens with birds, and any assays involving reptiles are conspicuous by their absence, despite the known sensitivity of this group to some EDCs (see Chapter 9 in this book).
Until many more operational data have been generated, it is impossible to be sure that the existing and planned vertebrate TGs will provide a sufficiently fine screen for chemicals with endocrine activity. The birds and reptiles seem to have been left behind to some extent, but it may eventually be shown that read-across from mammals, fish and amphibians will prove sufficiently reliable. However, the case of diclofenac in vultures reminds us that no chemical risk assessment program is ever likely to be capable of sieving out all problem chemicals without incurring unrealistic testing costs.
It will therefore be essential to integrate the relatively few screens and tests into efficient hazard assessment schemes that make optimal use of animals (i.e., are ethically acceptable) yet minimize the risks of false negatives and provide both mechanistic and apical data on suspected EDCs that will meet the needs of all regulatory authorities.
13.3 “NEW” MODES OF ENDOCRINE-DISRUPTING ACTION
All EDC-responsive in vivo assays that have been internationally standardized to date have been designed to detect and/or evaluate the effects of a limited range of substances—essentially those that can be described as having EATS modalities: that is, (anti-)estrogenic; (anti-)androgenic; thyroid-disrupting; and steroidogenesis-disrupting. These are the types of endocrine disruption that are reasonably well known and have been observed in the environment or in laboratory experiments with known chemicals. It might be expected that life cycle tests would reveal the apical effects of other potential endocrine modalities but not their mechanisms of action. However, some types of EDC probably cause effects that are not even detectable in currently planned life cycle assay procedures (see below section 13.4).
Several such suspected or possible EDC modalities are known, including effects on glucocorticoid signaling, the somatotropic axis, retinoid signaling, some aspects of thyroid hormone signaling, vitamin D signaling, peroxisome proliferator-activated receptor signaling, and epigenesis [9]. It is outside the scope of this chapter to describe these potential modalities in detail, and their relative importance in the real world remains to be seen. However, there is little doubt that future test programs will have to consider at least some of these possible mechanisms of endocrine action. Furthermore, as indicated earlier, even current life cycle testing may not be sensitive to some known and potential modalities. One example is the close involvement of the thyroid and other endocrine systems with smoltification in anadromous salmonid fish such as salmon [10]. It is known or suspected that some EDCs are able to interfere with the control of this process (e.g., [11,12]), but current standardized life cycle test procedures are not capable of studying smoltification because they do not involve salmonids. The same restriction on the value of current life cycle tests applies to some types of interference with the corticosteroid system that can impair responses to stress [13].
These considerations simply show that the routine regulatory testing of potential EDCs is far from being a mature discipline, and it is highly likely that additional procedures will be needed in due course in order to provide reassurance that all significant modes of endocrine action have been accounted for.
13.4 HOW SHOULD TESTS FOR EDCS BE DEPLOYED IN AN INTEGRATED FASHION?
At present, testing chemicals for ED properties is a relatively new concept and is not yet a routine or integrated feature of regulatory programs. The only regulatory assessment scheme actually in progress at the time of writing is Tier 1 of the U.S. Environmental Protection Agency’s Endocrine Disruptor Screening Program (EDSP) (www.epa.gov/endo/), and this is more in the nature of a trial run for screening tests rather than a full-scale search for endocrine properties across the whole chemical spectrum. Sixty-seven mainly pesticidal substances (not necessarily suspect EDCs) that cause widespread exposure to humans or the environment are being subjected to a large battery of candidate in vitro and in vivo endocrine screening assays, and those screening positive will then be subjected to more advanced Tier 2 in vivo tests that will be used for risk assessment purposes. However, it seems unlikely that such a battery of assays eventually will be needed to evaluate all chemicals. Rather, it is to be hoped that careful evaluation of the EDSP’s results, and of other data, will lead to a much more targeted approach that minimizes or eliminates animal use at the screening stage and reduces it at higher tiers of testing, should such use be deemed necessary in particular cases.
A key point is that evaluation of chemicals for ED properties, as in the EDSP, is still being thought of as a separate activity from more traditional chemical assessments, but efficiency demands that effective methods for detecting and measuring hazards (both endocrine and non-endocrine) ultimately should be deployed in an integrated strategy from the very start of any chemical assessment.
This type of integrated approach is beginning to receive discussion at the international level. For example, a recent OECD workshop on fish testing [14] concluded that a sufficiently complete range of endocrine and nonendocrine fish-based screens and tests has now been, or shortly will be, internationally standardized. This will permit the design of an integrated strategy for assessing the totality of currently understood chemical hazards that this group of organisms may face. A generic version of such a strategy was discussed and proposed as a basis for elaborating more detailed operational schemes under different jurisdictions (see Figure 13.1).
FIGURE 13.1 Scheme for assessing the hazards of chemicals, including endocrine disrupters, to fish [14].
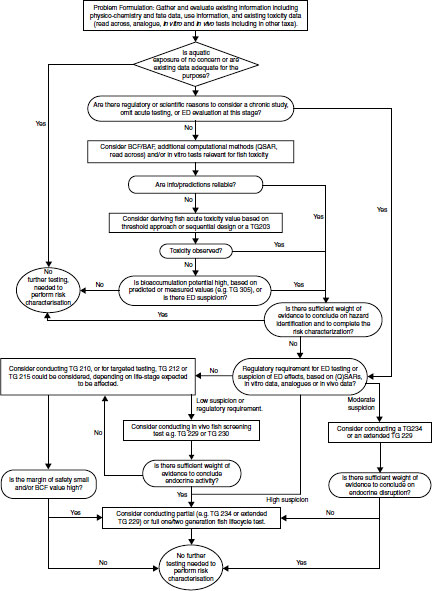
Consideration of this suggested generic approach shows that EDC-sensitive and more traditional assays are closely intertwined and in some cases serve both functions. Thus, for example, fish life cycle tests are potentially the most advanced assays available, irrespective of whether they are being used to assess the apical effects of EDCs or non-EDCs. If early data in a hazard assessment, potentially including those from in silico, in vitro, bio-accumulation, and acute toxicity studies, give rise to concern about possible longer-term effects, then the first question that should be asked is whether the WoE suggests that endocrine disruption may be a factor. If the answer is no, one proceeds down the conventional route of a Fish Early Life Stage Test (FELS—OECD TG 210) or similar, which might lead on to the possibility of partial or full life cycle testing only if margins of safety are small or the bioconcentration factor (BCF) in fish is high.
If the answer is yes (i.e., there are some data pointing to endocrine activity) but the existing WoE for possible endocrine activity is only low, one should then consider the need for an EDC-specific in vivo fish screen (i.e., OECD TG 229, TG 230, or GD 148). In this scenario, a positive screen might suggest the need for partial or full life cycle testing to more completely evaluate the possible ED hazard while a negative result would then trigger a default to the FELS. Moderate WoE for endocrine activity would, however, allow the in vivo screening step to be bypassed in favor of immediate partial life cycle testing (e.g., the Fish Sexual Development Test—FSDT; OECD TG 234; or a Partial Life Cycle Reproduction Test). A negative in these tests probably would be sufficient to permit risk characterization, while a positive might in turn lead on to life cycle testing. Finally, strong suspicion of ED properties generally would lead to the immediate conduct of a life cycle test.
These choices in turn throw up a question about which type of life cycle test to use in particular circumstances, a question that cannot yet be fully answered due to relative inexperience with these assays. However, choice may be dictated by the expected mode of action (e.g., if sexual development is the primary target of the EDC, then the FSDT may be the most appropriate first choice for a longer-term assay), or by whether transgenerational effects are expected (when the MMGT may be more suitable than the FFLCT). One possible clue to the likelihood of transgenerational effects may be the bioaccumulation potential of an EDC. Those with a high BCF in fish are more likely to be passed on as harmful residues from mothers to offspring via the eggs, implying that offspring will receive greater exposure in the MMGT than the FFLCT. Another type of transgenerational effect that could be caused by some EDCs is epigenesis [15], but as with high-BCF substances, it is not yet known if chemicals with epigenetic properties will be consistently more potent in multigeneration tests.
This proposed generic testing strategy for fish is capable of elaboration by individual regulatory authorities, in order to suit their local requirements, and it will be clear that it does not obviate the need for the involvement of experts in the decision-making process. Furthermore, it will need to be expanded to include consideration of aquatic vertebrates other than fish (e.g., amphibian screening assays—OECD TG 231 with Xenopus laevis and higher-tier tests with amphibians) (see Chapter 8) as well as of certain invertebrates when a more complete range of relevant and validated assays becomes available. Life cycle tests for insects with an aquatic life stage (e.g., OECD TG 233 with Chironomus spp.) and crustaceans (a harpacticoid copepod assay with Amphiascus tenuiramis and a mysid two-generation assay with Americamysis bahia are both in development by OECD) are already a practical possibility (see Chapters 4 and 5), and similar tests with molluscs such as Potamopyrgus antipodarum and Lymnaea stagnalis are not far off [7] (see Chapter 6). However, as indicated, validated screening assays for many invertebrates are not likely to be developed any time soon due to our relatively poor understanding of their mechanistic responses to EDCs at the hormonal level. Their potential use as surrogates for ethically less desirable tests with vertebrates is not at present feasible and may never be realized.
Finally, the generic testing strategy also will need to be expanded to consider terrestrial vertebrates (other than mammals, which will have been covered in any human risk assessment), potentially including reptiles and birds. This strategy initially will be driven by consideration of whether such organisms are likely to be exposed to the test substance, but if such exposure is expected, in vivo testing with reptiles or birds may be needed. At present, there are no internationally standardized tests with either reptiles or birds that are likely to be sensitive to EDCs, and the only such assay currently under development by OECD is an avian two-generation test using the Japanese quail, Coturnix japonica. However, Chapters 9 and 10 of this book provide some pointers to possible test developments with these taxa in the future.
13.5 USE OF WEIGHT OF EVIDENCE WHEN ASSESSING POSSIBLE EDCS
It may be apparent from the foregoing discussion that the use of WoE techniques (see [1] for a helpful discussion concerning WoE approaches with EDCs) will be an essential component of hazard evaluations of possible EDCs. While it is not the purpose of this chapter to describe such methods, it is important to explain why they are necessary. In a nutshell, there will almost always be a need for several different items of data about a substance before conclusions either about its status as an EDC or about possible next steps in a testing strategy for EDCs can be made. Put another way, there will rarely be a single test that is able both to identify a substance as possessing an endocrine mode of action, and to fully describe its adverse apical effects in a given species. This fact implies the need to consider multiple items of information before reaching a conclusion either that further testing is needed or that a substance is an EDC. Of course, this concept is already in use in chemical risk assessment schemes, but it will become even more important when they are expanded to detect possible EDCs. The need to consider many pieces of data has been recognized in a new OECD GD [16] that describes in detail how to evaluate the outcome of EDC-responsive assays in light of all existing data and also provides suggestions about a possible next testing step if more data are needed.
One crucial area where this applies is in the early stages of hazard assessment before in vivo ecotoxicity testing is considered, corresponding with the first and fourth boxes in Figure 13.1. At this point, it is vital to assemble all the information known about a substance that could give a clue about possible ED properties. This may include predictions from (quantitative) structure-activity relationships ([Q]SARs) about possible receptor-mediated activity (e.g., [17]; OECD QSAR Application Toolbox www.oecd.org/document/54/0,3343,en_2649_34373_42923638_1_1_1_1,00.html), results of in vitro tests for such activity (e.g. [18]), and information about endocrine activity and ecotoxicity in analogous compounds with similar chemical structures. The most important source of information at this stage could be in vivo studies with rodents and other mammals that are likely to have been conducted in support of the human risk assessment. Many of these studies can give clues about apical effects resulting from possible endocrine activity (e.g., the Extended One-Generation Reproductive Toxicity Study, OECD TG 443), and some like the uterotrophic and Hershberger assays (e.g., OECD TG 440 and 441) have been specifically designed to detect certain types of EDC.
The evaluation of all this initial information will rarely result in a clear-cut conclusion; instead, the outcome will lie on a spectrum of probabilities. For example, if the information is comprehensive and entirely negative with respect to ED properties, there may be an acceptable level of confidence, but not certainty, that the substance in question is not an EDC via the modes of action considered. In this scenario, it is still possible that the substance gives rise to endocrine-active metabolites that would not be detectable in current in vitro screens (which do not generally have metabolic competence). Likewise, current (Q)SARs for endocrine activity are still in their infancy, and they will fail to flag up certain potential EDCs. These uncertainties may lead some regulatory authorities to conclude that in vivo screening for endocrine activity is needed for all chemicals, but this would have huge financial, logistic, and ethical implications.
If the initial evidence on a chemical suggests that there may be some endocrine activity, its strength (i.e., the weight of that evidence) should be used to decide on the type of in vivo testing that may be required. For example, if the initial evidence is incomplete, it may be desirable to obtain more comprehensive in vitro data before proceeding. However, if the initial data are reasonably comprehensive but provide only low confidence that the substance may be an EDC, it probably will be appropriate to obtain some in vivo screening data such as that available for fish from OECD TG 229 or 230, or GD 148, or amphibians from TG 231. Negative results in these screens would allay concerns about certain types of endocrine disruption (i.e., estrogen/androgen/steroidogenesis/thyroid mediated), while positive results generally would result in consideration of the need for partial or full life cycle testing (as described for fish). If the initial data provide a stronger suspicion of possible endocrine activity, it probably will be most efficient to consider life cycle testing without an intermediate in vivo screening step.
It may be considered that read-across of conclusive endocrine data from a positive mammalian test would obviate the need for in vivo ecotoxicity testing, but as yet we have insufficient experience with these tests or read-across procedures to be confident of extrapolating risk assessments across vertebrate classes. As indicated, not the least problem is the difficulty of comparing data based on oral dosing (mammals) with data based on ambient exposure (fish and amphibians). However, if the chemical is to be regulated on the basis of its ED hazard alone, as in the European Union, some regulatory authorities may be content to categorize the substance as an EDC solely on the basis of positive mammalian data. Equally, negative mammalian data cannot at present be taken to imply that other vertebrate taxa will definitely fail to show ED responses.
13.6 CONCLUSIONS
Enough standardized and validated in vivo ecotoxicity tests with sensitivity to a range of EDCs are now available, or shortly will be available, to begin constructing efficient testing strategies that can be integrated into mainstream chemical assessments.
Assessments of the data from such tests will depend on consideration of the WoE not only from in vivo procedures but also from (Q)SARs, in vitro tests, and read-across from related chemicals and from in vivo tests in other taxa.
There are a number of gaps in the suite of standardized EDC-sensitive in vivo tests that need to be filled in order to provide a more comprehensive toolbox’ or chemical companies and regulatory authorities. The more flexibility that is made available in this tool box, the more efficient and targeted chemical evaluation programs will become.
Research is still needed on mechanisms of action of EDCs in invertebrates in order to permit the design of suitable screening tests. Furthermore, research on certain novel mechanisms of action of EDCs in both vertebrates and invertebrates will be required before it is possible to claim that the testing tool box is fully comprehensive.
REFERENCES
1. Borgert, C. J., Mihaich, E. M., Ortego, L. S., Bentley, K. S., Holmes, C. M., Levine, S. L., Becker, R. A. (2011). Hypothesis-driven weight of evidence framework for evaluating data within the US EPA’s Endocrine Disruptor Screening Program. Regulatory Toxicology and Pharmacology 61(2): 185–191.
2. Oaks, J. L., Gilbert, M., Virani, M. Z., Watson, R. T., Meteyer, C. U., Rideout, B. A., Shivaprasad, H. L., Ahmed, S., Chaudhry, M. J. I., Arshad, M., Mahmood, S., Ali, A., Khan, A. A. (2004). Diclofenac residues as the cause of vulture population declines in Pakistan. Nature 427: 630–633.
3. Matthiessen, P., Gibbs, P. E. (1998). Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environmental Toxicology and Chemistry 17: 37–43.
4. Matthiessen, P., Reynoldson, T., Billinghurst, Z., Brassard, D. W., Cameron, P., Chandler, G. T., Davies, I. M., Horiguchi, T., Mount, D. R., Oehlmann, J., Pottinger, T. G., Sibley, P. K., Thompson, H. M., Vethaak, A. D. (1999). Field assessment of endocrine disruption in invertebrates. In: DeFur, P. L., Crane, M., Ingersoll, C., Tattersfield, L. (eds.), Endocrine Disruption in Invertebrates: Endocrinology, Testing and Assessment. SETAC, Pensacola, FL pp. 199–270.
5. LeBlanc, G. A., Campbell, P. M., Den Besten, P., Brown, R. P., Chang, E. S., Coats, J. R., DeFur, P. L., Dhadialla, T., Edwards, J., Riddiford, L. M., Simpson, M. G., Snell, T. W., Thorndyke, M., Matsumura, F. (1999). The endocrinology of invertebrates. In: DeFur, P. L., Crane, M., Ingersoll, C., Tattersfield, L. (eds.), Endocrine Disruption in Invertebrates: Endocrinology, Testing and Assessment. SETAC, Pensacola, FL, pp. 23–106.
6. Oehlmann, J., Di Benedetto, P., Tillman, M., Duft, M., Oetken, M., Schulte-Oehlmann, U. (2007). Endocrine disruption in prosobranch molluscs: Evidence and ecological relevance. Ecotoxicology 16: 29–43.
7. Matthiessen, P. (2008). An assessment of endocrine disruption in mollusks, and the potential for developing internationally-standardised mollusk lifecycle test guidelines. Integrated Environmental Assessment and Management 4: 274–284.
8. Pickford, D. B. (2010). Screening chemicals for thyroid-disrupting activity: A critical comparison of mammalian and amphibian models. Critical Reviews in Toxicology 40: 845–892.
9. Organisation for Economic Cooperation and Development (2012). Detailed Review Paper on the State of the Science on Novel in vitro and in vivo Screening and Testing Methods and Endpoints for Evaluating Endocrine Disruptors. Series on Testing and Assessment no. 178, ENV/JM/MONO(2012)23, Organisation for Economic Cooperation and Development, Paris. 213 pp.
10. Bjornsson, B. T., Stefansson, S. O., McCormick, S. D. (2011). Environmental endocrinology of salmon smoltification. General and Comparative Endocrinology 170: 290–298.
11. Bangsgaard, K., Madsen, S. S., Korsgaard, B (2006). Effect of waterborne exposure to 4-tert-octylphenol and 17 beta-estradiol on smoltification and downstream migration in Atlantic salmon, Salmo salar. Aquatic Toxicology 80, 23–32.
12. Lower, N., Moore, A. (2007). The impact of a brominated flame retardant on smoltification and olfactory function in Atlantic salmon (Salmo salar L.) smolts. Marine and Freshwater Behaviour and Physiology 40: 267–284.
13. Pottinger, T. G. (2003). Interactions of endocrine-disrupting chemicals with stress responses in wildlife. Pure and Applied Chemistry 75: 2321–2333.
14. Organisation for Economic Cooperation and Development (2012). Fish Toxicity Testing Framework. Series on Testing and Assessment no. 171, Organisation for Economic Cooperation and Development, Paris. ENV/JM/MONO(2012)16, 174 pp.
15. Vandegehuchte, M. B., Janssen, C. R. (2011). Epigenetics and its implications for ecotoxicology. Ecotoxicology 20: 607–624.
16. Organisation for Economic Cooperation and Development (2012). Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. Series on Testing and Assessment no. 150, ENV/JM/MONO(2012)22, Organisation for Economic Cooperation and Development, Paris. 524 pp.
17. Organisation for Economic Cooperation and Development. (2006). Report on the Regulatory Uses and Application in OECD Member Countries of (Quantitative) Structure-Activity Relationships [(Q)SAR] Models in the Assessment of New and Existing Chemicals. Series on Testing and Assessment no. 58, OECD, Paris. 79 pp.
18. Organisation for Economic Cooperation and Development. (2010). Detailed Review Paper on Environmental Endocrine Disruptor Screening: The Use of Estrogen and Androgen Receptor Binding and Transactivation Assays in Fish. OECD Series on Testing and Assessment no. 135, ENV/JM/MONO(2010)34. OECD, Paris. 64 pp.