CHAPTER 7
Pollinators and Meadow Restoration

Brendan Colloran, Gretchen LeBuhn, and Mark Reynolds
Abstract. There are clear indications that climate change is altering plant and animal populations in ways that confound conservation efforts. The consequences of climate change may be particularly dire for montane and alpine bumble bee communities, which include species that are at the upper elevational and northern limits of their habitat range. We modeled the community dynamics within meadows (modeled aspatially) and migration among meadows (modeled spatially) for a set of 50 meadows in the Sierra Nevada over a span of 100 years to study the effects of meadow condition and climate change on montane bumble bee population dynamics in the Sierra Nevada. These results suggest that increasing the scale of current meadow restoration efforts may be an effective approach to slowing biodiversity loss in montane pollinator communities— and the plants that depend on their pollinator services—in the face of climate change.
Key Points
• Continued restoration of alpine meadows is key to increasing persistence of bumble bee species.
• Our models suggest that persistence of bumble bee species would be higher in undisturbed / restored meadows than in degraded ones in all climate change scenarios considered.
• For the range of climate change scenarios considered, species that emerge earlier appear to gain a competitive advantage from the earlier warming, and generally persist on the order of two to three decades longer than later-emergent species in undisturbed / restored meadows.
• In degraded meadows, drier conditions lead to a shorter resource-rich period when population can grow, which reduces persistence of species and removes the influence of the emergence timing.
• In the Sierra Nevada mountain range where altitude generally decreases toward the north, restoration will be critical in higher elevation meadows as the opportunity for poleward or upslope migration are limited because such sites are lacking.
INTRODUCTION
Meadows, which are wetlands or semi-wetlands in the subalpine and alpine zone, are some of the most threatened habitats in the Sierra Nevada, with over 80% of the meadows degraded (McKelvey et al. 1996). Meadow systems comprise less than 10% of the land area, and over the last 150 years, the hydrology of meadow systems has been dramatically changed by stream incision resulting from overgrazing (Odion et al. 1988, Kirchner et al. 1998, Blank et al. 2006), erosion (Micheli and Kirchner 2002a, Micheli and Kirchner 2002b), logging, housing, railroad, or road development (Ffolliott et al. 2004, Loheide and Gorelick 2007). The resulting stream incision leads to a lowering of the water table and a drying out of soil in the meadow, leading to changes in the composition of the vegetation and the timing of availability of floral resources (Loheide and Gorelick 2005).
Recently, there has been a move to restore some of these montane meadows. Restoration of meadow hydrology occurs primarily through the building of dams, called “pond and plug” restoration (Loheide and Gorelick 2005, Loheide and Gorelick 2007). In this type of restoration, dams are built at various points across the stream channel and water pools above the dam, rerouting flow, decreasing erosion, and increasing saturation upstream of the dam. This results in an increase in the water table depth and restoration of wet meadow vegetation (Loheide and Gorelick 2005, Loheide and Gorelick 2007). Meadows with restored hydrology have a larger area influenced by surface water and groundwater, and maintain a more diverse plant community with greater vegetation structure, particularly of riparian, emergent, and wet meadow species (e. g., Dobkin et al. 1998, Lang and Halpern 2007).
Bumble bees (genus Bombus, approximately 250 species worldwide) are one of the most important groups of pollinators for both native plants and crop plants, and their populations are declining worldwide (Williams 1986, Thorp and Shepard 2005, Cameron et al. 2011). Because they are typically found at higher latitudes and elevations than other bees, bumble bees are the primary pollinators in alpine and arctic communities, including the Sierra Nevada. Climate-driven changes in bumble bee communities, including phenology, may lead to decreased pollinator service for native plants and possibly shifts in the composition of both the plant and the pollinator communities (Williams 1986). In montane and alpine environments, these changes may have devastating effects on native plants and ecosystem processes, potentially creating an “extinction vortex” (Gilpin and Soule 1986, Hegland et al. 2009) in which declining bee populations could result in a reduction in pollinator services leading to the subsequent decline of plant populations, which in turn results in less foraging habitat for bees, reinforcing bee population decline.
Twenty species of bumble bees are known to inhabit California’s Sierra Nevada mountain range. These bees are sorted spatially by elevation, and since each bumble bee species has a unique seasonal emergence pattern, they are also sorted temporally by emergence time (Thorp et al. 1983). In the mid-elevations (1500–2500 m) of the Sierra, bumble bees emerge over an 8- to 12-week period (Nordby 2010). Bombus bifarius is the earliest species to appear after annual snowmelt (Nordby 2010). The next group to emerge consists of B. vosnesenskii, B. flavifrons, B. nevadensis, B. fervidus, B. centralis, B. vandykei, and B. psithyrus insularis. The late group includes B. appositus, B. californicus, B. griseocollis, and B. occidentalis. Californian bumble bees compete for nest sites in a limited number of abandoned rodent holes, and it has been shown that the availability of nest sites plays an important role in determining the structure of these bumble bee communities (McFrederick and LeBuhn 2006). This limit on the number of nest sites suggests that there is the potential for priority effects, if specific bee species have different physiological tolerances that influence their ability to compete for sites (Morin 1999, Fukami and Morin 2003, Price and Morin 2004).
In years where snowmelt is early and there are no late frosts, the early species may have a distinct advantage in securing nest sites and establishing colonies—and since colony growth is exponential after the nest is established, nests that are established early produce more workers and are able to garner more resources than nests that are established later in the season. On the other hand, species that emerge later face fewer risks, as they are less exposed to the cold weather and poor resource availability that is present early in the season. This sensitivity to climatic conditions, particularly early in the season, suggests that bees may be very vulnerable to changes in the phenology of alpine meadows (e. g., season length, timing of extreme events, and availability of resources), and that changes in climate may have dramatic effects on both community dynamics at any given elevation and the distribution of species across elevations.
Given the strong links between meadow community dynamics and climatic drivers, costs and benefits of conservation efforts are best assessed in the context of both current and future conditions. There are clear indications that climate change is altering plant and animal populations in ways that confound conservation efforts. Recent comprehensive meta-analyses (Parmesan and Yohe 2003, Root et al. 2003, Bartomeus et al. 2011) and a multi-taxa synthesis (Parmesan 2006) of changes in plant and animal populations suggest that recent patterns of global warming are already affecting the range, behavior, phenology, and other attributes of many species. In North America, species ranges are expected to respond to temperature increases by shifting north and moving to higher elevations (Parmesan 2006). However, the consequences may be dire for montane and alpine communities, some of which are already at the tops of mountains or the northernmost parts of North America, and which therefore have little opportunity to migrate. Particularly when combined with competition for resources, changes in climate may have dramatic effects on both community dynamics at any given elevation and the distribution of species across elevations (Bowers 1985). Moreover, the consequences of climate change may be intensified by habitat degradation—in the Sierra Nevada, for example, changes in meadow hydrology affect the availability of resources, possibly exacerbating climate-driven changes in bumble bee communities.
The combination of meadow degradation and climate change may have serious effects on bumble bee communities by changing the quantity and phenology of resources. Fortunately, recent work has suggested that meadow restoration can improve the hydrology of degraded meadows (Loheide and Gorelick 2005). In order to explore the connections between climate change and the benefits of restoring meadow hydrology, we developed a model to examine how meadow restoration might influence bumble bee population dynamics in the Sierra Nevada under various climate change scenarios.
This research is a first step to understanding how these pollinators may respond to climate change. We know of no other work that has modeled the effects of climate change on bees in these environments.
METHODS
Overview of the Model
Our model simulates community dynamics within meadows (modeled aspatially) and migration among meadows (modeled spatially) for a set of 50 meadows in the Sierra Nevada over a span of 100 years. We compare the persistence of bumble bee species in meadows with degraded hydrology to that of meadows with restored hydrology under four climate scenarios. Persistence is defined as the number of years individuals of that species are found in a meadow. Loheide and Gorelick (2007) found restored meadows and those that have not been degraded in the Northern Sierra have similar hydrology, so we lumped them into a single category (“restored”). Each scenario was simulated 500 times under both hydrology regimes (degraded and restored).
Meadows and Species Pool
To generate the meadows in our model, we started with size, position, and elevation data for a set of 61 meadows in the Northern Sierra that ranged in elevation from 1750 to 2400 m (Hatfield and LeBuhn 2007). To simplify our spatial migration model, we treated these meadows as disks; simplifying the geometry of the meadows in this way caused some to overlap, which we merged into a single larger meadow with a total area equal to the sum of the areas of the two meadows merged. The final elevation of the merged meadow was the average of the elevations of the original meadows weighted by the meadow areas. This procedure left us with a set of 50 meadows. We assigned each meadow a maximum carrying capacity (the number of bees it is able to support) based on its area, and a fixed, nonrandom number of nest sites based on its area (1–50 ha–1). All nest sites are identical in our model, and may be occupied by any species. We do not consider any possible effects of meadow hydrology on availability of nest sites.
The hydrological quality of each meadow affects resource availability within that meadow. We used field capacity, a measure of the amount of moisture that soil can hold, to capture the hydrological quality of the meadows. For restored meadows, the model assumed a starting field capacity of 27 cm; for degraded meadows, this is reduced by 40% to 16.5 cm. In any run of our simulation, all of the meadows are either restored or degraded—we do not consider scenarios in which some meadows are restored and others are degraded.
We consider 10 species, each of which was assigned a distinct “degree days” requirement that triggered its emergence from overwinter hibernation (a degree day is a standardized measure of heating and cooling calculated as the integral of a function of time that varies with temperature). For the purpose of this model, the species are otherwise identical, and are not intended to represent particular species. Since their emergence timing is their only distinguishing feature, we may refer to the species by “species number” or “emergence number” interchangeably. Initially, we seeded the meadows with hibernating queens of all 10 species. The number of queens of each species in each meadow was set by the meadow size, and thus was the same for each simulation. Each time a queen was added, its species type was drawn at random from a pool of all potential species, each of which had different emergence times.
Community and Population Dynamics
Each day of the simulation, all meadows accumulated degree days based on the regional temperature (determined by the climate model described below), which is common to all meadows, and adjusted for each meadow’s specific elevation. If a meadow has accumulated enough degree days to trigger the emergence of one of the species, then all the queens of that species hibernating in that meadow emerge and begin their search for a nest. These queens either nest in the meadow in which they hibernated or migrate to another meadow if they cannot locate a nest in their initial meadow.
Our simple spatial model of migration assumes that queens travel in a straight line on a random heading from their meadow of origin. If this flight path comes within 200 m of a meadow, we assume that the queen successfully detects the meadow and searches it for a nest site; if there are no meadows within 200 m of this flight path, the queen dies.
A queen that successfully migrates to a new meadow searches for a nest; her probability of finding a nest in any given meadow is determined by how many unoccupied nest sites are available. If she cannot locate an empty nest in this new meadow, she again attempts to migrate to another meadow, repeating these steps until she has either successfully found a nest or died during migration. Our model assumes that the entire process of emergence, nest location, and migration occurs during one day; once all queens have either found nests or died, the simulation proceeds to the next day, repeating these steps until the hibernating queens of each species have emerged.
If a queen successfully locates a nest in a meadow, she enters a 21-day foraging period, during which she may die based on the temperature in the meadow—as the temperature decreases, the probability of mortality increases. If she survives the foraging period, her first brood of 15 workers become active, and begins gathering resources for the nest, allowing the queen to stop foraging and produce more workers. At this point, the size of the active nest increases according to the simple Lotka–Volterra model
Nt + 1 = Nt + (r * Nt (1 − Nt/Kt))
where Nt is the number of workers in a nest, r is the growth rate of the number of workers in the nest (which is the same for all species), and Kt is the resources currently available in the meadow.
The above model of nest growth applies to all species and all meadows, but resources available in the meadow (Kt) vary based on meadow size, degree days accumulated, and water availability in the meadow (which is in turn determined by the hydrological quality of the meadow). We modeled water availability by using a modified Hargreaves equation to estimate evapotranspiration (Droogers and Allen 2002) and the Thornthwaite–Mather procedure for calculating soil-moisture balance (Steenhuis and Van der Molen 1986).
For simplicity, we assume that each season of colony life ends on the 280th day of the year (which is in early October). At that point, the queens of all existing nests die, all nest sites are vacated and become available for colonization the following season, and nests produce new queens in direct proportion to the number of workers in the nest (one new queen for every 10 workers). These new queens hibernate in the meadow in which they were born. To model overwinter mortality, the model assumed a standard overwinter mortality rate of 60% for queens of all species.
Climate and Weather Model
To capture large-scale temperature and precipitation patterns in the Northern Sierra under a range of climate scenarios, we used projected monthly precipitation and temperature data for the years 2000–2099 under the A2 (“higher” emissions), A1B (“middle” emissions), and B1 (“lower” emissions) scenarios described in the IPCC Special Report on Emissions Scenarios (Nakicenovic and Swart 2000). These data were obtained from the Lawrence Livermore National Laboratory, Bureau of Reclamation, and Santa Clara University downscaled climate projections derived from the World Climate Research Programme’s Coupled Model Inter-comparison Project phase 3 multi-model dataset. Temperature trends for the Northern Sierra under these emissions scenarios are depicted in Figure 7.1. None of these scenarios display any significant trends in precipitation quantity over time, and the seasonal timing of precipitation is essentially the same under all three scenarios.
These climate projections gave us monthly temperature and precipitation averages for each year of the simulation run, but did not provide the daily weather information required by our model. To generate these data in a way that would realistically capture natural variability and extreme events, we based daily temperature and precipitation amounts for each year of the simulation on a randomly selected year of observed temperature and precipitation data from the Sierraville Ranger Station from 1957 to 2007. In order to make these daily observations conform to the monthly averages given by the climate projections, we performed a histopolation procedure, adding a smooth trend to the observed daily time series data that adjusted the monthly averages up or down as needed to match the average for the climate projection.
As a null climate model, we chose a year of historical weather data at random for each year of the 100 years in a model run, simulating a climate scenario with no interannual trend.
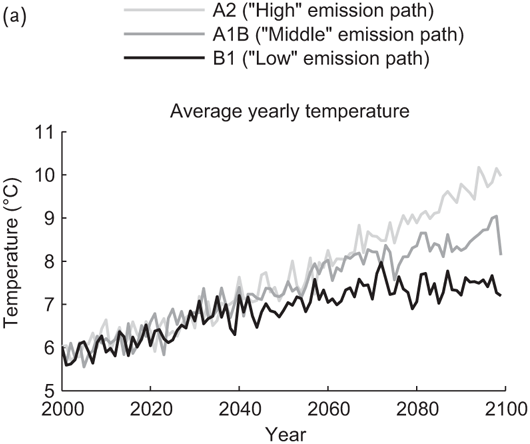
FIGURE 7.1: (a) Projected annual average temperature for the area surrounding the Sierraville Ranger Station for 2000–2100 under the A2, A1B, and B1 emission scenarios. (b) Projected average daily precipitation for the area surrounding the Sierraville Ranger Station for 2000–2100 under the A2, A1B, and B1 emission scenarios.
RESULTS
Community Structure
While most species decrease in their probability of persistence under climate change, the earlier-emerging species are more likely to persist. Under both the restored and degraded meadow hydrology regimes, all three climate change scenarios favor earlier-emerging species relative to our null climate scenario (Figure 7.2). Under the null scenario, all species fare worse in degraded meadows. This also holds in all three climate change scenarios for all but the last species to emerge. Surprisingly, in degraded meadows the null climate scenario results in the lowest mean persistence times for every species. In restored meadows, we see that some species persist for a much greater duration than others. These differences in persistence times are attenuated in degraded meadows. This flattening of persistence times indicates that those species that are most likely to persist in restored meadows are the most severely affected by meadow degradation.
Community Size
In both the degraded and restored meadows, the A2, A1B, and B1 climate projections result in approximately the same number of species persisting at any given year. The median number of species persisting under the null model follows a trajectory similar to that of the climate change scenarios in restored meadows, but is lower in degraded meadows (Figure 7.3). The rate of species loss is much greater in degraded meadows than restored meadows, and by year 100 the expected number of species persisting in degraded meadows has fallen to around one under all climate scenarios, whereas in restored meadows the expected number of species persisting at year 100 is around two for all climate scenarios. In particular, under all three climate change scenarios, about one additional species persists to year 100 in restored meadows, and under the null scenario, about two more species persist in restored meadows.
DISCUSSION
Meadows with restored hydrology remain wet later into the summer drought, which in our model means that floral resources remain available later into the summer, and hence bumble bee populations are able to continue to increase in size for a longer period of time. Consequently, restored meadows in our model larger communities of bumble bees for longer periods of time than degraded meadows (Figure 7.3). In all cases, early-emerging species do better under climate change than under current climate conditions because their peak nest sizes are larger (Figure 7.2). In restored meadows, late-emerging species have lower persistence under climate change scenarios than under current climate conditions because their peak nest sizes are smaller, resulting in decreases in new queen production. The same lower persistence time is not observed in degraded meadows. The magnitude of the effect of climate change is less for degraded meadows because these meadows already have diminished ecological function and the time for nest population growth is already reduced because floral resources are reduced. While the magnitude of effect of climate change is less, the loss of bumble bee species in degraded meadows is dramatically higher than that in restored meadows under all scenarios.
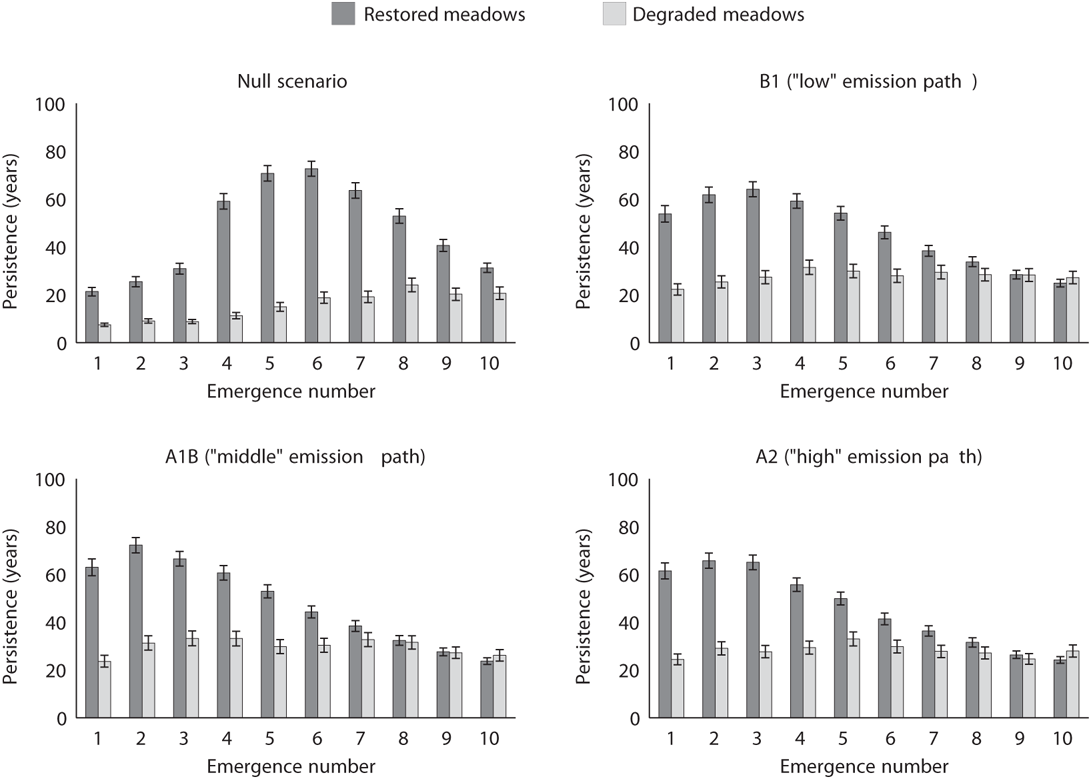
FIGURE 7.2: Mean number of years (starting from 2000) that each species persists in both hydrologic regimes. Each panel depicts one climate scenario. The sample mean is computed across all 500 model runs; error bars are 95% confidence intervals. Persistence times are greater in restored meadows.
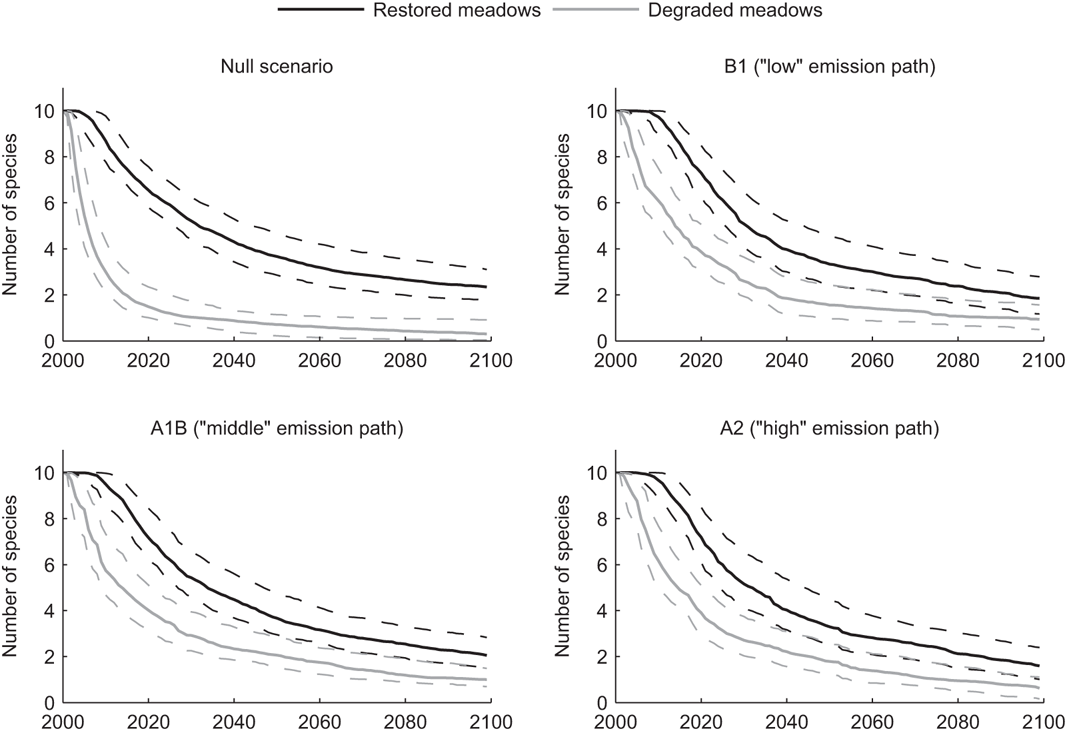
FIGURE 7.3: Resampled estimates of the median number of species persisting in this region for each year from 2000 to 2100 in both hydrologic regimes. Each panel depicts one climate scenario. The dashed lines are the upper and lower limits of the interquartile range. Under all climate scenarios, more species persist in restored meadows at every year of the simulation.
Because of their social structure, bumble bees are susceptible to losses in genetic variation when populations are highly fragmented (Chapman and Bourke 2001, Darvill et al. 2006, Ellis et al. 2006). Sociality causes a decreased effective population size because most individuals are sterile workers (Packer and Owen 2001). This reduced genetic diversity may hamper their ability to respond to environmental change. Data on gene flow in bumble bees suggest that for many species long-range dispersal is unlikely (Darvill et al. 2006) and species that have become isolated have rapidly gone extinct even when their habitat persists (Goulson 2003). In the Sierra Nevada, isolation of higher elevation bumble bee species is a potential threat because there are no high-elevation meadows in the Northern Sierra and, therefore, no routes for migration to suitable high-elevation habitats.
A key to understanding the effects of climate change on bumble bee communities will be the changes in the plant community, particularly changes in plant phenology. In this model, the increase in early season temperatures and change in rainfall patterns lead to a shift in both the peak of mean resources and the date at which resources no longer become available in a meadow. The increase in early season temperatures and resources decreases the probability of early season mortality for those species that emerge first. This increase in success of early season species is coupled with a decline in late-emerging species. This decline may simply be a numerical effect where early population growth leads to exploitation of available resources and carrying capacity is reached prior to the emergence of the later species (Louette and De Meester 2007). While the probability of mortality decreases in the early spring under the climate change scenarios, resource availability declines in the early summer, leading to a shorter length of time available for bee worker production. Since the reproductive success of colonies in this model is linearly related to the number of workers produced, the reduced size of late-emerging colonies has a significant effect on their persistence. These results are consistent with a meta-analysis of bumble bees on three continents, which showed that bumble bees with later colony initiation dates were more likely to have declined (Williams et al. 2009)
The model suggests that extending the length of the growing season by improving a meadow’s ability to retain moisture may have significant positive effects on the bumble bee community. However, the changes in temperature and season length may cause unexpected problems for some of the high-elevation habitat specialists that are not explored in our model. These problems may be caused directly by a physiological inability of these species to cope with changes to their habitat, or less directly by species adapted to lower elevations migrating upward and increasing competition for resources in high-elevation habitats (Theurillat and Guisan 2001, Halloy and Mark 2003, Pauli et al. 2007).
The benefits to plant species of maintaining larger pollinator communities may be substantial. In the Andes, bees pollinate over 50% of the species in the Andean zone (60% of the plant species between 2200 and 2600 m, over 40% of the species between 2700 and 3100 m, and 13% between 3200 and 3600 m) (Arroyo et al. 1982). For primarily outcrossing plant species, a decline in gene flow may increase the probability of extinction. If migration rates vary across either plant or animal species, there may be significant changes in both plant and animal communities and in ecosystem function (Root et al. 2003). If pollinators and plant phenologies become uncoupled, there may be a negative feedback between the loss of bumble bee species and the maintenance of plant communities (Inouye 2008).
Coadaptation of patterns of emergence, arrival or flowering, or other resources in alpine systems is common. For example, numerous migratory birds’ arrival time coincides with peak resources, the emergence time of many insect taxa match the emergence or flowering time of their host plants, and there is a clear sequence of flowering phenologies. While the responses of individual taxa to changes in climate are unpredictable, the model suggests that late emerging or arriving species may be at risk. Consistent with these results, data on pollen records from subalpine meadows suggest that plants in those meadows with shorter growing seasons are more sensitive to climate change (Gavin and Brubaker 1999).
SOLUTIONS, ADAPTION, AND LESSONS
Meadows are some of the most threatened ecosystems in North America (McKelvey et al. 1996). Our results indicate that one of the most effective strategies for managing climate change impacts for montane and alpine meadow pollinator communities may be doing what conservation practitioners and resource managers are already doing—restoring meadows. This model suggests that meadow restoration will be paramount to maintaining diverse bumble bee communities in the face of climate change.
Late-emerging bee species that have lower probabilities of persisting under climate change will respond to climate change in one of three ways: migration, adaptation, or extinction. In the Sierra, the opportunity for northward migration may be limited because the amount of available high-elevation habitat is markedly less toward the northern end of the Sierra Nevada range. This suggests that the potential for finding equivalent habitat by migrating northward may not exist. This is especially true for high-elevation species like B. appositus, B. sylvicola, and B. balteatus. While some species may be able to adapt to changes in their habitats, there currently are no data on what determines the elevational stratification of bumble bee species—it may be that bumble bee distributions are determined by thermal tolerance (Arroyo et al. 1982). Alternatively, distributions may be determined by constraints on flight physiology and the outcome of competitive interactions at different air densities as suggested for hummingbirds (Altshuler and Dudley 2006). Depending on the cause of this elevational stratification, the opportunity for adaptation may be limited.
Conservation practitioners and resource managers are understandably challenged by how to effectively protect, restore, and manage species, natural communities, and ecosystems in the face of climate change. The consequences of climate change for montane and alpine communities are especially vexing, because some of these communities are already at their upper elevational and northern limits. Conservationists will justifiably need to perform habitat “triage” focusing efforts on conservation of habitats most likely to persist under climate change scenarios and leaving species and communities dependent on declining habitats to adapt, go extinct, or be rescued by interventions such as managed relocation.
This model of the effects of meadow condition and climate change on montane bumble bee population dynamics in the Sierra Nevada suggests that meadow restoration is an effective strategy for ameliorating climate change threats to montane bee communities. Restoring montane meadows is an opportunity to increase multiple ecosystem service values. By stabilizing the hydrology in these meadows, there should be less and slower loss of water from the system, decreased flooding, and improved habitat for plants and other wildlife. Rather than “writing these ecosystems off,” increasing the pace and scale of current meadow restoration efforts may be an effective approach to conserving montane pollinator communities, and the plants that depend on their pollinator services, in the face of climate change. It has been said that the essential ecological consequence of climate change is that it changes everything. Conserving montane pollinator communities through restoration may be one instance where continuing to do what we are doing already in terms of restoration activities is a wise response to climate change and particularly important in high- and mid-elevation meadows.
Manager Comments
Brendan Colloran
in conversation with
Tina Mark
Colloran: Have you observed changes in the meadow ecosystems that you manage that seem linked to changes in climate? As climate continues to change, what ecological changes do you expect to see?
Mark: We’re seeing changes but at this point climate change does not seem to be the biggest driver of change. The most significant changes we are seeing in montane meadows of the Tahoe area are conifer encroachment likely related to lack of fire and also a drop in water table from historic stream incision. In the long term, these factors may be influenced by climate change. There are also positive changes (rewetting) in meadows related to reduced grazing pressure and meadow engineering (restoration).
Colloran: How do you see this information fitting in with the work that you do to manage / conserve montane meadow systems in California? What kinds of decisions could this type of research be used to inform?
Mark: This information can help the Forest Service in developing effective approaches to managing use of meadows by livestock, which meadows need hydrologic restoration. Also, it may benefit meadow restoration including conifer removal. It can also help understanding the effects of climate change on meadow size and hydrology. There is some work already underway on prescribed fire in meadows of Sierraville Ranger District on the Tahoe National Forest and on the Inyo National Forest.
Colloran: Is this an area where stronger or continued partnership with academic researchers is desired / valued? Can you give an example of an ecological study that would be of particular interest to you, and that would have the potential to influence management actions?
Mark: Yes, partnerships with academic researchers can help identify new issues and approaches for management. Some examples of areas of ecological study that would have the potential to influence management include design of meadow restoration and conservation of species of management concern (e. g., state and federal listed endangered species such as willow flycatcher); experiments on factors affecting rate of conifer encroachment in meadows; and long-term monitoring of climate change effects on meadows. Additional needs include public education and citizen science on wildflowers and pollinators.
Colloran: When you think about your ability to change management approaches or shift conservation priorities in this system, what do you see as the main constraints? Are there particular areas of study (ecological science, climate science, policy, data management, etc.) that could help overcome this constraint?
Mark: An important issue (or constraint in many cases) for conservation and management priorities is the concept of managing for single species needs versus overall ecological function and resilience. Society and current legal protection are most concerned with rare species (e. g., mountain yellow-legged frog), yet the greatest long-term benefits to species and ecosystems could be achieved in managing for resilient and healthy ecosystems. The other constraints include limited funding, lack of public awareness of the issues, and high turnover (and low replacement) of experienced staff. Areas of study that would help overcome constraints include studies that incorporate species functional groups as indicators of ecological resilience and function rather than studies that incorporate only single species or special status species.
Colloran: What are the key factors that are currently used to guide restoration priorities for alpine meadow communities? Have these changed at all in light of observed and projected changes in climate?
Mark: Stream degradation factors such as stream incision and altered hydrology, along with restoration feasibility, guide priorities. The role of these factors in guiding restoration priorities has not changed in light of climate change.
Colloran: Are benefits to pollinators currently being considered, and / or is any monitoring of bees underway or being considered?
Mark: Benefits to pollinators are considered and are a big part of public awareness campaigns. Bees are being considered to being added to sensitive / management species list for Tahoe area National Forests. There is no systematic bee monitoring currently underway. Plant species composition of meadows is being extensively monitored, but no guidelines are being given as to which plant species / pollinator relationships are important to monitor.
Colloran: Are there other key ecological services that are provided by meadows that could be modeled in a similar way to help understand the vulnerabilities and priorities for meadow restoration?
Mark: Modeling of hydrologic and biological responses to restoration could help understanding of vulnerabilities and priorities for meadow restoration. A new meadow guide has been developed which classifies meadow hydrologic types (Weixelman et al. 2011). This new publication has the potential to help guide meadow restoration efforts and to clarify the different types of meadows and restoration possibilities to fit certain meadow types.
Colloran: Understanding hydrologic impacts of climate change, especially in alpine systems where we are concerned with both precipitation in general and the depth and timing of snowpack, is a major challenge. In this system, can you think of restoration approaches or factors to consider in conservation planning that future research could focus on that would help develop adaptation strategies that are robust to a variety of different possible futures?
Mark: Future research could focus on coupling hydrologic and biological restoration goals for resilience, management, and adaptation to various climate change scenarios. A major question is the overall effect on hydrology due to lack of fire, overly dense forested areas, and subsequent effects on water availability as compared to effects on hydrology due to climate change. Teasing out these effects is crucial to management.
LITERATURE CITED
Weixelman, D. A., B. Hill, D. J. Cooper, E. L. Berlow, J. H. Viers, S. E. Purdy, A. G. Merrill, and S. E. Gross. 2011. A Field Key to Meadow Hydrogeomorphic Types for the Sierra Nevada and Southern Cascade Ranges in California. General Technical Report R5-TP-034, U.S. Department of Agriculture, Forest Service, Pacific Southwest Region, Vallejo, CA, 34 pp.
LITERATURE CITED
Altshuler, D. and R. Dudley. 2006. The physiology and mechanics of avian flight at high altitude. Integrative and Comparative Biology 46:6–71.
Arroyo, M. T. K., R. Primack, and J. Armesto. 1982. Community studies in pollination ecology in the high temperate Andes of central Chile. I. Pollination mechanisms and altitudinal variation. American Journal of Botany 69:82–97.
Bartomeus, I., J. Ascher, D. Wagner, B. N. Danforth, S. Colla, S. Kornbluth, and R. Winfree. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences of the United States of America 108(51):20645–20649.
Blank, R. R., T. Svejcar, and G. Riegel. 2006. Soil attributes in a Sierra Nevada riparian meadow as influenced by grazing. Rangeland Ecology and Management 59(3):321–329.
Bowers, M. A. 1985. Experimental analyses of competition between two species of bumble bees (Hymenoptera: Apidae). Oecologia 67:224–230.
Cameron, S. A., J. D. Lozier, J. P. Strange, J. B. Koch, N. Cordes, L. F. Solter, and T. L. Griswold. 2011. Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences of the United States of America 108:662–667.
Chapman, R. E. and A. F. Bourke. 2001. The influence of sociality on the conservation biology of social insects. Ecology Letters 4(6):650–662.
Darvill, B., J. Ellis, G. Lye, and D. Goulson. 2006. Population structure and inbreeding in a rare and declining bumble bee, Bombus muscorum (Hymenoptera: Apidae). Molecular Ecology 15:601–611.
Dobkin, D. S., A. C. Rich, and W. H. Pyle. 1998. Habitat and avifaunal recovery from livestock grazing in a riparian meadow system of the northwestern Great Basin. Conservation Biology 12(1):209–221.
Droogers, P. and R. Allen 2002. Estimating reference evapotranspiration under inaccurate data conditions. Irrigation and Drainage Systems 16:33–45.
Ellis, J. S., M. E. Knight, B. Darvill, and D. Goulson. 2006. Extremely low effective population sizes, genetic structuring and reduced genetic diversity in a threatened bumble bee species, Bombus sylvarum (Hymenoptera: Apidae). Molecular Ecology 15:4375–4386.
Ffolliott, P. F., L. F. DeBano, M. B. Baker, D. G. Neary, and K. N. Brooks. 2004. Hydrology and impacts of disturbances on hydrologic function. In D. G. Neary, L. F. DeBano, M. B. Baker, and P. F. Ffolliott (eds), Riparian Areas of the Southwestern United States: Hydrology, Ecology, and Management. CRC Press, Boca Raton, FL.
Fukami, T. and P. J. Morin. 2003. Productivity-biodiversity relationships depend on the history of community assembly. Nature 424:423–426.
Gavin, D. and L. Brubaker. 1999. A 6000-year soil pollen record of subalpine meadow vegetation in the Olympic Mountains, Washington, USA. Journal of Ecology 87:106–122.
Gilpin, M. E. and M. E. Soulé. 1986. Minimum viable populations: processes of species extinction. In M. E. Soule (ed.), Conservation Biology: The Science of Scarcity and Diversity. Sinauer Press, Sunderland, MA. 19–34.
Goulson, D. 2003. Bumblebees: Their Behavior and Ecology. Oxford University Press, Oxford, UK.
Halloy, S. R. P. and A. F. Mark. 2003. Climate-change effects on alpine plant biodiversity: A New Zealand perspective on quantifying the threat. Arctic, Antarctic, and Alpine Research 35:248–254.
Hatfield, R. and G. LeBuhn. 2007. Patch and landscape factors shape community assemblage of bumble bees, Bombus spp. (Hymenoptera: Apidae), in montane meadows. Biological Conservation 139:150–158.
Hegland, S. J., A. Nielsen, A. Lázaro, A.-L. Bjerknes, and Ø. Totland. 2009. How does climate warming affect plant-pollinator interactions? Ecology Letters 12:184–195.
Inouye, D. 2008. Effects of climate change on phenology, frost damage and floral abundance of montane wildflowers. Ecology 89:35–362.
Kirchner, J. W., L. Micheli, and J. D. Farrington. 1998. Effects of Herbaceous Riparian Vegetation on Streambank Stability. Technical Completion Report, Project W-872. University of California Water Resources Center, Berkeley, CA, USA.
Knapp, R. A. and K. R. Matthews. 1996. Livestock grazing, golden trout, and streams in the Golden Trout Wilderness, California: Impacts and management implications. North American Journal of Fisheries Management 16:805–820.
Lang, N. L. and C. B. Halpern. 2007. The soil seed bank of a montane meadow: Consequences of conifer encroachment and implications for restoration. Canadian Journal of Botany 85:557–569.
Loheide, S. and S. Gorelick. 2005. A high resolution evapotranspiration mapping algorithm (ETMA) with hydroecological applications at riparian restoration sites. Remote Sensing Environment 98:182–200.
Loheide, S. and S. Gorelick. 2007. Riparian hydroecology: A coupled model of the observed interactions between groundwater flow and meadow vegetation patterning. Water Resources Research 43:W07414. doi: 10.1029 / 2006WR005233.
Louette, G. and L. De Meester. 2007. Predation and priority effects in experimental zooplankton communities. Oikos 116:419–426.
McFrederick, Q. and G. LeBuhn. 2006. Are urban parks refuges for bumble bees? Bombus spp. (Hymenoptera: Apidae). Biological Conservation 129:372–382.
Mckelvey, K. S., C. N. Skinner, C. Chang, D. C. Erman, S. J. Husari, D. J. Parsons, J. W. van Wagendonk, and C. P. Weatherspoon. 1996. Sierra Nevada Ecosystem Project: Final Report to Congress, Vol. II. Assessments and Scientific Basis for Management Options. Centers for Water and Wildland Resources, University of California, Davis, CA.
Micheli, E. R. and J. W. Kirchner. 2002a. Effects of wet meadow riparian vegetation on streambank erosion. 1. Remote sensing measurements of streambank migration and erodibility. Earth Surface Processes and Landforms 27:627–639.
Micheli, E. R. and J. W. Kirchner. 2002b. Effects of wet meadow riparian vegetation on streambank erosion. 2. Measurements of vegetated bank strength and consequences for failure mechanics. Earth Surface Processes and Landforms 27:687–697.
Morin, P. J. 1999. Community Ecology, 2nd ed. Blackwell Science, Malden, MA.
Nakicenovic, N. and R. Swart (eds). 2000. IPCC Special Report on Emissions Scenarios. Cambridge University Press, Cambridge, UK.
Nordby, A. C. 2010. Influence of Emergence Phenology and Elevation on Community Structure of Bumble Bees. Master’s Thesis, San Francisco State University.
Odion, D. C., T. L. Dudley, and C. M. D’Antonio. 1988. Cattle grazing in southeastern Sierran meadows: Ecosystem change and prospects for recovery. In C. A. Hall and V. Doyle-Jones (eds), Plant Biology of Eastern California, Mary DeDecker Symposium. White Mountain Research Station, Los Angeles, CA, USA.
Packer, L. and R. Owen. 2001. Population genetic aspects of pollinator decline. Conservation Ecology 5:4.
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37:637–669.
Parmesan, C., N. Ryrholm, C. Stefanescu, J. K. Hill, C. D. Thomas, H. Descimon, B. Huntley, L. Kaila, J. Kullberg, T. Tammaru et al. 1999. Poleward shift of butterfly species’ ranges associated with regional warming. Nature 399:579–583.
Parmesan, C. and G. Yohe, 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42.
Pauli, H., M. Gottfied, I. K. Reiter, C. Klettner, and G. Grabherr. 2007. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology 13:147–156.
Price, J. E. and P. J. Morin. 2004. Colonization history determines alternate community states in a food web of intraguild predators. Ecology 85:1017–1028.
Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig, and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421:57–60.
Steenhuis, T. and W. Van der Molen. 1986. Thornthwaite-Mather procedure as a simple engineering method to predict recharge. Journal of Hydrology 84:221–229.
Theurillat, J. and A. Guisan. 2001. Potential impact of climate change on vegetation in the European Alps: A review. Climate Change 50:77–109.
Thorp, R., D. S. Horning, and L. L. Dunning. 1983. Bumble Bees and Cuckoo Bumble Bees of California. University of California Press, Berkeley, CA.
Thorp, R. W., and M. D. Shepherd. 2005. Profile: Subgenus Bombus. In Red List of Pollinator Insects of North America CD-ROM Version 1 (May 2005). The Xerces Society for Invertebrate Conservation, Portland, OR.
Williams, P. H. 1986. Environmental change and the distributions of British bumble bees (Bombus Latr.). Bee World 67:50–61.
Williams, P., S. Colla, and Z. Xie. 2009. Bumblebee vulnerability: Common correlates of winners and losers across three continents. Conservation Biology 23(4):931–940.