CHAPTER 4
Modeling Krill in the California Current

A 2005 CASE STUDY
Jeffrey G. Dorman
Abstract. Examining ecosystem response in coastal upwelling regions to variable atmospheric conditions can help us understand how sensitive these ecosystems may be to climate-driven atmospheric perturbations. A coupled ocean circulation model (ROMS) and a model representing the biology of a prey species (krill) was used to compare predator species during a typical year off northern California (2001) and a year in which the onset of upwelling occurred later and weaker (2005). Decreases in predator survival (salmon) and reproductive success (seabirds) in 2005 were related to poor ocean-feeding (krill) conditions. Hence, these commercially important regions need to be managed from an ecosystem-based approach that depends on a better understanding of the impacts of climate change on atmosphere and biology of the region.
Key Points
• Anomalous physical conditions (i.e., climate change effects on water temperature, and extent of upwelling) can influence the highest trophic levels within upwelling ecosystems.
• Shifts in the timing and intensity of upwelling can disrupt predator–prey interaction in the California Current.
• Single species fishery management is insufficient in light of the ecosystem variability expected with climate change. Continued implementation of Ecosystem-Based Fishery Management (EBFM) techniques to account for climate-driven ecosystem variability will be important for coastal resource management.
• Marine Protected Areas (MPAs) may not serve their designated purposes in light of changing oceanographic conditions. Maintaining flexibility in the design of MPAs over their lifetime will ensure they are able to serve their intended purpose in light of environmental variability due to climate change
INTRODUCTION
The California Current System (CCS) is one of the four major coastal upwelling regions in the world’s oceans. These regions are some of the most productive ecosystems in the ocean, and despite occupying less than 1% of the ocean’s surface area, they contribute over 20% of the global commercial fish catch. Within California, there are over 100 commercial fisheries that account for annual revenues of over 100 million dollars (Pacific Fisheries Information Network 1981–2011) and large numbers of coastal jobs. As such, it is of great interest to understand how climate change could impact these important coastal resources.
In most of the world’s oceans, primary productivity in surface waters is considered “nutrient-limited,” as any nutrients required for photosynthesis and plant growth that enter surface waters are quickly utilized. Waters that are below the depth where light is sufficient for photosynthesis, which can range from 10 to 100 m depending on the water clarity, are typically high in nutrients, and photosynthesis in this zone is said to be “light-limited.” The exchange of water (and the nutrients in the water) between deep and surface regions is limited in most regions due to the density difference between warm surface water (less dense) and cold deep water (more dense). The surface waters within upwelling regions are so productive because the alongshore winds provide a means to draw up cold, nutrient-rich water from the deep ocean and essentially fertilize the well-lit surface waters. The upwelled nutrients are quickly utilized by small plant species (phytoplankton), which create “blooms” of phyto plankton in surface waters. The phytoplankton are fed on by small zooplankton (copepods, krill) and small fish (anchovy and herring), which are in turn fed upon by many commercially important species (hake, salmon, rockfish, sablefish, squid). Without the wind-driven upwelling of nutrients to surface waters, the base of the food chain (phytoplankton) would have low abundance and productivity, and the resulting higher trophic level productivity would be greatly reduced. The nutrient-driven phytoplankton blooms and the small number of trophic steps from phytoplankton to commercially important predator species are the primary reasons that the CCS is one of the most productive regions for commercial fisheries in the world.
Winds are the driver of the productivity of upwelling systems, and any change in atmospheric conditions due to climate change will influence the biological productivity of coastal regions through changes in wind strength, direction, and / or timing. Regional modeling studies of the CCS indicate that changes in atmospheric conditions are likely (Snyder et al. 2003), and there is evidence of increase in wind strength in upwelling regions (Bakun 1990). Changes in wind patterns can affect physical attributes of the water in many different ways (density structure, temperature, currents), which in turn affect individual organism physiology (metabolic rates and duration of the larval life stage), species populations (larval dispersal, size structure, range shifts), and entire communities (changes in predator–prey dynamics). Increasing our understanding of how organisms, populations, and communities will most likely respond to these changes will greatly aid the management of California’s coastal resources in a changing climate.
To help assemble information needed to manage ocean resources as climate changes, we are examining the potential climate change impacts on the biological productivity of coastal upwelling regions, with a focus on the krill species Euphausia pacifica. E. pacifica is a common species of zooplankton throughout the California Current (Brinton 1962) and plays an important role in the regional food web (Field et al. 2006). E. pacifica feeds primarily on the upwelling region’s diatom-rich phytoplankton blooms, and is preyed upon by a myriad of higher trophic level predators including many commercially important fishes (hake, salmon, rockfish) (Genin et al. 1988, Yamamura et al. 1998, Tanasichuk 1999) and seabirds (Ainley et al. 1996). As the primary productivity in the region is tied to climatic events (e.g., upwelling-favorable winds), and the krill provide a direct path to higher trophic levels, changes in their population biology in response to changing climate events could reverberate up to the higher tropic levels. Decreases in E. pacifica abundance due to atmospheric forcing (unfavorable upwelling winds) are hypothesized to be the likely cause of two recent years (2005 and 2006) of reproductive failure in a seabird population (Cassin’s auklet; see Box 4.1) on the Farallon Islands (Sydeman et al. 2006), and notable declines in salmon returns in 2008 and 2009 that resulted in the closure of the commercial salmon fishery (see Box 4.1; Lindley et al. 2009). These are pointed examples of how understanding the response of important prey species to climate-driven ocean conditions will enable better management of the organisms that are dependent upon them as a food source.
BOX 4.1 • Two recent examples of dramatic changes to upper trophic level marine populations
Cassin’s auklet (Ptychoramphus aleuticus) and Chinook salmon (Oncorhynchus tshawytscha) have captured public attention and exposed how climate change may be interrupting important tropic interactions. In 2005 and 2006, the Cassin’s auklet population at the Farallon Islands experienced near total reproductive failure due to inadequate food resources. In 2007, record low numbers of juvenile salmon returned to the Sacramento River, ultimately resulting in the closure of the 2008 commercial and recreational salmon fishery in California (Box Figure 4.1.1).
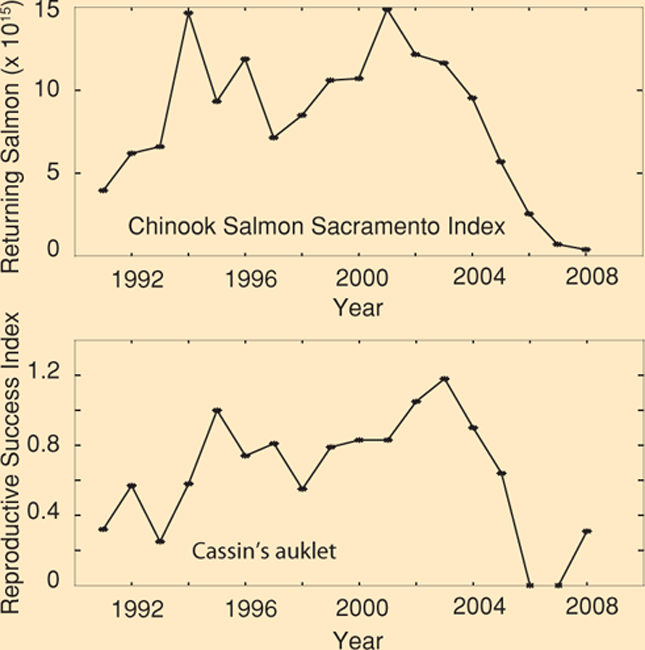
BOX FIGURE 4.1.1: The Chinook Salmon Sacramento Index (sum of escapement, ocean harvest and river harvest) and the reproductive success of Cassin’s auklets on the Farallon Islands from 1990 to 2008. Sacramento Index data from O’Farrell et al. (2009) and on Cassin’s auklets reproductive success from monitoring conducted by US Fish and Wildlife Service and Point Blue.
Cassin’s Auklets
Cassin’s auklets are diving marine birds that feed on zooplankton and small fishes over the continental shelf off California. During the breeding season on the Farallon Islands, the adults lay eggs in early April, the eggs hatch in May, and adults feed chicks from May through early July. The breeding and chick rearing period coincides with the onset of upwelling-favorable conditions and increases in abundances of krill species Euphausia pacifica and Thysanoessa spinifera. Both of these species are an important part of their diet. In spring of 2005, egg laying by Cassin’s auklets was delayed by almost a month and all eggs were abandoned within weeks of laying (Sydeman et al. 2006).
Abandonment of eggs is a survival strategy in long-lived birds to essentially cut losses when reproductive conditions are poor and ensure adult survival so that reproduction will occur in subsequent years. The reproductive failure in 2005 coincided with delayed upwelling and anomalously low primary and secondary productivity early in the year. Events of this type highlight the sensitivity of higher trophic levels to the potential impacts of climate change on the coastal ocean. In 2006, another year of reduced upwelling-favorable winds and warmer surface temperatures (especially during the spring months), a high percentage of Cassin’s auklet eggs did not hatch due to abandonment, and only a few chicks survived to fledgling. Reproductive failure of this nature had never been observed in the 35 years of monitoring the Cassin’s auklet breeding colony at the Farallon Islands (Sydeman et al. 2006).
Chinook Salmon
The Sacramento River salmon run is one of the largest on the West Coast of the United States and provides a significant portion of the salmon caught commercially and recreationally off Oregon and California. Salmon begin their life in freshwater, spend the majority of their lives feeding in the coastal ocean, and return to freshwater as adults to spawn after 3–4 years (see Quinones and Moyle in this volume). Some juvenile salmon, called “jacks,” return to the freshwater environment a year early and their number is considered a good indicator of the number of adults that will return to spawn in the subsequent year. In the fall of 2007, the number of returning jacks (<2000 salmon) was the fewest since records have been kept and well below the average number (∼40,000 salmon). This prompted the unprecedented closure of the 2008 commercial and recreational salmon fishery in Oregon and California. As salmon live across such varied habitats, there could be many reasons for the decline in abundance. However, as stock estimates for Oregon and British Columbia rivers were also depressed, it is likely that ocean conditions are at least partially to blame. At least some of the salmon that returned to spawn in the fall of 2008 were juveniles in 2005, and entered the ocean to feed on krill when ocean productivity was very low. It is possible that the reduced productivity that led to reproductive failure in Cassin’s auklets also led to increased mortality in juvenile salmon, decreased juvenile returns in 2007, and a closed salmon fishery in 2008.
METHODS
An oceanographic model, a Nutrient-Phytoplankton-Zooplankton-Detritus (NPZD) model, and an individual-based model parameterized for the krill species E. pacifica were utilized and linked to model the response of prey species to changing ocean conditions. We ran the models for the years 2001 and 2005 to compare a “normal year” (2001) with the conditions of 2005 that led to anomalously low higher trophic level success.
The coastal ocean was simulated using the Regional Ocean Modeling System (ROMS) (Shchepetkin and McWilliams 2005, Haidvogel et al. 2008) over a region of the eastern Pacific Ocean from Newport, Oregon (∼44° 30′N latitude), to Point Conception, California (∼35° N latitude), and up to 450 km offshore. To run ROMS, inputs of atmospheric conditions are required that drive currents (via winds) and heat and cool the ocean (with radiation fluxes, air temperature, humidity, and precipitation). Atmospheric conditions were provided at three-hour intervals from the North American Regional Reanalysis model dataset provided by the National Centers for Environmental Prediction (Mesinger et al. 2006). Ocean conditions at the edges of the modeled region were provided from the global ocean model Estimating the Coastal Circulation of the Ocean (ECCO2) (Menemenlis et al. 2008). A simple NPZD model (Powell et al. 2006) was incorporated into the ROMS model to provide a food source (phytoplankton) for the simulated E. pacifica.
The population biology of E. pacifica was simulated using the Individual-Based Model (IBM) POPCYCLE (Batchelder and Miller 1989, Batchelder et al. 2002). The use of an IBM to simulate E. pacifica’s population biology allows parameters for bioenergetics (ingestion, respiration, assimilation) to vary with attributes of the individual (e.g., size, age, sex), and allows a more accurate representation of discrete events such as reproduction or mortality. As we have incorporated the IBM into the three-dimensional ROMS domain, organism movements and behaviors can be included, along with known variability in those behaviors. For each time-step of the IBM, data on temperature, salinity, current velocity, and phytoplankton abundance are retrieved from the ROMS model output. Each krill individual in the IBM then undergoes growth, stage development, evaluation for reproduction (female only), evaluation for mortality, and spatial position update based on the corresponding ROMS and NPZD data (Figure 4.1).
The bioenergetics of the model were parameterized for the species E. pacifica based on extensive laboratory studies on feeding (Ohman 1984), growth, and development (Ross 1982a, Ross 1982b, Feinberg et al. 2006). For every time-step of the IBM, each krill individual is evaluated for growth based on food resources (phytoplankton) and on the physical environment (temperature) from its corresponding location within the ROMS model. Krill were seeded at a weight (40 µg C) representing an early larval stage based on field data from northern California (Dorman et al. 2005). Krill then advance through life stages (based on weight gain), are evaluated for reproduction (based on life stage, sex, and reproductive parameters) and starvation (based on potential weight loss under food limited conditions), and krill location is updated within the model domain based on currents from the ROMS model. Individual krill are removed from the population either through starvation or by being transported beyond the domain of the physical model. The IBM was seeded with approximately 5000 krill that were evenly distributed over the continental shelf (within 60 km of the coast) between Point Arena, California (∼38 N latitude) to the southern model boundary. Data were collected from the model runs of 200 days, beginning on January 5 of 2001 and 2005.
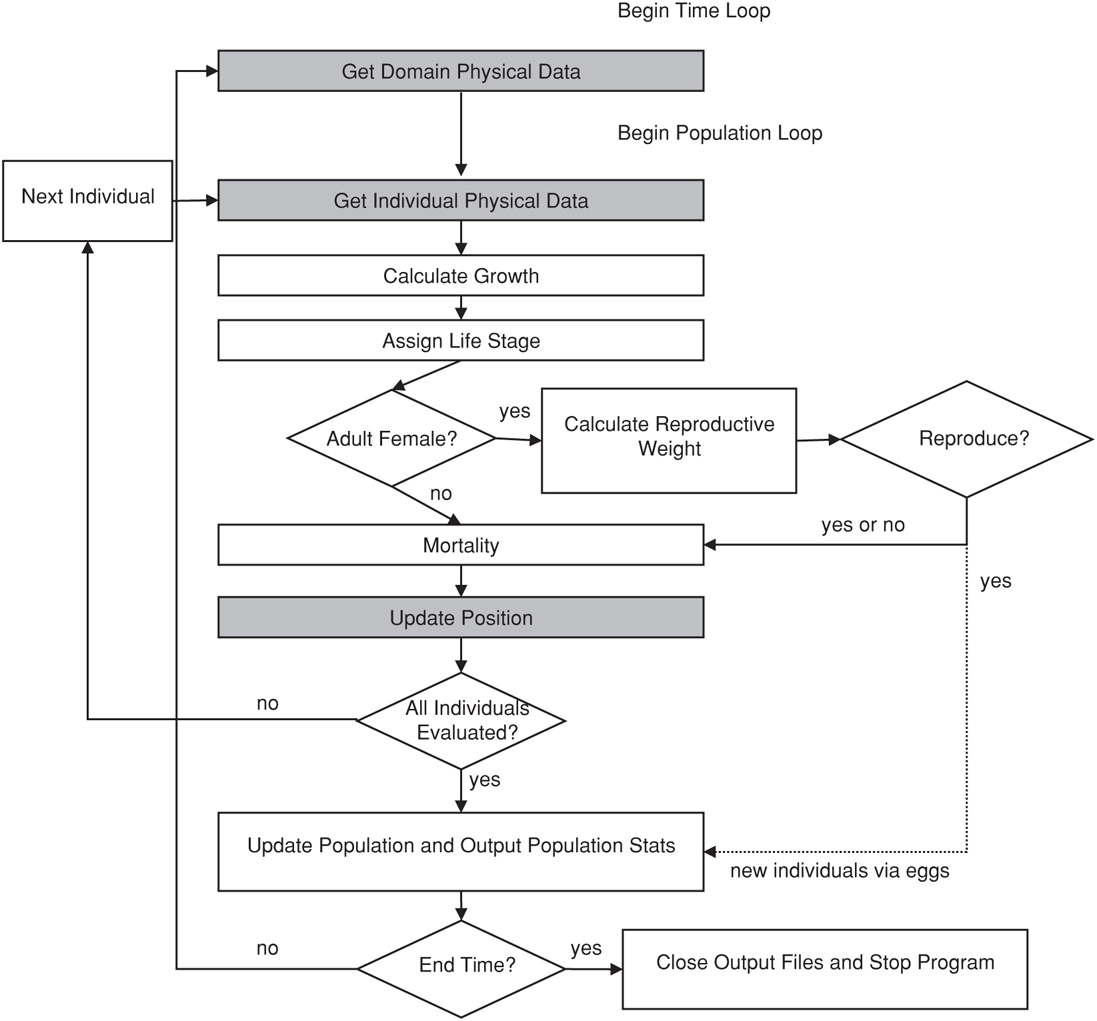
FIGURE 4.1: The structure of the individual-based model POPCYCLE that was used to model changes in krill.
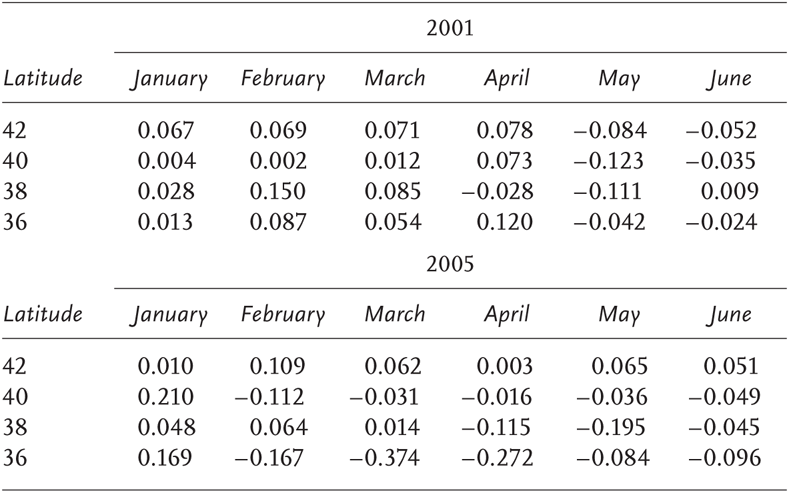
TABLE 4.1
Monthly mean along shore current velocity from locations approximately 30 km offshore. Poleward flow is represented by positive values and flow toward the equator by negative values
NOTE THE STRONG POLEWARD FLOW IN JANUARY 2005
The use of an IBM that includes stage- or size-specific parameters allows the inclusion of life history traits such as reproduction or growth rates that vary with life stage or size. The modeled krill for this study undergoes variable diel vertical migration based on both size and life stage. This results in differences between the depths occupied by adults and larvae during a 24-hour cycle, thereby exposing various life stages to potentially differing currents, and allowing us to more realistically simulate potential changes in the spatial distribution of krill as ocean conditions change.
RESULTS
We compared modeled alongshore currents between 2001 and 2005 from locations 30 km offshore at 36°, 38°, 40°, and 42° N latitude (Figure 4.2). Model results indicate anomalously strong and northward (poleward, upwelling unfavorable) currents in the coastal region of interest during January 2005 (Table 4.1). Upwelling-favorable (equatorward) currents were fully established during May and June in 2001, and one month earlier in 2005 except in the northernmost regions of the model domain.
Northward krill advection by currents was most evident during January 2005, resulting in 10.6% of simulated krill being advected north of Cape Mendocino (∼40° 20′N latitude), compared with 0.6% in January 2001 (Figure 4.3).
Mean krill weight was significantly greater, for much of the model run time, in 2001 than in 2005 (Figure 4.4). The model results suggest that krill during 2001 and 2005 gained a similar amount of weight until approximately year-day 40, after which krill in 2001 gained 0.35 µg more carbon per day on average than those in 2005. As a result of this difference in weight gain, at the end of the model run, average krill weight was significantly greater in 2001 (mean = 178.7 µg C) than in 2005 (mean = 120.9 µg C) (t = 18.88, df2001 = 1088, df2005 = 545, p < 0.001). An analysis of the 100 modeled krill that gained the greatest weight over the course of the model run (approximately 2% of the total population) also showed significantly more weight gain during 2001 (mean = 321.7 µg C) than in 2005 (mean = 192.0 µg C) (t = 19.1, df2001 = 99, df2005 = 99, p < 0.001) (Figure 4.5).

FIGURE 4.2: Alongshore and cross-shelf surface current velocity at 30 km offshore of (a) 42° North, (b) 40° North, (c) 38° North, and (d) 36° North. Poleward and onshore flows are represented by positive values and negative values indicating flow toward the equator and offshore flows.
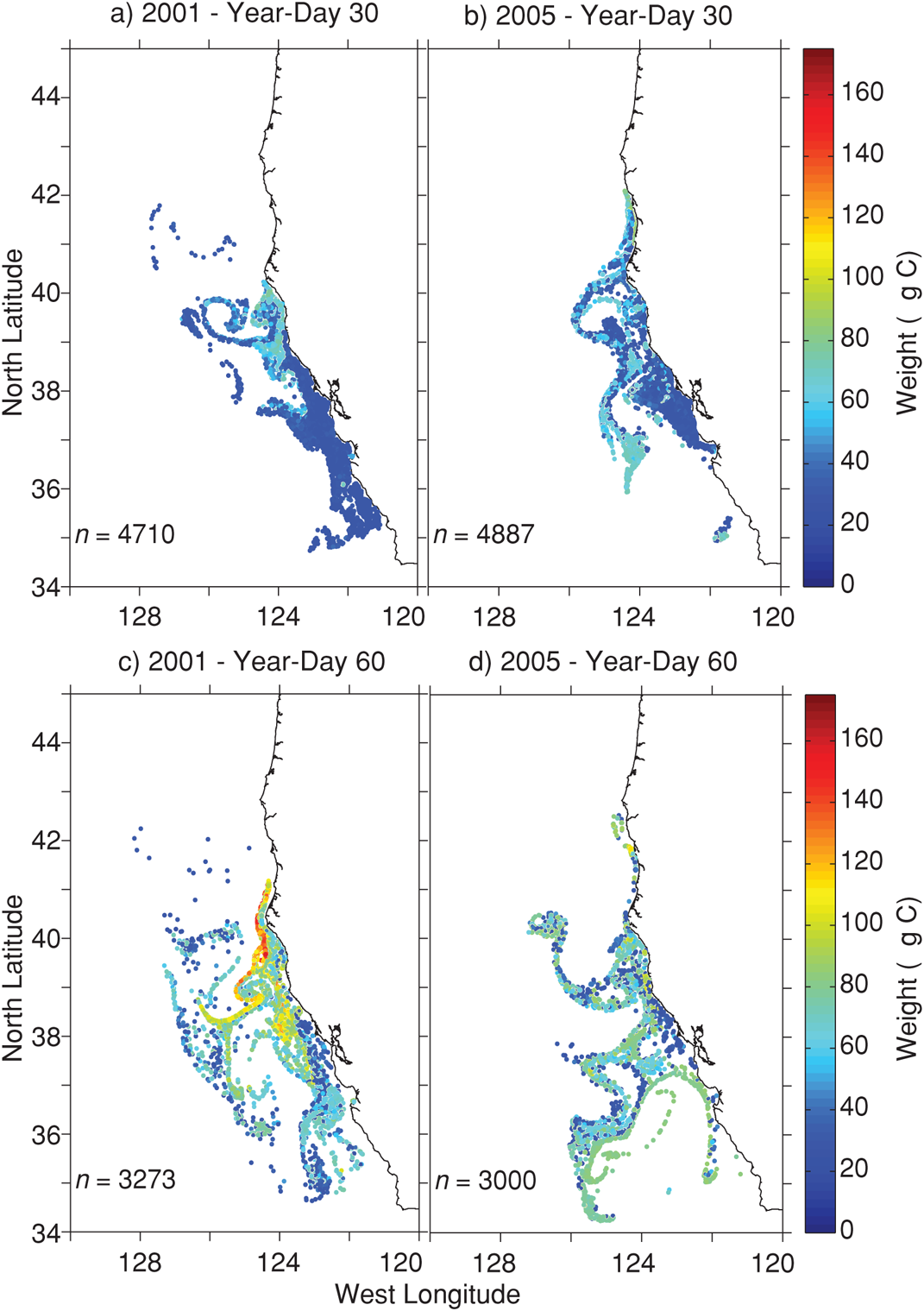
FIGURE 4.3: Krill distribution and weight on year-day 30 of (a) 2001 and (b) 2005, and year-day 60 of (c) 2001 and (d) 2005.

FIGURE 4.4: Mean population weight of all live krill within the model domain during 2001 and 2005, and the mean weight of the 100 individuals that gained the most weight during the model run during 2001 and 2005. Error bars represent 95% confidence intervals of the mean.
The models suggest that food limitation was an important factor limiting population growth. Starvation of the initial seeding of krill was evident during both 2001 and 2005 with 31.9% and 45.2% of individuals starving, respectively, without molting to the next life stage (Figure 4.5). For those individuals that did gain enough weight to molt to the next life stage, furcilia IV, and beyond, starvation was less in 2001 than in 2005, with 3.8% and 21.8% of krill starving, respectively.
DISCUSSION
These simulations indicate that higher starvation mortality of juvenile and adult krill was the primary reason for the lower abundance of E. pacifica observed during the spring and summer of 2005 in the central California Current. The greater modeled mortality in 2005 was caused by anomalously low phytoplankton food sources during periods of typical high primary production, and was influenced by northward advection of krill to regions that typically experience lower primary productivity at this time of year.
During both 2001 and 2005, there was high starvation of krill early on in the model run (∼year-day 34). High starvation is to be expected during winter months due to the reduced wintertime primary productivity, yet starvation continued on through the spring months in much greater numbers in 2005 than in 2001 due to a delayed onset of upwelling during 2005. Advection also played a role in increasing winter mortality as much of the coastal region experienced strongly poleward currents during January 2005. This resulted in many krill being transported to the north of Cape Mendocino where seasonal upwelling does not typically begin until early summer. Thus, these krill were transported to a region still months away from any significant upwelling of nutrients, and over 90% of the modeled population that was transported to the north died in 2005. Increases in wintertime starvation of E. pacifica like the ones suggested by these results would ultimately reduce the abundances of this key prey species during the spring months, as there is little observational evidence of any significant reproduction by E. pacifica occurring during winter months that would maintain population size. Thus, decreases in the wintertime population would have negative impacts on predators that utilize the krill population in early spring, before reproduction begins again with the onset of upwelling.
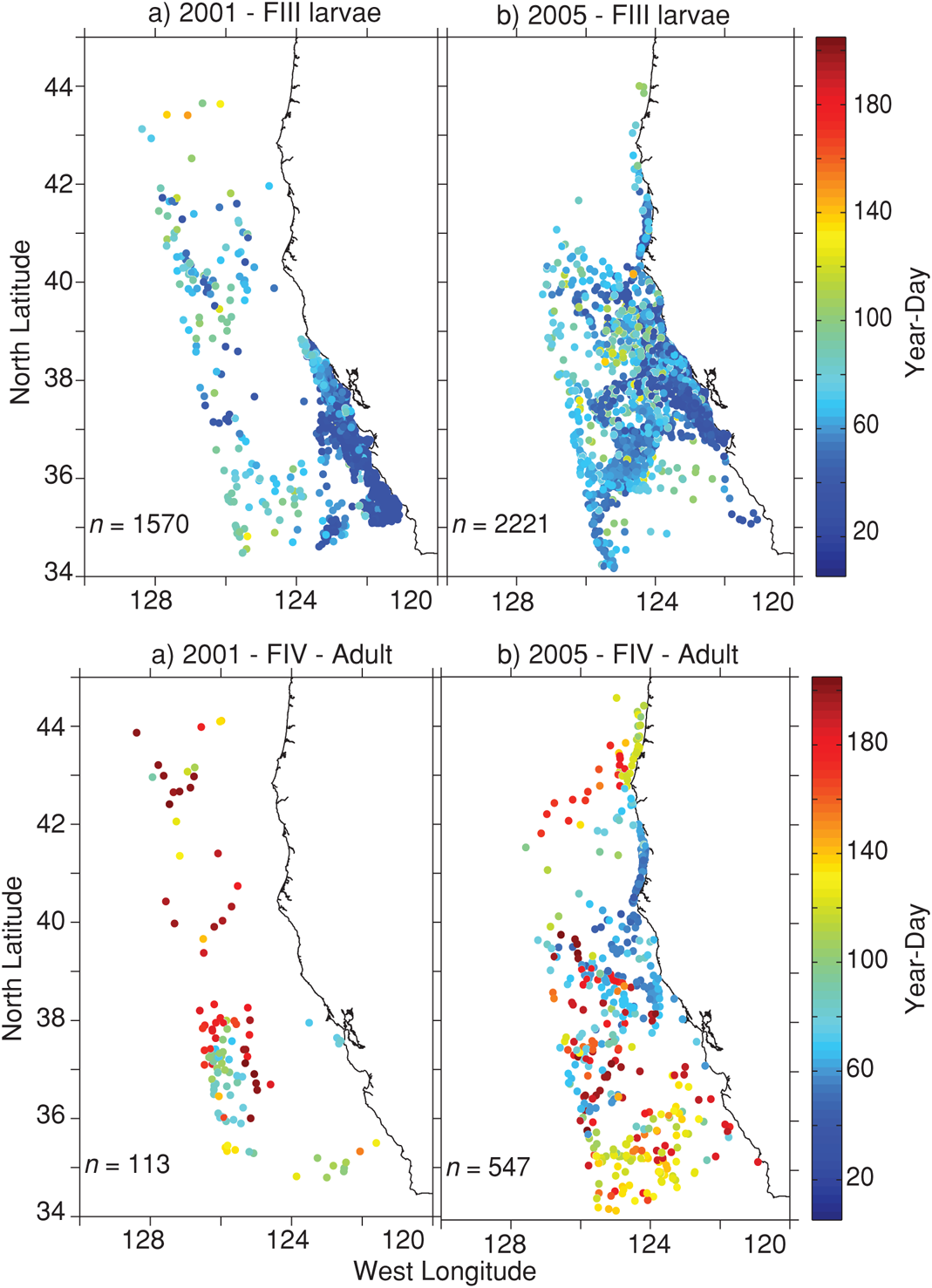
FIGURE 4.5: Starvation location and time of furcilia III larval stage krill during (a) 2001 and (b) 2005, and starvation location and time of later stage krill (furcilia IV through adult) during (c) 2001 and (d) 2005.
In addition to increased starvation, those krill that did survive gained less weight over the course of the model run during 2005. Growth of krill during the early part (January–March) of any year is typically a period of slow growth (or actual weight loss) as this is not a time of strong upwelling in the region. During 2005, the mean weight of the population was significantly less than during 2001, with the greatest difference in weight gain occurring in the spring (April–June). This is due to the unusually late onset of upwelling during 2005. Smaller krill in an ecosystem equates to less krill biomass, and impacts predators through their need to increase the time and energy spent foraging, which also increases their exposure to predators higher up in the food chain.
While the entire population of krill is of interest as a food source for higher trophic levels, only a small percentage of the population will survive to adulthood and reproduce. Those individuals that have gained the most weight during the course of the model run are representative of krill that will reach adulthood the fastest, and provide information on the potential timing of future reproductive events. A comparison of the 100 krill that gained the most weight over the course of the model run revealed a greater disparity in weight gain between 2001 and 2005 than when comparing the entire population, with greater weight gain in 2001. Thus, in addition to suggesting population-level impacts on current krill, these simulations suggest that in 2005 those krill that would have produced the next generation of larvae would have required more time to mature, resulting in greater exposure to potential predators, and would have reproduced later in the year than the 2001 population.
The impacts of the sort of anomalous conditions experienced during 2005 in the California Current have been the source of much interest (see special section of Geophysical Research Letters 2006, Volume 33, Issue 2, Jahncke et al. 2008). A season of delayed upwelling to the extent that was observed in 2005 allows a glimpse into how anomalous atmospheric conditions influenced the entire food web of the California Current up to the highest trophic levels (see Box 4.1). Our results mechanistically explore how the spatial and temporal distribution of krill, a key prey species, might have played a role in the low production observed at these higher trophic levels.
SOLUTIONS, ADAPTATIONS, AND LESSONS
The impacts of climate change on zooplankton in the California Current, and specifically on the krill species E. pacifica, are far from certain. The anomalous conditions from 2005 that were modeled in this case study are not necessarily indicative of the types of conditions that we should expect under a warmer climate. However, these types of modeling results highlight the possible ramifications of changing ocean conditions on a key prey species of many higher trophic levels in the California Current (see Box 4.1). This interconnection between upwelling-favorable winds and krill, and krill with many top predators, highlights the importance of shifting the management of our coastal resources to a broad ecosystem-based approach.
Traditionally, fisheries management decisions in the United States have considered species-scale variability, with little focus on ecosystem variability and interactions between trophic levels. Single-species techniques are valuable, but are insufficient by themselves in light of the potential ecosystem-wide changes that will likely result from climate change. Ecosystem-scale assessments must also be incorporated into fisheries management to make sense of system variability and the large-scale impacts of climate change. The framework for Ecosystem-Based Fishery Management (EBFM) was laid out by the Ecosystem Principles Advisory Panel (1999) in response to the Sustainable Fisheries Act (SFA) of 1996, which calls for fisheries to be managed while taking the biological complexities and the overall health of the ecosystem into account. EBFM is a move away from single-species fishery models to a more holistic view of the ecosystem that may incorporate prey-species population dynamics, habitat conditions, and environmental variation. Where data on these parameters are not available, the SFA urges erring on the side of caution. Implementation of EBFM is an ongoing process (Field and Francis 2006) in California and will be essential to managing fisheries in response to climate change.

FIGURE 4.6: Three impacts (in bold text) of increasing temperatures and increased atmospheric CO2. The physical and biological paths through which these changes might impact productivity and the potential impacts on productivity are noted.
1. (Mendelssohn et al. 2003, Palacios et al. 2004, Bograd and Lynn 2003)
2. (Palacios et al. 2004, Capotondi et al. 2012)
3. (O’Connor et al. 2007, Pörtner and Farrell 2008)
4. (Beaugrand et al. 2002, Lindley and Daykin 2005)
5. (Bakun 1990, Schwing and Mendelssohn 1997, Snyder et al. 2003, Bograd et al. 2009, García-Reyes and Largier 2012)
6. (Cury and Roy 1989, Gargett 1997, Botsford et al. 2003, Botsford et al. 2006, García-Reyes and Largier 2012)
7. (Chan et al. 2008, Bograd et al. 2008, Pierce et al. 2012)
8. (Mackas et al. 1998, Edwards and Richardson 2004, Costello et al. 2006, Text Box 4.1)
9. (Fabry et al. 2008, Doney et al. 2009)
The primary hindrance to effective implementation of EBFM is the uncertainty regarding the basic ecosystem changes that are expected (Figure 4.6). It is understood how increased atmospheric CO2 will directly impact the physical ocean characteristics of the California Current (increasing sea surface temperatures, increasing upwelling favorable winds, decreasing oceanic pH). These changes will influence a myriad of other physical characteristics (water column stratification, ocean currents, salinity, dissolved oxygen levels), all of which can impact the biology of the coastal ocean. In many cases, we have a fairly complete understanding of how a change in one single process will impact biological productivity, but our level of understanding is often lacking when considering how changes in multiple physical factors will impact the biology of the region. For example, increased upwelling favorable winds may cause upwelling of more nutrients into surface waters, but increased sea surface temperatures will cause greater thermal stratification (and thus a stronger barrier) between warm nutrient-poor surface water and cold nutrient-rich deep waters. Nutrients will only be upwelling into surface waters if the increase in wind strength is great enough to overcome the increased strength of thermal stratification. Extrapolating the expected changes in physics to the biology of individual organisms and food webs of the California Current introduces even more uncertainty into our understanding. Research to clarify the impacts of climate change on the biological productivity of the coastal ocean is needed in the following areas: Ocean ecosystem response, species-specific response, and food web response.
Ocean Ecosystem Response
Significant work has been accomplished toward understanding the impacts of climate change on the global atmosphere using large-scale climate models. As California’s coastal ocean is primarily atmospheric driven, emphasis is also needed on applying these warmer atmospheric conditions to California’s upwelling system. Ocean models forced by an atmosphere containing increased CO2 concentrations will provide a better understanding of the oceanographic conditions necessary (currents, nutrients, temperature, pH, oxygen) to predict lower trophic level biological responses (phytoplankton and zooplankton productivity) using knowledge of ecosystems and individual species. It should be noted that many of these models do not take pH or temperature into consideration, but they can provide a first approximation of the impacts of climate change on primary producers and consumers.
Species-Specific Response
There are many commercially important species in California’s coastal ocean and each species will have a unique biological response to climate change. For example, species that have calcareous exoskeletons or shells may be more susceptible to changing ocean pH levels (increased mortality due to a weakening of calcareous structures), while pelagic fish may be affected by increasing temperature (range shifts, changes in physiology). Research on the dominant species responses (physiology, range and phenology shifts, population biology, etc.) to changing ocean conditions needs to be a priority if we are to effectively manage these populations and the pelagic ecosystem with climate change considerations.
Food Web Response
Perhaps the biggest void in our understanding of how climate change may impact coastal ecosystems relates to how food-web dynamics will likely change in response to a warmer climate and more acidic ocean. A greater understanding of changing species interactions (competition and predator–prey dynamics) based on changing abundances and spatial ranges is critical to understanding the flow of trophic energy to the highest levels. Putting together the species-specific research on the major components of the coastal food web will be an important step in understanding the impacts of climate change on California’s coastal ocean.
Finally, managers of marine resources will need to maintain flexibility in management plans to incorporate new understandings of the impacts of climate change on the coastal ocean. Marine Protected Areas (MPAs), which are primarily “no take” zones for one or many species, may not serve their designed purpose of promoting population recovery under a warmer ocean (range shifts of organisms, decreased larval dispersal distances due to faster development) or if located in regions of increased hypoxia. Fishery seasons may need to be adjusted later or earlier as shifts in the timing of reproduction or migration occur. Adaptive management of these resources will be crucial to maintaining sustainable fisheries as atmospheric CO2 increases.
Manager Comments
Jeff G. Dorman
in conversation with
Dan Howard
Dorman: Can you provide an introduction to Cordell Bank, and describe how you expect climate change to impact the region?
Howard: Cordell Bank National Marine Sanctuary (CBNMS) was designated in 1989 and is one of the 14 federally protected marine and great lake areas in the National Marine Sanctuary System. Under the authority of the National Marine Sanctuaries Act, CBNMS provides comprehensive and coordinated conservation and management of the marine resources within the sanctuary’s 399 square nautical miles. CBNMS is an offshore site located about 43 nautical miles northwest of San Francisco off the coast of Marin and Sonoma County. The sanctuary encompasses an offshore area roughly between Bodega Bay and Point Reyes from six nautical miles offshore to 30 nautical miles from shore. The centerpiece of the sanctuary is a granite bank located 18 nautical miles west of Point Reyes that is 4.5 miles wide by 9.5 miles long. The rocky bank emerges from the soft sediments of the continental shelf, with the upper pinnacles reaching within 115 ft of the ocean’s surface, and shelf depths at the base of the Bank in roughly 300–400 ft of water. This is an area of special significance due to its position in the California Current and unique geological and oceanic features that create productive conditions supportive of diverse and abundant marine life.
Dorman: How do you expect climate change to impact CBNMS?
Howard: It is difficult to predict the sequence of change that will occur within the cool temperate California Current Ecosystem including CBNMS. But since these organisms and communities have evolved within an oceanographic system that is driven by an annual productivity cycle that is fueled by upwelling, any long-term change affecting the timing or intensity of upwelling would have significant impacts on early life stages, trophic interactions, and community structure. For example, many fishes and sessile invertebrates inhabiting Cordell Bank release their gametes or larvae into the water column in late winter or early spring. This release is timed to coincide with the onset of the annual productivity cycle (upwelling season) so their young can maximize feeding opportunities and therefore increase their odds of surviving. There would be high mortality in these early life stages if the timing or intensity of the annual productivity cycle changed. There are many similar stories including the Cassin’s Auklets dependence on early season productivity that you covered in your chapter. I expect that climate change will restructure the current composition of our regional ocean community. As changes occur in upwelling, sea surface temperature or ocean acidification, those species that cannot adapt will move or die and a new suite of organisms will assume their ecological roles, and what we now consider as our typical ocean community may look very different in the future.
Dorman: Are there any aspects of climate change that stand out to you as a primary threat to conservation and management goals established for the CBNMS?
Howard: One of the reasons Cordell Bank is so productive is the fact that it sits on the very edge of the continental shelf on a little peninsula surrounded by deep water on three sides. This position may also make Cordell Bank susceptible to hypoxic events related to the Oxygen Minimum Zone (OMZ). If increased upwelling related to climate change draws the OMZ up onto the shelf, it could potentially devastate the benthic invertebrates that cover the upper reaches of the bank.
It has also been documented that Humboldt or Jumbo squid (Dosidicus gigas) are associated with the OMZ during the day. The area west of Cordell Bank has been a hotspot for catching these squid since they moved into the area several years ago. If climate change shifted the OMZ shallower and closer to the bank, rockfish populations could be in jeopardy from increased predation by the squid. Regulations implemented by the Pacific Fisheries Management Council have prohibited all commercial and recreational fishing for rockfish at Cordell Bank since 2003 to allow overfished rockfish populations to recover. It is unclear what kind of impact jumbo squid would have on these recovering populations. The persistent presence of jumbo squid at Cordell Bank may be an indication that ecosystem-level changes are already occurring.
Dorman: How are the staffs at CBNMS responding to the challenge of understanding climate change?
Howard: We have participated in a two-day workshop hosted by the Gulf of the Farallones National Marine Sanctuary (GFNMS) that included CBNMS staff and the broad cross-section of the local scientific community to identify potential climate change drivers and local impacts.
We have also formed a joint working group composed of local scientists, sanctuary staff, and science advisors from the Cordell Bank and Gulf of the Farallones sanctuary advisory councils. This working group will help develop a site scenario for both sanctuaries that will include research and monitoring strategies, management and policy strategies, education and outreach focusing on changing behavior strategies, and operation strategies.
Finally, CBNMS and GFNMS are outfitting our research vessel with instrumentation and developing an at sea sampling plan to monitor ocean acidification in coordination with NOAA Pacific Marine Environmental Laboratory in Seattle, WA.
Dorman: Based on your experiences so far, what do you see as the major hindrances to implementing management strategies that incorporate potential changes in climate?
Howard: I think one of the biggest challenges is the scale of the problem. We manage discreet areas with a limited geography, but many of the drivers associated with climate change are operating on a regional or global scale. So as the ocean gets more acidic and threatens the stability of local food webs, it is hard to implement management actions in local sanctuaries that can effectively address this global issue. Another stumbling block that was identified by both the U. S. Commission on Ocean Policy and the Pew Ocean Commission is the number of different management authorities that have some type of jurisdiction in the ocean. Different missions and overlapping authority can stymie management actions.
LITERATURE CITED
Ainley, D. G., L. B. Spear, and S. G. Allen. 1996. Variation in the diet of Cassin’s auklet reveals spatial, seasonal, and decadal occurrence patterns of euphausiids off California, USA. Marine Ecology Progress Series 137:1–10.
Bakun, A. 1990. Global climate change and intensification of coastal ocean upwelling. Science 247:198–201.
Batchelder, H. P. and C. B. Miller. 1989. Life history and population dynamics of Metridia pacifica: Results from simulation modelling. Ecological Modelling 48:113–136.
Batchelder, H. P., C. A. Edwards, and T. M. Powell. 2002. Individual-based models of copepod populations in coastal upwelling regions: Implications of physiologically and environmentally influenced diel vertical migration on demographic success and nearshore retention. Progress in Oceanography 53:307–333.
Beaugrand, G., P. C. Reid, F. Ibañez, J. A. Lindley, and M. Edwards. 2002. Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296:1692–1694.
Bograd, S. J. and R. J. Lynn. 2003. Long-term variability in the southern California current system. Deep-Sea Research II 50:2355–2370.
Bograd, S. J., C. G. Castro, E. DiLorenzo, D. M. Palacios, H. Bailey, W. Gilly, and F. P. Chavez. 2008. Oxygen declines and the shoaling of the hypoxic boundary in the California current. Geophysical Research Letters 35, L12607. doi:10.1029 / 2008GL034185.
Bograd, S. J., I. Schroeder, N. Sarkar, X. Qiu, W. J. Sydeman, and F. B. Schwing. 2009. Phenology of coastal upwelling in the California Current. Geophysical Research Letters 36:L01602. doi:10.1029 / 2008GL035933.
Brinton, E. 1962. The distribution of Pacific euphausiids. Bulletin of the Scripps Institute of Oceanography 8:51–269.
Botsford, L. W., C. A. Lawrence, E. P. Dever, A. Hastings, and J. Largier. 2003. Wind strength and biological productivity in upwelling systems: An idealized study. Fisheries Oceanography 12(4–5):245–259.
Botsford, L. W., C. A. Lawrence, E. P. Dever, A. Hastings, and J. Largier. 2006. Effects of variable winds on biological productivity on continental shelves in coastal upwelling systems. Deep-Sea Research Part II 53:3116–3140.
Capotondi, A., M. A. Alexander, N. A. Bond, E. N. Curchitser, and J. D. Scott. 2012. Enhanced upper ocean stratification with climate change in the CMIP3 models. Journal of Geophysical Research 117:C04031. doi:10.1029 / 2011JC007409.
Chan, F., J. A. Barth, J. Lubchenco, A. Kirincich, H. Weeks, W. T. Peterson, and B. A. Menge. 2008. Emergence of anoxia in the California current large marine ecosystem. Science 319:920.
Costello, J. H., B. K. Sullivan, and D. J. Gifford. 2006. A physical-biological interaction underlying variable phonological responses to climate change by coastal zooplankton. Journal of Plankton Research 28(11):1099–1105.
Cury, P. and C. Roy. 1989. Optimal environmental window and pelagic fish recruitment success in upwelling areas. Canadian Journal of Fisheries and Aquatic Sciences 46:670–680.
Doney, S. C., V. J. Fabry, R. A. Feely, and J. A. Kleypas. 2009. Ocean acidification: The other CO2 problem. Annual Review of Marine Science 1:169–192.
Dorman, J. G., S. M. Bollens, and A. M. Slaughter. 2005. Population biology of euphausiids off northern California and effects of short timescale wind events on Euphausia pacifica. Marine Ecology Progress Series 288:183–198.
Edwards, M. and A. J. Richardson. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–884.
Fabry, V. J., B. A. Seibel, R. A. Feely, and J. C. Orr. 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science 65:414–432.
Feinberg, L. R., C. T. Shaw, and W. T. Peterson, 2006. Larval development of Euphausia pacifica in the laboratory: Variability in the developmental pathways. Marine Ecology Progress Series 316:127–137.
Field, J. C. and R. C. Francis. 2006. Considering ecosystem-based fisheries management in the California Current. Marine Policy 30:552–569.
Field, J. C., R. C. Francis, and K. Aydin. 2006. Top-down modeling and bottom-up dynamics: Linking a fisheries-based ecosystem model with climate hypotheses in the Northern California Current. Progress in Oceanography 68:238–270.
García-Reyes, M. and J. L. Largier. 2012. Seasonality of coastal upwelling off central and northern California: New insights, including temporal and spatial variability. Journal of Geophysical Research 117:C03028. doi:10.1029 / 2011JC007629.
Gargett, A. E. 1997. The optimal stability “window”: a mechanism underlying decadal fluctuations in the North Pacific salmon stocks? Fisheries Oceanography 6(2):109–117.
Genin, A., L. Haury, and P. Greenblatt. 1988. Interactions of migrating zooplankton with shallow topography: Predation by rockfishes and intensification of patchiness. Deep Sea Research 2:151–175.
Haidvogel, D. B., H. Arango, W. P. Budgell, B. D. Cornuelle, E. Curchitser, E. Di Lorenzo, K. Fennel, W. R. Geyer, A. J. Hermann, L. Lanerolle et al. 2008. Ocean forecasting in terrain-following coordinates: Formulation and skill assessment of the Regional Ocean Modeling System. Journal of Computational Physics 227:3595–3624.
Jahncke, J., B. L. Saenz, C. L. Abraham, C. Rintoul, R. W. Bradley, and W. J. Sydeman. 2008. Ecosystem responses to short-term climate variability in the Gulf of the Farallones, California. Progress in Oceanography 77:182–193.
Lindley, J. A. and S. Daykin. 2005. Variations in the distributions of Centropages chierchiae and Temora stylifera (Copepoda: Calanoida) in the north-eastern Atlantic Ocean and western European shelf waters. ICES Journal of Marine Science 62:869–877.
Lindley, S. T., C. B. Grimes, M. S. Mohr, W. Peterson, J. Stein, J. T. Anderson, L. W. Botsford, D. L. Bottom, C. A. Busack, T. K. Collier et al. 2009. What Caused the Sacramento River Fall Chinook Stock Collapse? Report to the Pacific Fishery Management Council.
Mackas, D. L., R. Goldblatt, and A. G. Lewis. 1998. Interdecadal variation in developmental timing of Neocalanus plumchrus populations at Ocean Station P in the subarctic North Pacific. Canadian Journal of Fisheries and Aquatic Sciences 55:1878–1893.
Mendelssohn, R., F. B. Schwing, and S. J. Bograd. 2003. Spatial structure of subsurface temperature variability in the California Current, 1950–1993. Journal of Geophysical Research 108(C3):3093. doi:10.1029 / 2002JC001568.
Menemenlis, D., J. Campin, P. Heimbach, C. Hill, T. Lee, A. Nguyen, M. Schodlok, and H. Zhang. 2008. ECCO2: High resolution global ocean and sea ice data synthesis. Mercator Ocean Quarterly 31:3–21.
Mesinger, F., G. DiMego, E. Kalnay, K. Mitchell, P. C. Shafran, W. Ebisuzaki, D. Jović, J. Woollen, E. Rogers, E. H. Berbery et al. 2006. North American regional reanalysis. Bulletin of the American Meteorological Society 87:343–360.
O’Connor, M. I., J. F. Bruno, S. D. Gaines, B. S. Halpern, S. E. Lester, B. P. Kinlan, J. M. Weiss. 2007. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proceedings of the National Academy of Sciences 104(4):1266–1271.
O’Farrell, M. R., M. S. Mohr, M. L. Palmer-Zwahlen, and A. M. Grover. 2009. The Sacramento Index. Report to the Pacific Fishery Management Council.
Ohman, M. D. 1984. Omnivory by Euphausia pacifica: The role of copepod prey. Marine Ecology Progress Series 19:125–131.
Pacific Fisheries Information Network (PacFIN). 1981–2011. Commercial Landed Catch: Metric Tons, Revenue, Price / Pound. California All Species Report 308. Pacific States Marine Fisheries Commission. http://pacfin.psmfc.org/pacfin_pub/all_species_pub/woc_r308.php.
Palacios, D. M., S. J. Bograd, R. Mendelssohn, and F. B. Schwing. 2004. Long-term and seasonal trends in stratification in the California Current, 1950–1993. Journal of Geophysical Research 109:C10016. doi:10.1029 / 2004JC002380.
Pierce, S. D., J. A. Barth, R. K. Shearman, and A. Y. Erofeev. 2012. Declining oxygen in the Northeast Pacific. Journal of Physical Oceanography 42:495–501.
Pörtner, H. O. and A. P. Farrell. 2008. Physiology and climate change. Science 302:690–692.
Powell, T. M., C. V. W. Lewis, E. N. Curchitser, D. B. Haidvogel, A. J. Hermann, and E. L. Dobbins. 2006. Results from a three-dimensional, nested biological-physical model of the California Current System and comparisons with statistics from satellite imagery. Journal of Geophysical Research 111:C07018. doi:10.1029 / 2004 / JC002506.
Ross, R. M. 1982a. Energetics of Euphausia pacifica. I. Effects of body carbon and nitrogen and temperature on measured and predicted production. Marine Biology 68:1–13.
Ross, R. M. 1982b. Energetics of Euphausia pacifica. II. Complete carbon and nitrogen budgets at 8° and 12°C throughout the life span. Marine Biology 68:15–23.
Schwing, F. B. and R. Mendelssohn. 1997. Increased coastal upwelling in the California Current System. Journal of Geophysical Research 102(C2):3421–3438.
Shchepetkin, A. F. and J. C. McWilliams. 2005. The regional ocean modeling system (ROMS): a split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Modelling 9:347–404.
Snyder, M. A., L. C. Sloan, N. S. Diffenbaugh, and J. L. Bell. 2003. Future climate change and upwelling in the California Current. Geophysical Research Letters 30(15):1823. doi:10.1029 / 2003GL017647.
Sydeman, W. J., R. W. Bradley, P. Warzybok, C. L. Abraham, J. Jahncke, K. D. Hyrenbach, V. Kousky, J. M. Hipfner, and M. D. Ohman. 2006. Planktivorous auklet Ptychoramphus aleuticus responses to ocean climate, 2005: Unusual atmospheric blocking? Geophysical Research Letters 33:L22S09. doi:10.1029 / 2006GL026736.
Tanasichuk, R. W. 1999. Interannual variation in the availability and utilization of euphausiids as prey for Pacific hake (Merluccius productus) along the south-west coast of Vancouver Island. Fisheries Oceanography 8:150–156.
Yamamura, O., T. Inada, and K. Shimazaki. 1998. Predation on Euphausia pacifica by demersal fishes: Predation impact and influence of physical variability. Marine Biology 132:195–208.