CHAPTER 8
Elevational Shifts in Breeding Birds in the Southern California Desert Region

Lori Hargrove and John T. Rotenberry
Abstract. The biogeographical distribution of a species is generally limited by the set of environmental conditions (ecological “niche,” including climate and habitat) to which the species is best adapted. Distribution limits often occur along ecological gradients such as temperature isotherms or transition zones between habitat types. If limiting environmental conditions change, then we expect populations at distribution margins to show evidence of expansion or retraction in association with that change. In this study, we test for distributional shifts in breeding birds along an arid elevation gradient in the Santa Rosa Mountains of southern California that is undergoing rapid climate change (locally, mean maximum temperature in spring at the low end of this gradient has increased by 5.0°C since 1961). Increasing temperatures and aridity in this system are expected to cause upward shifts in the elevational distributions of species. Over the past 26 years, five bird species (out of 28 tested) showed statistically significant shifts, all upward in elevation (average increase 496 m). The average shift for all 28 species was upward (average increase 116 m), but desert species had stronger upward shifts (average increase 171 m) compared to montane species (average increase 60 m). Our results reveal rapid shifts in avian distributions as predicted by climate warming, which has profound implications for this arid ecosystem, which include potential altered community structure and novel species–habitat associations. Management strategies designed to conserve biodiversity will need to incorporate this rapid dynamism into future conservation efforts.
Key Points
• Long-term monitoring is necessary, especially along gradients, and often straddling multiple management boundaries.
• Upper limits of species are expected to expand upward in elevation, while lower limits of species are expected to contract upward in elevation.
• Maintain habitat connectivity along elevation gradients.
• Not all species will shift at the same rate, due to differences in physiology, demography, site tenacity, dispersal, and local differences in climatic change and other environmental factors.
• Monitor fitness measures of marginal populations that are both shifting and not shifting to determine stressors and mechanisms of distribution shifts.
INTRODUCTION
Biogeography, or the spatial and temporal distribution of species, is a fundamental aspect of ecology and evolution, and understanding distributional change is valuable to conservation and management of species. Across the range of a species, locations with higher abundances tend to be associated with more favorable conditions, while abundances tend to taper off toward range limits where conditions become unfavorable (Brown et al. 1995, Brown et al. 1996). In vagile organisms such as birds, the behaviors of habitat selection and territoriality largely determine spatial distribution, and are expected to link adaptive traits of individuals to suitable habitat. When there are no barriers to dispersal, range limits are often linked to physiological or reproductive constraints directly or indirectly associated with climate. Consequently, species often show strong distributional patterns when surveyed along latitudinal and, especially, elevational gradients because of climatic differences. In birds, climate may be a direct limiting factor on distributions when associated with a species’ physiological temperature tolerance limits, and it may also affect species’ distributions indirectly through influence on patterns in vegetation and resource availability. Distributional studies of birds often identify climate as an important range boundary predictor (e. g., Root 1988, Bohning-Gaese and Lemoine 2004), while vegetation structure and composition are often identified as important predictors of bird distribution and abundance locally (Cody 1985, Wiens 1989, Block and Brennan 1993).
To the extent that species’ distributions reflect climatic limitations, distributions are expected to shift in response to climate change unless these shifts are constrained by some form of barrier or resource limitation. For example, if average temperatures increase, then distributions are expected to expand where a species is cold-limited, and retract where it is heat-limited. Many observed changes in species’ distributions are consistent with the hypothesis that they represent a response to climate change. Poleward latitudinal range expansions are well documented and demonstrate a “fingerprint” of global warming (Walther et al. 2002, Parmesan and Yohe 2003, Root et al. 2003, Parmesan 2006, Chen et al. 2011). However, range retractions and elevational shifts are less well known (Shoo et al. 2006, Thomas et al. 2006). Strong climatic gradients can be found with changes in elevation over a relatively short distance, and thus provide a potentially sensitive system for detecting distributional shifts in association with climate change. Examples of recent upward elevational shifts in species assemblages consistent with climate change effects include those for vascular plants (Walther et al. 2005, Kelly and Goulden 2008, le Roux and McGeouch 2008, Lenoir et al. 2008, Brusca et al. 2013, Kopp and Cleland 2014), insects (Konvicka et al. 2003, Franco et al. 2006, Wilson et al. 2007, Baessler et al. 2013), reptiles and amphibians (Raxworthy et al. 2008), small mammals (Moritz et al. 2008), and birds (Reif and Flousek 2012, Freeman and Freeman 2014), but there have been many inconsistencies as well, especially for birds (Archaux 2004, Brommer and Møller 2010, Lenoir et al. 2010, Forero-Medina et al. 2011, Tingley et al. 2012). Although birds are expected to be responsive to climate change and there is evidence of close tracking of ranges with climate (Tingley et al. 2009), fine-scale studies of elevational shifts in birds are greatly needed but are few (Sekercioglu et al. 2008). Ideally, tests of elevational shifts should also be linked to mechanistic studies that determine how climate change affects the demography of local populations, and how that varies among regions and species.
Elevation-based studies usually address the effects of climate change on high-elevation montane species rather than low-elevation desert species, and predictions tend to emphasize extinction risk due to warmer temperatures at high-elevation sites (e. g., Thomas et al. 2006). Although it is often thought that global warming will cause relatively minor impacts on desert species (e. g., Sala et al. 2000, Thomas et al. 2004), little is known about biological responses to climate change in arid ecosystems. Desert species are strongly responsive to variation in precipitation, and may be particularly sensitive to changes in both temperature and precipitation (e. g., McKechnie and Wolf 2010, Sinervo et al. 2010). Yet, how climate parameters change and how ecosystems respond to those changes may vary widely among regions. In the Chihuahuan Desert, the replacement of grassland by desert scrub has been attributed to an increase in winter precipitation (Brown et al. 1997). The desert regions of southern California are generally predicted to become warmer and drier, and variation in extreme events such as floods and droughts is expected to increase (e. g., Hayhoe et al. 2004, Seager et al. 2007). In deserts, although inter-annual variance in productivity associated with variation in rainfall tends to be high, increasing variance in annual rainfall can reduce population viability (survival and reproduction) by intensifying droughts (Saltz et al. 2006). Changes in drought periodicity and intensity may have even stronger impacts on desert populations than gradually shifting temperature and precipitation means (Albright et al. 2010, Barrows et al. 2010). Although a high rate of turnover is predicted for birds in the southern California deserts due to rapid temperature increases (Stralberg et al. 2009), it is unclear if desert species will be able to simply shift into higher-elevation areas (Hargrove and Rotenberry 2011a).
Along a desert-to-mountain transition, distributional shifts of both desert and montane species can be tested simultaneously. To test for distributional shifts in association with climate change, we quantified current distributions of breeding birds along a desert-to-mountain elevational gradient in southern California that is undergoing rapid climate change. These distributions were then compared to those recorded 26 years earlier using identical methods (Mayhew 1981, Weathers 1983). A warmer, more arid climate is predicted to cause an upward elevational shift in distributions of both desert and montane species. For desert species, the upper elevational limit is expected to advance upward, while for montane species, the lower elevational limit is expected to retract upward. Here, the results of this comparison are followed by a discussion of the possible implications of climate change for desert ecosystems, with suggestions for research needs and updates to management strategies.
METHODS
We conducted our study along the “Deep Canyon Transect” located at the Philip L. Boyd Deep Canyon Desert Research Center, part of the University of California Natural Reserve System, and extending into the San Bernardino National Forest on the north- and east-facing slopes of the Santa Rosa Mountains in central Riverside County, California (Figure 8.1). The Deep Canyon Transect spans an elevational range from near sea level to 2600 m over a distance of 35 km along the transition between the Peninsular Ranges and Colorado Desert. The Peninsular Ranges, which include the Santa Rosa Mountains, run north–south and form a rain shadow for the Colorado Desert to the east. The Colorado Desert is an extension of the Sonoran Desert, and includes areas that rank among the hottest, driest places on earth (Meigs 1953, UNEP 1997). The vegetation varies from Sonoran desert scrub at lower elevations to chaparral and pinyon-juniper woodland at mid-elevations and mixed coniferous woodland at upper elevations (Figure 8.2).
In 1979, a series of plot transects were established along the Deep Canyon Transect, each of varying length and orientation, but typically 1-km long (Mayhew 1981). These transects were systematically surveyed multiple times each year (typically 2–3 times each spring), and all observations of vertebrates were recorded (Mayhew 1981, Weathers 1983). In the spring of 2005–2007, using the same methods, we conducted repeat surveys at 15 of the existing plot transects that were most consistently surveyed and that were stratified along an elevation range of 200–2400 m. We compared our results to data collected at these same locations during the first three years of the original surveys, 1979–1981, using only morning surveys during spring months with good weather conditions. The analyses were restricted to 28 breeding bird species. We excluded species that were rare, nonbreeding migrants, wide-ranging foragers (e. g., swifts overhead), not always identified to species (e. g., hummingbirds), or completely absent during one of the two survey periods. Although the elevational ranges and distributional limits varied widely by species, we categorized species as “desert” if they tended to be more restricted to lower-elevation sites in this study area (with an upper limit distributional margin falling within the study area) or “montane” if they tended to be more restricted to higher-elevation sites (with a lower limit distributional margin falling within the study area).
FIGURE 8.1: Location of the Deep Canyon Transect. Located at the transition between the South Coast Peninsular Ranges and Colorado Desert, Southern California. Basemap source: ESRI.

FIGURE 8.2: The range of vegetation types surveyed along the Deep Canyon Transect. A. Chaparral and oak-conifer woodland (elevation 1800 m). B. Chaparral and pinyonjuniper woodland (elevation 1200 m). C. Mid-elevation desert scrub (elevation 900 m). D. Sonoran desert scrub and Palo Verde Wash (elevation 200 m).
To provide context for our bird observation data, we analyzed temperature and precipitation data from two local weather stations, one on the desert floor at an elevation of 292 m and one in the mountains at an elevation of 1640 m (WRCC 2007, M. Fisher, pers. comm.). We tested long-term trends for maximum temperature (yearly and spring averages of mean monthly maximum temperatures), minimum temperature (yearly and spring averages of mean monthly minimum temperatures), and precipitation (July–June rain-year). To compare cumulative precipitation between the two survey periods, we summed precipitation over a five-year period (each period containing the three survey years plus two preceding years).
To test for distributional shifts, we used methods that were robust to any differences in absolute abundance between the two time periods that might have arisen, for example, due to observer differences. For each species, we calculated abundance as the average number of birds detected over a 1-km transect for each of the 15 sites, for each three-year period. We calculated a weighted mean elevation for each species for each three-year period as the sum of elevations at which the species was present, each multiplied by the abundance of that species at that site, and divided by the total abundance for that species over all sites. We constructed cumulative frequency distribution curves for each species by summing its proportional abundance from its lowest to highest altitudinal occurrence (Box 8.1). Thus, cumulative frequency at the highest elevation site is always 100%. A Kolmogorov–Smirnov (K-S) test was used to test for any difference in the cumulative elevational distribution of individual species between the two periods (Box 8.1), and a paired-sample t-test was used to test for upward elevational shifts in weighted mean elevations at the community level. Three community groups were considered: All species, desert species alone, and montane species alone.
BOX 8.1 • Elevational Shifts in the Mountain Quail Distribution
To test for elevational shifts in distributions, we compared observations of 28 bird species along an elevational gradient between two three-year time periods at Deep Canyon, 26 years apart. We quantified patterns of bird abundance along this gradient using the data collected during the breeding season at 15 sites ranging in elevation from 200 to 2400 m, using the same survey methods during both time periods.
In this example for mountain quail (Oreortyx pictus), the elevational distributions are shown for the two time periods (1979–1981 in green vs. 2005–2007 in orange; Box Figure 8.1.1). For each time period, the graph shows the cumulative abundance of mountain quail observed at each of the 15 sites as elevation increases from 200 to 2400 m. Thus, the cumulative frequency distribution curves show the sum of the species’ proportional abundance from its lowest to highest altitudinal occurrence, such that the cumulative frequency at the highest elevation site is always 100%.
In 2005–2007, we found fewer mountain quail at lower-elevation sites compared to 1979–1981 (shown by the rightward shift in the distribution), indicating that there was an upward shift in the elevational distribution of abundance for this species. A Kolmogorov–Smirnov test is a nonparametric statistical test used to compare differences between two cumulative distributions. For the mountain quail, this test indicated a difference between the two distributions that was statistically significant (p < 0.05). Of the 28 species tested in this way, five showed a statistically significant difference. All of these five species had a strong upward shift in weighted mean elevation.

BOX FIGURE 8.1.1: Upward shift in the elevational breeding distribution of mountain quail (Oreortyx pictus) over a 26-year time period, Boyd Deep Canyon Desert Research Center, Riverside County, California. Elevational distributions are shown for the two time periods [1979–1981 (green squares) vs. 2005–2007 (orange triangles); K-S test: p < 0.05]. Photo credit: Peter LaTourrette / birdphotography.com.
RESULTS
In the desert (Boyd Deep Canyon Desert Research Center, elevation 292 m), mean maximum temperature increased by 3.8°C from 1962 to 2006, while there was no change in mean minimum temperature. The increase in mean maximum temperature was even greater during the main breeding season when surveys were conducted: 5.0°C since 1961 (r2 = 0.48, March–June, Figure 8.3). In contrast, at a montane site near the upper end of the transect (Idyllwild, elevation 1640 m), there was no trend in the mean maximum temperature, but the mean minimum temperature increased by 1.7°C from 1960 to 2006, with no difference between annual (r2 = 0.39) and spring (r2 = 0.16, Figure 8.3) averages. In the desert, there was no long-term trend in precipitation over the 45 years from 1962 to 2007, but the most recent survey period, 2003–2007, had 44% less cumulative precipitation than the survey period 26 years ago, 1977–1981 (each period containing the three survey years plus two preceding years, using July–June rain-years).
Five individual species (out of 28 tested) showed statistically significant differences in their cumulative elevational distributions (K-S test, p < 0.05, n = 15; Box 8.1, Table 8.1). All five had an upward shift in their weighted mean elevation, with an average increase of 496 m. Moreover, there was an upward elevational shift in the avian community as a whole (paired-sample t-test, p < 0.01, n = 28 species). The average weighted mean elevation for all 28 species increased by 116 m. Species categorized as “desert” showed a statistically significant average elevational shift of 171 m upward (pairedsample t-test, p < 0.05, n = 14), whereas the shift in “montane” species, although upward an average of 60 m, was not significant (paired-sample t-test, p = 0.12, n = 14).
DISCUSSION
These results suggest that significant elevational shifts in breeding bird distributions are possible over a relatively short time. The upward shifts we observed are consistent with expectations given the warmer, drier conditions now versus 26 years ago. Compared to montane species, desert species were more likely to show upward shifts. These shifts in distribution occurred in a diverse group of breeding bird species, each likely to have a different set of direct and indirect ecological links to changes in climate. Response to temperature and precipitation can be direct (e. g., mortality and nest timing), or mediated through biotic factors linked to climate (e. g., habitat and food availability).
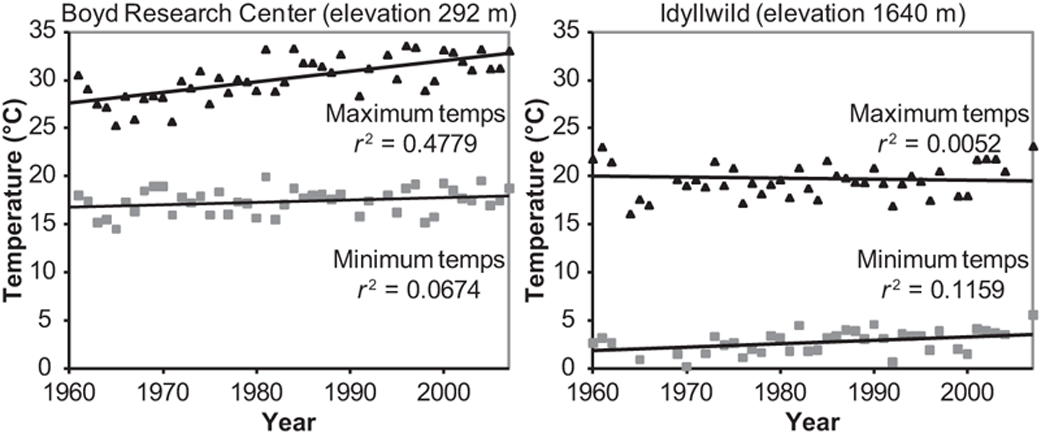
FIGURE 8.3: Spring mean maximum and minimum temperature trends in the desert (left) and mountains (right), 1960–2007. Values are daily maximum and minimum temperatures, averaged for each spring (March–June), and r2 denotes proportion of variance in temperature statistically explained by linear regression on year. (Boyd Deep Canyon Desert Research Center, elevation 292 m, and Idyllwild, elevation 1640 m.)
In desert birds, physiological adaptations to heat and aridity likely involve decreases in metabolic rates and water loss (Tieleman and Williams 2000). Increasing maximum temperatures at desert sites can exceed tolerance limits, while increasing minimum temperatures at montane sites can improve suitability. Drought is known to cause reproductive failure in many bird species, even in relatively arid environments (e. g., Bolger et al. 2005, Albright et al. 2010). Many bird species are associated strongly with certain habitats, and there is a suggestion that vegetation is similarly shifting upward in this area (Kelly and Goulden 2008). This is likely associated with recent die-off of desert shrubs at low elevations (Miriti et al. 2007). Thus, although changing climate can have direct effects on species’ distributions, biotic interactions (such as species–habitat relationships) can amplify the effects of climate change if one component of the relationship is more sensitive than another. Therefore, biotic interactions should be an important consideration in predictive distribution modeling too (Preston et al. 2008, Angert et al. 2013).
In this study system, desert species were especially likely to show upward elevational shifts. Although it is often thought that desert species will exhibit relatively little negative impact of global warming because desertification will create more desert habitat (e. g., Sala et al. 2000, Thomas et al. 2004), desert species are likely to be closer to their limits of temperature and precipitation tolerance than species of more humid habitats. Even though some desert species may be able to adapt to further increases in temperature and aridity within their current range, it is unlikely that adaptive evolutionary processes can keep pace with the high rate of temperature increase in this system. Results from the North American Breeding Bird Survey indicate decreasing population trends overall for the Sonoran and Mojave deserts from 1966 to 2007 (Sauer et al. 2008), but more study is needed on the causes of these population declines. With increasing desertification throughout the region, relatively mobile species such as birds may be able to shift into new areas as they become habitable. However, in southern California, higher elevations are limited in extent, and in the more mesic coastal areas, natural habitats that might otherwise undergo conversion to desert vegetation types are highly fragmented by urbanization. In addition, there is unlikely to be any “backfill” or immigration by new species if lower elevations become increasingly inhospitable to current desert species—the Colorado Desert is already one of the hottest and driest areas on earth. Although this study excluded rare species that were absent during one of the two time periods, no obvious trends were apparent with respect to species additions and extirpations at low versus high elevations.
TABLE 8.1
Species in order of weighted mean elevation (m) in 1979–1981 at 15 sites, Boyd Deep Canyon Desert Research Center, Riverside County

NOTE: “D” (desert) indicates that the species was more common at low-elevation sites in this study system, while “M” (montane) indicates that the species was more common at higher-elevation sites. Elevational shift is the difference in weighted mean elevation between the two time periods (1979–1981 vs. 2005–2007). Positive values indicate upward elevational shifts. The Kolmogorov–Smirnov test was used to compare the cumulative elevational distributions between the two time periods.
a Data combined for two hybridizing species (California and Gambel’s quail).
b Pinyon jays were highly localized at intermediate elevation sites.
SOLUTIONS, ADAPTATION, AND LESSONS
Predictions or forecasts from most climate-change models are averaged over large geographical spaces and long periods of time. However, organisms and their habitats are affected by local temperature, precipitation, and short-term extreme events (e. g., McKechnie and Wolf 2010). The distributional shifts observed in this study system over a 26-year period could be partly due to short-term climate fluctuations, but this rapid dynamism suggests that longer-term effects could be equally pronounced. Elevational distributions of birds were found to be relatively static over a three-year period (Hargrove and Rotenberry 2011b), which further suggests that these 26-year shifts are due to longer-term changes. The strong differences in temperature within this study system with opposing temperature trends only a short distance apart are likely to have very different biological effects. Thus, climate-change predictions at a much finer scale are needed to manage biodiversity for these challenges. Furthermore, although trends in temperature and precipitation extremes are less well known, they are even more likely to be biologically relevant than annual averages.
Understanding distributional change is valuable to conservation management. As species may shift across management boundaries, management perspectives must evolve to encompass broader spatial and temporal scales. However, little is known about the rates and mechanisms of range shifts, and there is great uncertainty as to the differences in responsiveness among species. Additional monitoring is needed along elevational gradients and at other transition zones where distribution limits occur. Ideally, monitoring should be linked to mechanistic studies as well. For example, during drought years in this study system, breeding success of desert species tends to be greater at higher elevations, while reproductive failure is more likely on the desert floor, which may be exacerbated by warmer temperatures (Hargrove 2010). However, the link between demographic processes and range shifts remains weak, and both ecological and evolutionary studies of distribution limits are greatly needed (Hoffmann and Blows 1994, Parmesan et al. 2005, Travis et al. 2013). Nevertheless, it is clear that management strategies will need to incorporate dynamic processes into future conservation efforts and encompass broader spatial and temporal scales across management boundaries.
ACKNOWLEDGMENTS
We are grateful to Al Muth and Mark Fisher for assistance with field access and historical data, and to Myung-Bok Lee for data entry. We thank the Biological Impacts of Climate Change in California group and Phil Unitt for comments on the research and manuscript. Field work was supported by a Biological Impacts of Climate Change in California Research grant from the California Energy Commission’s Public Interest Energy Research Environmental Area and Point Reyes Bird Observatory Conservation Science, the California Department of Parks and Recreation, a California Desert Research Fund from the Community Foundation Serving Riverside and San Bernardino Counties, a Mildred E. Mathias Graduate Student Research grant from the University of California Natural Reserve System, a Mewaldt-King Student Research grant from the Cooper Ornithological Society, and a Ralph W. Schreiber Ornithology Research Award from the Los Angeles Audubon Society.
Manager Comments
Lori Hargrove
in conversation with
Mark Fisher, Allan Muth, Jenny Rechel, and Anne Poopatanapong
Hargrove: As climate continues to change, what ecological changes do you expect to see in southern California’s ecosystems?
Fisher and Muth: At Boyd Deep Canyon, we have observed changes in the distribution of plants, the timing of flowering, and mortality of plant species that seem linked to a warming and drying climate. For example, the perennial plants Encelia farinosa and Ambrosia dumosa are missing from large parts of the creosote bush scrub where they were once among the top 10 dominant species. The cactus Opuntia basilaris begins flowering about a month earlier than when we both came here in the early 1980s. A prolonged drought resulted in over 75% mortality of the cactus Cylindropuntia bigelovii at one site. We expect increased mortality of species at the hotter and drier margins of their distributions in conjunction with an overall decrease in precipitation and increase in temperature. Since the hotter, drier margins are typically at lower elevation, there should be a net uphill movement in species distributions as demonstrated with birds in this case study.
Rechel: I work in the San Jacinto Mountains on the San Jacinto Ranger District. In our area, I expect to see the density of shrubs increasing in the mid-elevations and in the area dominated currently by oak woodlands, and expect to record increased density of Quercus species in the upper elevations with conifer species. More generally, I expect to observe an overall drier climate, with more common spikes in storm events, thereby resulting in increased erosion in stream channels (I have already observed this in several areas). I also expect to observe angiosperms flowering earlier in the spring and expect to see different angiosperm species and grasses flowering based on soil moisture resulting from too dry or too wet winter precipitation conditions.
Poopatanapong: In the San Bernardino National Forest (SBNF), we are already seeing changes, such as shorter spring flowering periods. We are also seeing Quino checkerspot butterflies in places where they have not been documented scientifically before—we are seeing them at higher elevations and farther to the east. In 2010, we saw significant increase in the individuals sighted, which may suggest that the species is actually breeding on the forest that we have not seen before. Also, on the topic of possible range shifts, we haven’t seen species that we expect to see on the district, such as the northern flying squirrel. The SBNF is not actively searching for them, but no one seems to see them anymore on the mountain range. We have also seen desert species such as great-tailed grackle in areas that one would not typically see them such as Lake Hemet. It appears that they are expanding their distribution westward for some reason.
Hargrove: In your experience, what do you see as key vulnerabilities of the ecosystems you manage? Are they viewed as highly vulnerable to climate change?
Fisher and Muth: The increased variability in weather that is associated with climate change can make the marginal ecosystems most susceptible to extirpations of endemic species, especially those species that are unable to disperse upward because of specific habitat preferences. The Colorado Desert division of the Sonoran Desert is the driest, hottest desert in the United States, and is thus extremely vulnerable to the effects of climate change in comparison with more insulated ecosystems.
We have recently documented extreme variation in reproductive output in the endangered lizard, Uma inornata, that might lead to localized extirpation during prolonged droughts. This should be replicated by many other species that are likewise restricted to a specific substrate type or other limited habitat feature.
Rechel: In terms of how systems in this region are viewed, it depends on the definition of “highly vulnerable” and the answer varies by species groups. But I would say that the upper elevations of conifer forests are the most vulnerable. Additionally, avian species that occur in the mid-elevation oak woodlands are expected to move up in elevation and potentially compete with existing conifer forest species.
For the most part, I do believe the forest personnel view the San Bernardino as vulnerable to climate change. The biologists do, and local researchers do, but resource managers likely view vulnerable ecosystems as part of the overall resource management complex of issues. Fire personnel may or may not; if they do, I suspect this vulnerability relates to increases in temperatures and longer droughts and drier winters, thus increasing fire risk in the chaparral; especially at lower elevations.
Hargrove: How do you see the information from this case study and other similar research fitting in with the work that you do in management?
Fisher and Muth: The Deep Canyon Transect, including this UC Reserve and its surrounding matrix comprised primarily of National Forest and BLM lands, contains an elevational gradient that includes plant communities ranging from low-elevation creosote bush scrub to high-elevation yellow pine forest, with chaparral and pinyon-juniper woodland plant communities at mid-elevations. Many plant and animal taxa are restricted to each of these communities and come into contact or near contact at the transition zones. An example of a management decision made from what was learned from this study would be to restrict disturbance and perturbation (e. g., no exclusionary fencing or removal experiments) so as to avoid interference with the future uphill movement of species in response to climate change.
Poopatanapong: For the San Bernardino, information from researchers is used to help complete the biological assessments and evaluations needed for the NEPA process. Since the Forest Service does not actively survey for many wildlife species, any information gathered by partners is extremely helpful. More generally, having research on the national forest is always desired. At each national forest, the Forest Service does not have funding, staff, and ability to actively conduct research—nor is it the mission of the agency. Having the opportunity to use the data gathered by students and other researchers really helps all groups work together to better manage the land.
Rechel: For the San Bernardino and Angeles National Forests, climate change information is important to use in conjunction with existing longterm (20+ years) datasets of avian population and habitat use questions, especially related to changes in fire regimes in Mediterranean ecosystems.
LITERATURE CITED
Albright, T. P., A. M. Pidgeon, C. D. Rittenhouse, M. K. Clayton, C. H. Flather, P. D. Culbert, B. D. Wardlow, and V. C. Radeloff. 2010. Effects of drought on avian community structure. Global Change Biology 16:2158–2170.
Angert, A. L., S. L. LaDeau, and R. S. Ostfeld. 2013. Climate change and species interactions: Ways forward. Annals of the New York Academy of Sciences 1297:1–7.
Archaux, F. 2004. Breeding upwards when climate is becoming warmer: No bird response in the French Alps. Ibis 146:138–144.
Baessler, C., T. Hothorn, R. Brandl, and J. Muller. 2013. Insects overshoot the expected upslope shift caused by climate warming. PLOS ONE 8:e65842.
Barrows, C. W., J. T. Rotenberry, and M. F. Allen. 2010. Assessing sensitivity to climate change and drought variability of a sand dune endemic lizard. Biological Conservation 143:731–736.
Block, W. M. and L. A. Brennan. 1993. The habitat concept in ornithology: Theory and applications. Current Ornithology 11:35–91.
Bohning-Gaese, K. and N. Lemoine. 2004. Importance of climate change for the ranges, communities and conservation of birds. Advances in Ecological Research 35:211–236.
Bolger, D. T., M. A. Patten, and D. C. Bostock. 2005. Avian reproductive failure in response to an extreme climatic event. Oecologia 142:398–406.
Brommer, J. E. and A. P. Møller. 2010. Range margins, climate change, and ecology. In A. P. Møller, W. Fiedler, and P. Berthold (eds), Effects of Climate Change on Birds. Oxford University Press, Oxford. 249–274.
Brown, J. H., D. W. Mehlman, and G. C. Stevens. 1995. Spatial variation in abundance. Ecology 76:2028–2043.
Brown, J. H., G. C. Stevens, and D. M. Kaufman. 1996. The geographic range: Size, shape, boundaries, and internal structure. Annual Reviews of Ecology and Systematics. 27:597–623.
Brown, J. H., T. J. Valone, and C. G. Curtin. 1997. Reorganization of an arid ecosystem in response to recent climate change. Proceedings of the National Academies of Science of the United States of America 94:9729–9733.
Brusca, R. C., J. F. Wiens, W. M. Meyer, J. Eble, K. Franklin, J. T. Overpeck, and W. Moore. 2013. Dramatic response to climate change in the Southwest: Robert Whittaker’s 1963 Arizona Mountain plant transect revisited. Ecology and Evolution 3:3307–3319.
Chen, I. C., J. K. Hill, R. Ohlemuller, D. B. Roy, and C. D. Thomas. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026.
Cody, M. L. (ed.). 1985. Habitat Selection in Birds. Academic Press, Orlando, FL.
Forero-Medina, G., J. Terborgh, S. J. Socolar, and S. L. Pimm. 2011. Elevational ranges of birds on a tropical montane gradient lag behind warming temperatures. PLOS ONE 6:e28535.
Franco, A. M. A., J. K. Hill, C. Kitschke, Y. C. Collingham, D. B. Roy, R. Fox, B. Huntley, and C. D. Thomas. 2006. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Global Change Biology 12:1545–1553.
Freeman, B. G. and A. M. C. Freeman. 2014. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proceedings of the National Academies of Science of the United States of America 111:4490–4494.
Hargrove, L. 2010. Limits to species’ distributions: Spatial structure and dynamics of breeding bird populations along an ecological gradient. Dissertation. University of California, Riverside, CA. http://proquest.umi.com/pqdweb?did=2019822781&sid=1&Fmt=2&clientId=48051&RQT=309&VName=PQD.
Hargrove, L. and J. T. Rotenberry. 2011a. Breeding success at the range margin of a desert species: Implications for a climate-induced elevational shift. Oikos 120:1568–1576.
Hargrove, L. and J. T. Rotenberry. 2011b. Spatial structure and dynamics of breeding bird populations at a distribution margin, southern California. Journal of Biogeography 38:1708–1716.
Hayhoe, K., D. Cayan, C. B. Field, P. C. Frumhoff, E. P. Maurer, N. L. Miller, S. C. Moser, S. H. Schneider, K. N. Cahill, E. E. Cleland et al. 2004. Emissions pathways, climate change, and impacts on California. Proceedings of the National Academies of Science of the United States of America 101:12422–12427.
Hoffmann, A. A. and M. W. Blows. 1994. Species borders: Ecological and evolutionary perspectives. Trends in Ecology and Evolution 9:223–227.
Kelly, A. E. and M. L. Goulden. 2008. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academies of Science of the United States of America 105:11823–11826.
Konvicka, M., M. Mardova, J. Benes, Z. Fric, and P. Kepka. 2003. Uphill shifts in distribution of butterflies in the Czech Republic: Effects of changing climate detected on a regional scale. Global Ecology & Biogeography 12:403–410.
Kopp, C. W. and E. E. Cleland. 2014. Shifts in plant species elevational range limits and abundances observed over nearly five decades in a western North America mountain range. Journal of Vegetation Science 25:135–146.
le Roux, P. C. and M. A. McGeoch. 2008. Rapid range expansion and community reorganization in response to warming. Global Change Biology 14:2950–2962.
Lenoir, J., J. C. Gegout, P. A. Marquet, P. de Ruffray, and H. Brisse. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771.
Lenoir, J., J. C. Gegout, A. Guisan, P. Vittoz, T. Wohlgemuth, N. E. Zimmermann, S. Dullinger, H. Pauli, W. Willner, and J. C. Svenning. 2010. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 33:295–303.
Mayhew, W. W. 1981. Line Transects and Photo Sites on the Deep Canyon Transect. University of California Natural Land and Water Reserves System, Philip L. Boyd Deep Canyon Desert Research Center, Riverside, CA.
McKechnie, A. E. and B. O. Wolf. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biology Letters 6:253–256.
Meigs, P. 1953. World distribution of arid and semi-arid homoclimates. Reviews of Research on Arid Zone Hydrology. UNESCO, Paris. 203–209.
Miriti, M. N., S. Rodriguez-Buritica, S. J. Wright, and H. F. Howe. 2007. Episodic death across species of desert shrubs. Ecology 88:32–36.
Moritz, C., J. L. Patton, C. J. Conroy, J. L. Parra, G. C. White, and S. R. Beissinger. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322:261–264.
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics 37:637–669.
Parmesan, C. and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42.
Parmesan, C., S. Gaines, L. Gonzalez, D. M. Kaufman, J. Kingsolver, A. T. Peterson, and R. Sagarin. 2005. Empirical perspectives on species borders: From traditional biogeography to global change. Oikos 1:58–75.
Preston, K. L., J. T. Rotenberry, R. A. Redak, and M. F. Allen. 2008. Habitat shifts of endangered species under altered climate conditions: Importance of biotic interactions. Global Change Biology 14:1–15.
Raxworthy, C. J., R. G. Pearson, N. Rabibisoa, A. M. Rakotondrazafy, J. Ramanamanjato, A. P. Raselimanana, S. Wu, R. A. Nussbaum, and D. A. Stone. 2008. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: A preliminary appraisal for the highest massif in Madagascar. Global Change Biology 14:1703–1720.
Reif, J. and J. Flousek. 2012. The role of species’ ecological traits in climatically driven altitudinal range shifts of central European birds. Oikos 121:1053–1060.
Root, T. 1988. Environmental factors associated with avian distributional boundaries. Journal of Biogeography 15:489–505.
Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig, and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421:57–60.
Sala, O. E., F. S. Chapin III, J. J. Armesto, E. Barlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. Huenneke, R. B. Jackson, A. P. Kinzig et al. 2000. Global biodiversity scenarios for the year 2100. Science 287:1770–1774.
Saltz, D., D. I. Rubenstein, and G. C. White. 2006. The impact of increased environmental stochasticity due to climate change on the dynamics of Asiatic wild ass. Conservation Biology 20:1402–1409.
Sauer, J. R., J. E. Hines, and J. Fallon. 2008. The North American Breeding Bird Survey, Results and Analysis 1966 - 2007. Version 5.15.2008. USGS Patuxent Wildlife Research Center, Laurel, MD.
Seager, R., M. Ting, I. Held, Y. Kushnir, J. Lu, G. Vecchi, H. P. Huang, N. Harnik, A. Leetmaa, N. C. Lau et al. 2007. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316:1181–1184.
Sekercioglu, C. H., S. H. Schneider, J. P. Fay, and S. R. Loarie. 2008. Climate change, elevational range shifts, and bird extinctions. Conservation Biology 22:140–150.
Shoo, L. P., S. E. Williams, and J. M. Hero. 2006. Detecting climate change induced range shifts: Where and how should we be looking? Austral Ecology 31:22–29.
Sinervo, B., F. Méndez-de-la-Cruz, D. B. Miles, B. Heulin, E. Bastiaans, M. Villagrán-Santa Cruz, R. Lara-Resendiz, N. Martínez-Méndez, M. L. Calderón-Espinosa, R. N. Meza-Lázaro et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899.
Stralberg, D., D. Jongsomjit, C. A. Howell, M. A. Snyder, J. D. Alexander, J. A. Wiens, and T. L. Root. 2009. Re-shuffling of species with climate disruption: A no-analog future for California birds? PLOS ONE 4:1–8.
Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B. F. N. Erasmus, M. Ferreira de Siqueira, A. Grainger, L. Hannah et al. 2004. Extinction risk from climate change. Nature 427:145–148.
Thomas, C. D., A. M. A. Franco, and J. K. Hill. 2006. Range retractions and extinction in the face of climate warming. Trends in Ecology and Evolution 21:415–416.
Tieleman, B. I. and J. B. Williams. 2000. The adjustment of avian metabolic rates and water fluxes to desert environments. Physiological and Biochemical Zoology 73:461–479.
Tingley, M. W., W. B. Monahan, S. R. Beissinger, and C. Moritz. 2009. Birds track their Grinnellian niche through a century of climate change. Proceedings of the National Academies of Science of the United States of America 106: 19637–19643.
Tingley, M. W., M. S. Koo, C. Moritz, A. C. Rush, and S. R. Beissinger. 2012. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Global Change Biology 18:3279–3290.
Travis, J. M. J., M. Delgado, G. Bocedi, M. Baguette, K. Bartoń, D. Bonte, I. Boulangeat, J. A. Hodgson, A. Kubisch, V. Penteriani et al. 2013. Dispersal and species’ responses to climate change. Oikos 122:1532–1540.
UNEP. 1997. World Atlas of Desertification. United Nations Environment Programme, Edward Arnold Publishers, London.
Walther, G., E. Post, P. Convey, A. Menzel, C. Parmesan, T. J. C. Beebee, J. Fromentin, O. Hoegh-Guldberg, and F. Bairlein. 2002. Ecological responses to recent climate change. Nature 416:389–395.
Walther, G., S. Beibner, and C. A. Burga. 2005. Trends in the upward shift of alpine plants. Journal of Vegetation Science 16:541–548.
Weathers, W. W. 1983. Birds of Southern California’s Deep Canyon. University of California Press, Berkeley, CA.
Wiens, J. A. 1989. The Ecology of Bird Communities. Cambridge University Press, Cambridge, UK.
Wilson, R. J., D. Gutierrez, J. Gutierrez, and V. J. Monserrat. 2007. An elevational shift in butterfly species richness and composition accompanying recent climate change. Global Change Biology 13:1837–1884.
WRCC (Western Regional Climate Center). 2007. Idyllwild Fire Department, California. http://www.wrcc.dri.edu (accessed on October 1).