CHAPTER 10
Species Invasions

LINKING CHANGES IN PLANT COMPOSITION TO CHANGES IN CLIMATE
Laura Koteen
Abstract. California’s grasslands have been dramatically altered by the invasion of nonnative annual grasses from Mediterranean Europe. Along the coast, this invasion has displaced much of the region’s once dominant native perennial grasses. This research investigates the effects of this displacement in community composition on carbon cycling and storage at two sites in coastal northern California. The broader goal is to understand how community changes, brought on by species invasion, have contributed to global climate change through shifting the balance of carbon storage from the soil and plant tissues to the atmosphere. Our coastal research sites had native vegetation growing adjacent to locations where nonnative grasses had invaded. We tracked the processes that affect inputs and outputs of carbon to and from the soil to understand the causes of soil carbon loss following nonnative grass invasion. We found that nonnative grass invasion has resulted in the transfer of an added 30–60 Mg of carbon per hectare from the soil to the atmosphere since the invasion of annual grasses began in the 1700s, becoming widespread by the 1860s. Restoration in select areas may over time be able to reverse the impacts on soil carbon storage described in this chapter. Restoring native grasslands and preventing further large-scale invasion events could contribute to climate change mitigation by reducing carbon lost from California’s natural areas.
Key Points
• Changes in land use and the invasion of nonnative plants can reduce the ability of ecosystems to store carbon.
• In California’s grasslands, the displacement of native perennial grasses by nonnative annual grasses causes a loss of soil carbon.
• Land cover change is both a cause and effect of climate change because climate is the outcome of interactions between the land surface and the atmosphere.
• Native grassland restoration will likely increase carbon storage if established protocols are followed, if the protocols are maintained until perennial grasses become well established and if the climatic zones of the sites selected support relict stands of native grasses.
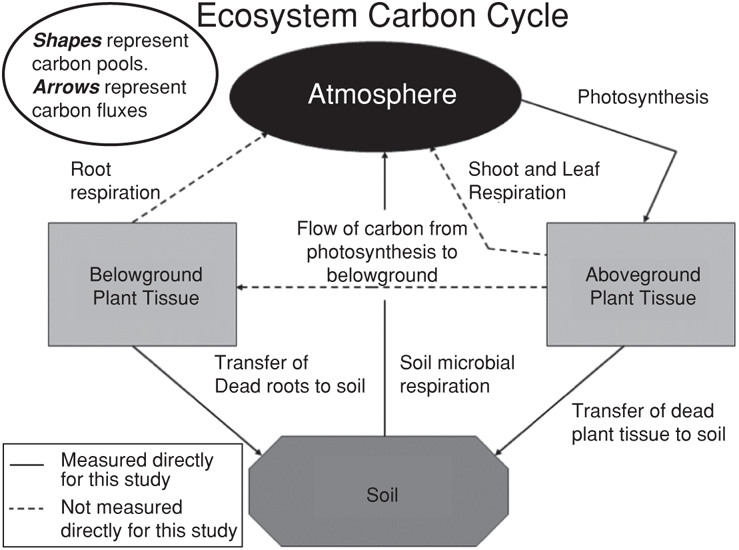
FIGURE 10.1: An illustration of the study design assessing carbon flux among the primary reservoirs of carbon in grassland ecosystems: the atmosphere, plant biomass, and soil.
INTRODUCTION
Since the onset of European settlement, California’s ecosystems have been radically altered. Among the most dramatic of changes is the widespread invasion of nonnative Eurasian grasses into the state’s grasslands. Beginning in the 18th century, this invasion has caused the near extirpation of California’s native perennial bunch grasses across millions of acres of grassland habitat. Indeed, the displacement of native-grassland vegetation in California is one of the most complete and extensively documented land-cover changes worldwide (Seabloom et al. 2003b). Yet, it is but one example of a phenomenon that is much more widespread.
Because they are so common and a primary cause of biodiversity loss, land-use and land-cover change have long been a focus of ecological research. What may be less appreciated is the effect of land-cover change on climate, and more specifically, on the balance of carbon storage between the terrestrial environment and the atmosphere. Historically, land-use and land-cover change account for one quarter to one-half of all terrestrial-carbon losses to the atmosphere, and thus rival fossil fuel combustion as a cause of global climate change (House et al. 2005). Biological invasion is among the causes of land-cover change, and a growing number of investigations have now linked species invasion directly to carbon cycle changes (Christian and Wilson 1999, Ehrenfeld 2003, Liao et al. 2008). For this research, we sought to characterize how the invasion of nonnative grasses has altered carbon cycling and storage in California’s grasslands.
Land-use and land-cover change can affect global climate change by interrupting the natural processes of carbon exchange that are regulated by plant growth, soil microbial activity, and climate. They affect ecosystem carbon pool sizes and flux rates that can shift the balance of carbon between the atmosphere and storage in both vegetation and soils, which in turn can alter the global greenhouse effect. Carbon pools are the reservoirs in which carbon is stored and include plant tissues, the soil, and the atmosphere. Fluxes describe the rates of exchange of carbon among ecosystem pools. The flux of carbon from the atmosphere to plants occurs via photosynthesis (Figure 10.1). Carbon flux from plant storage to the soil occurs when plant tissues decay and are incorporated into the soil complex by soil microbes and fauna, the process by which plant tissue becomes a part of the soil carbon pool. Carbon flux from the soil to the atmosphere occurs primarily through microbial consumption and respiration of soil organic matter in the form of CO2. As this study documents, even a seemingly subtle change in species composition (i. e., from one grass type to another) can affect climate by altering ecosystem carbon pool sizes and / or flux rates.
Here we compare carbon pools and fluxes at two locations where both native and nonnative grass types are found in similar environmental settings. Because most grassland carbon is stored in soil (Schlesinger 1997), our first goal was to determine if differences in soil carbon storage exist between native and nonnative grass types. Our second goal was to understand the specific aspects of carbon cycling and storage that differ between native and nonnative grasslands, which might explain soil carbon differences. We began with three hypotheses: (1) Differences in soil carbon result from differences in the amount of root and aboveground carbon that enters the soil due to turnover of plant tissues each year. (2) Differences in soil carbon result from differences in the chemical composition of plant tissues, affecting rates of soil organic matter accumulation and decomposition. (3) Differences in soil carbon result from differences in soil climate beneath the different grassland types. Soil climate and the tissue chemistry of senesced plant matter control the size, activity, and composition of the soil microbial community. These microbial community properties in turn affect flux rates of soil carbon to the atmosphere. Differences in annual plant growth as well as the flux size of plant tissue that enters the soil each year address the first hypothesis.
We predicted that nonnative grass invasion would cause a drop in ecosystem carbon storage due to a shift from perennial plants (natives) to annual ones (nonnative). Each type of plant is associated with a different suite of traits that allows survival through long summer drought. Being perennial, California’s native grasses maintain a year-round connection with the soil and atmosphere. Deep roots that exploit the full volume of soil for water, a dense aboveground structure that inhibits soil evaporation, and high root production enable their survival through seasonal water scarcity (Figure 10.2, left). Attributes that promote soil carbon accumulation also fit into a strategy of water conservation, as soil organic matter is capable of storing more water than mineral soil (Hudson 1994). In contrast, the nonnative grasses are annuals. They complete their life cycle before the onset of summer drought; they grow from seed each year when autumn rains begin and senesce in April or May. Aboveground, annual grasses are relatively sparse, allowing radiation to penetrate to the soil surface and causing intense drying of the upper soil profile (Figure 10.2, right). Soil desiccation near the surface also results from the annual grass root system, which is concentrated in the top 20 cm.
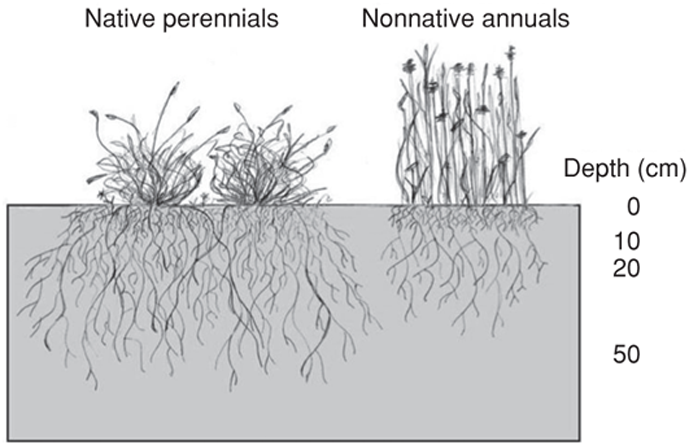
FIGURE 10.2: California’s native perennial and nonnative annual grasses. Grass morphology at the time of peak biomass in spring in the native perennial bunch grass community (left) and nonnative annual grass community (right).
METHODS
Site Description
We established research plots in two grassland sites: The Tennessee Valley site, which resides in the headlands of the Golden Gate National Recreation Area (elevation 220 m), and the Bolinas Lagoon Preserve site, located on a private preserve outside Bolinas, California (elevation 168 m; Figure 10.3). Both sites are within a kilometer of the coast and experience a climate with distinct wet and dry seasons. Mean annual precipitation is approximately 900 mm, with high interannual variability (±300 mm standard deviation). The rainy season typically occurs from late October through April, but can extend into May or June. Summers are warm and dry, but moderated by coastal fog. Vegetation at both sites was predominantly grassland interspersed with patches of shrubs. We set up research plots at Tennessee Valley on northeast-facing slopes that ranged from flat to 2%. Slopes at the Bolinas Lagoon Preserve were south-facing and ranged from flat to 5%. The latter site was generally drier than the Tennessee Valley site due the extended direct solar exposure this grassland receives. Soils at both sites are composed of a well-drained sandy loam on bedrock derived from sandstone and shale (Soil Conservation Service 1985).
FIGURE 10.3: A map of the study area and field site locations
At each site in the spring of 2003, we set up 2 × 2 m research plots in relatively pure patches of native perennial and nonnative annual grass communities: 10 plots at the Bolinas Lagoon Preserve site, equally divided between native and nonnative grass communities, and 15 plots at the Tennessee Valley site, with five in each of the two native grass communities and five in the nonnative community. Patches were chosen for their similarity in soil properties, land-use history, slope, and aspect. In this study design, native plots represent preinvasion conditions. The relative carbon-storage status in nonnative plots represents the cumulative carbon cycle changes that had accrued since the time of annual grass invasion. The dominant nonnative grass species at Tennessee Valley were slender wild oat, Avena barbata, Italian rye grass, Lolium multiflorum, rattlesnake grass, Briza maxima, and small and rattail fescue, Vulpia spp. The dominant native perennial grasses at this site were Hall’s bentgrass, Agrostis halli, a rhizomatous grass, with continuous aboveground cover, and red fescue, Festuca rubra, a bunch grass. In general, bunch grasses are more typical of native perennial grasses in California. At the Bolinas Lagoon Preserve, the five nonnative plots are dominated by the annual grass, false brome, Brachypodium distachyon, but also contain common wild oats, Avena fatua, and rattlesnake grass, B. maxima. The five native bunch grass plots at this site were dominated by a mix of purple needlegrass, Nassella pulchra, and California brome, Bromus carinatus, or by blue wild rye, Elymus glaucus.
Measurements of Soil Carbon Pool Size
At each site, we measured the standing pools of soil carbon in 10 cm intervals by extracting seven soil cores from the surface to 50 cm depth per plot). From each of these cores, we determined the percentage of carbon in each depth interval and then multiplied by the mass of soil in the core to determine the total mass of carbon for each depth.
HYPOTHESIS 1: DIFFERENCES IN BIOMASS AMOUNTS (ANNUAL PRODUCTIVITY) We harvested grasses in 0.25 m2 areas in the late spring of 2004 and 2005, and estimated the total annual productivity as equal to total standing aboveground biomass at the time of peak growth (Scurlock et al. 2002, Corbin and D’Antonio 2004, Lauenroth et al. 2006). To determine the belowground flux, we extracted 3.5 cm diameter soil cores from 0–10, 10–20 and 20–50 cm depth in January and April 2004, for nonnatives, and January and June 2004, for natives. Samples were collected at the time of minimum and peak biomass for each grass type (Corbin and D’Antonio 2004, Corbin and D’Antonio 2010). Subsequently, roots were separated from soil to obtain root-density estimates per soil volume. The annual belowground flux was calculated as the difference between root density at peak and minimum biomass plus an estimate of the roots that had decomposed in the intervening time period.
For the years of our study, annual precipitation was below average for the water year 2003–2004 (average of 89% and 83% for Tennessee Valley and the Bolinas Lagoon Preserve, respectively), and higher than average for the water years 2004–2005 (117% and 131%) and 2005–2006 (162% and 144%). We did not measure aboveground productivity directly in 2006 at either site, or root productivity in 2005 or 2006. Instead, we estimated these carbon pools based on reports from the literature that found a positive relationship between aboveground and belowground productivity and precipitation (Jackson and Roy 1986, Dukes et al. 2005, Chou et al. 2008). We also observed an increase in aboveground grass productivity after higher rainfall in 2005.
HYPOTHESIS 2: DIFFERENCES IN PLANT TISSUE CHEMICAL COMPOSITION (LITTER QUALITY) To estimate differences in the chemical composition of plant tissue between native and nonnative grass types, we performed leaf- and root-litter decomposition experiments in each plot (Koteen et al. 2011). Known amounts of senesced plant tissue were sewn into mesh bags and the rate of mass loss tracked over time (Wieder and Lang 1982). Differences in the rate of mass loss served as an indication of differences in tissue chemical composition between grass types, as tissues that are less palatable to soil microbes take longer to decompose. We initiated leaf decomposition experiments in 2003–2004 and 2004–2005, and tracked root-litter decomposition in 2005–2006. To bolster these findings, we also conducted an analysis of secondary compounds in leaf and root samples according to methods outlined in the study of McClaugherty et al. (1985) and Ryan et al. (1990).
HYPOTHESIS 3: DIFFERENCES IN SOIL CLIMATE IN DIFFERENT GRASS TYPES We measured soil respiration on a monthly basis from January 2005 to June 2006 at five sites within each plot, using the LI-COR 6400 infrared gas analyzer (LI-COR Inc., Lincoln, NE). To isolate the respiration of carbon by soil microbes from respiration by roots, we removed the aboveground vegetation from the soil collars where soil respiration measurements were performed, and kept them free of vegetation over the period of observation. Nonetheless, root respiration undoubtedly accounts for some unknown fraction of the amounts we report, as respiration from neighboring roots could still enter the soil column beneath each collar (Pumpanen et al. 2003).
To understand the role of soil climate at different soil depths in promoting or inhibiting soil respiration, we measured soil moisture in 5 cm intervals to 40 cm depth using a soil-moisture-profile probe (Delta-T Devices, Cambridge, UK) regularly over the course of the study. We also measured soil temperature at the surface and at 5, 15 and 35 cm depth, hourly, using HOBO data loggers (Onset Computer Corporation, Bourne, MA) from 2003 to 2006.
To compare the total soil respiration between grass types on an annual basis, we modeled soil respiration for the water years from 2003 to 2006 based on an analysis of the correlation between soil climate variables and measured soil respiration. Because we had nearly continuous measurements of soil moisture and temperature, we used these correlations to compute hourly soil respiration for each grass type, and to produce annual sums. To quantify the level of uncertainty in our modeled annual soil respiration values, we began with the error estimates from the correlation between soil respiration with soil moisture and temperature for each grass type. We next developed estimates for the probability distribution of our model parameters using the open source software program, WinBUGS, and then computed error estimates for each hour of the year via a Markov chain Monte Carlo simulation in Matlab (Verbeeck et al. 2006).
Differences between mean biomass values, flux rates, and litter-decay constants between grass types were determined using ANOVA, with five plots per grass community, for five grass communities across two sites.
RESULTS
As predicted, we found consistently higher pools of carbon in soils of the native perennial grass community relative to the nonnative. At the Bolinas Lagoon site, we found a drop in soil carbon equivalent to 30 Mg per hectare. At the Tennessee Valley site, drops in soil carbon amounted to a loss of 60 Mg per hectare. This difference was most notable at soil depths below 30 cm, but was also evident near the top of the soil profile, at both sites (Table 10.1 and Figures 10.4a and 10.4b). Therefore, we concluded that a net transfer of carbon from the soil to the atmosphere followed nonnative grass invasion. We found support for two out of three hypotheses that explain how this transfer occurred.
Reasons for Differences in Soil Carbon Storage
HYPOTHESIS 1: DIFFERENCES IN BIOMASS AMOUNTS (PLANT PRODUCTIVITY) We found support for our first hypothesis. In 2004, we found higher productivity in the native perennial community relative to nonnative annuals in both aboveground and belowground tissues (Table 10.1). Moreover, the native bunch grasses in our study produced significantly greater fine-root biomass at all soil depths than the nonnative annuals (Table 10.1 and Figures 10.4c and 10.4d). The rhizomatous grass, A. halli, also produced significantly greater root biomass below 10 cm depth. However, root production was significantly lower than the native bunch grasses. High annual rainfall and an extended growing season in 2005 corresponded with a much higher total production in annual and perennial grass types than in 2004. Belowground productivity at both sites in 2005 was significantly higher in all native grasses than in nonnative grasses at all soil depths. Aboveground productivity the same year was not significantly different between grass types at Tennessee Valley, but nonnative aboveground productivity was significantly higher in the nonnative grass type at the Bolinas Lagoon Preserve. In 2006, we assumed that both aboveground and belowground productivity were similar to 2005, given that it was also a high rainfall year.
HYPOTHESIS 2: DIFFERENCES IN TISSUE CHEMICAL COMPOSITION (LITTER QUALITY) We found only small differences in litter quality across the sites, with one exception. From aboveground litter and root decomposition experiments, we found significant differences in litter quality between native and nonnative grass types only for the roots at the Tennessee Valley site. At this site, we found the roots of the native perennial bunch grass, F. rubra, decomposed significantly more slowly than those of the other Agrostis and nonnative grass communities, with the first-order decomposition constant (k = 0.28 for F. rubra, k = 0.52 for A. halli, the native rhizomatous grass, and k = 0.52 for nonnative grass type, p = 0.001). Here, the constant values indicate a rate of mass loss over time, with a higher value indicating a faster rate of decomposition. The analysis of secondary compounds in leaf and root litter generally corroborated the findings of the decomposition assays.
TABLE 10.1
Productivity, respiration, and soil carbon at the Tennessee Valley (TV) and Bolinas Lagoon Preserve (BLP) sites in units of kg m−2
Native and nonnative grass communities for the water years 2003–2004, 2004–2005, and 2005–2006
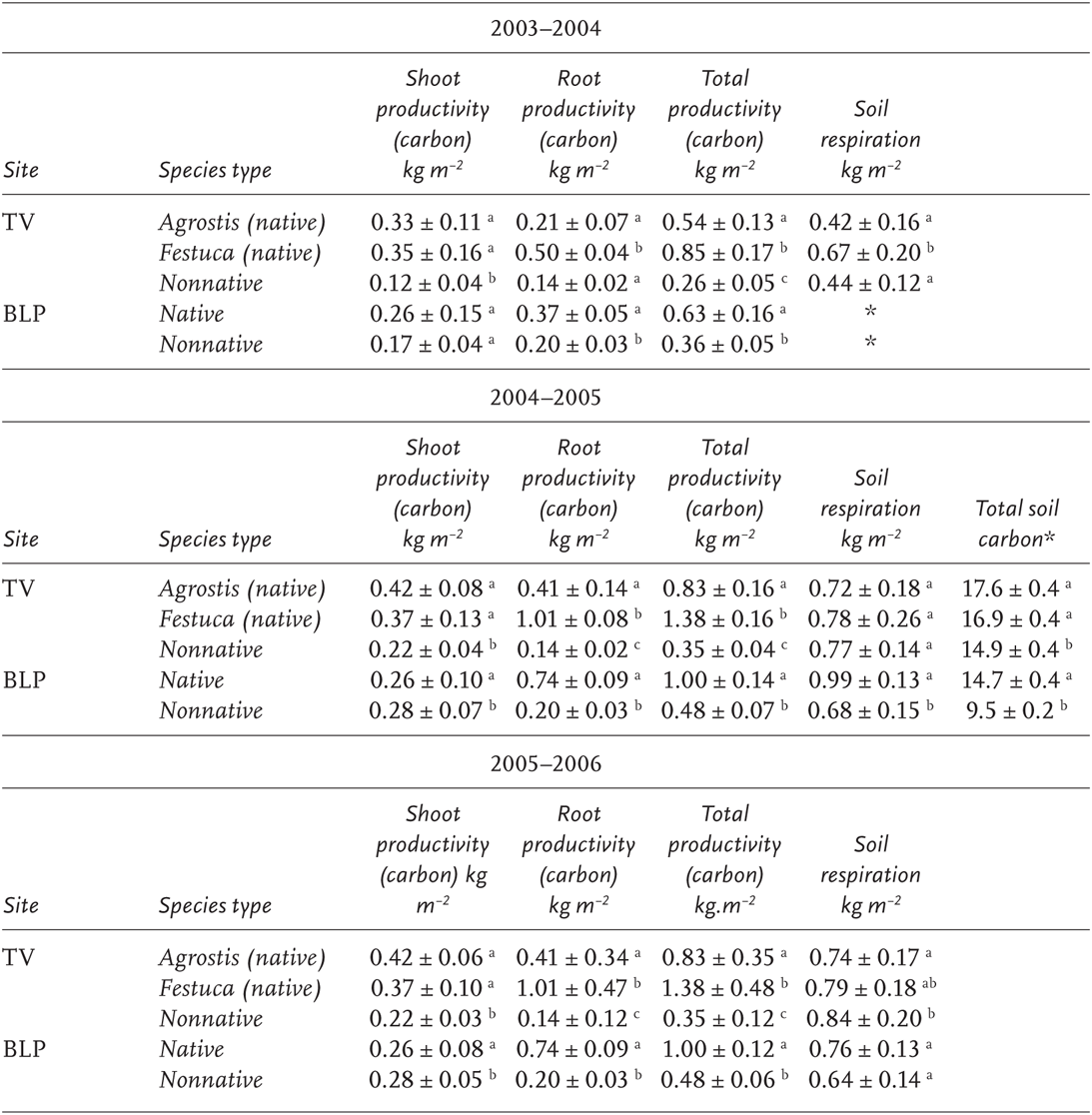
NOTE: Error values represent ±1 standard deviation, n = 5. ANOVA was used to detect differences between mean values. Different letters indicate significant differences at p < 0.05 among measurement categories, and only apply within individual sites.
*The soil carbon values reported here are from a single sampling assay completed in 2005, n = 7 cores per plot. Similar values were found in a 2003 sampling. Letters indicate significant differences among grass communities.
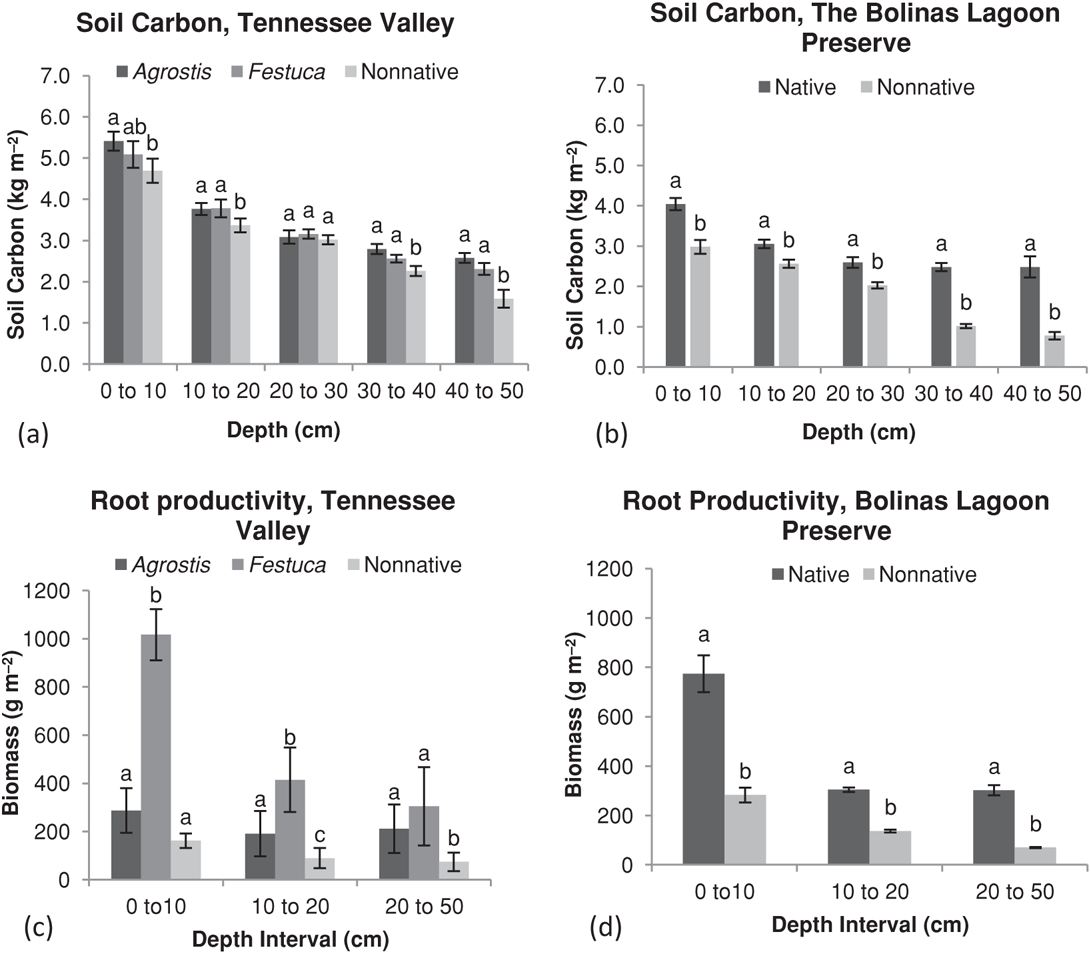
FIGURE 10.4: Soil carbon storage and root productivity as a function of soil depth. Soil carbon storage in native perennial and nonnative annual grass communities at (a) Tennessee Valley and (b) the Bolinas Lagoon Preserve. Annual root productivity for all grass types averaged between the dry year 2003–2004 and the wet year 2004–2005 at (c) Tennessee Valley and (d) the Bolinas Lagoon Preserve. At the Tennessee Valley site, Festuca is a native perennial bunch grass and Agrostis is a native perennial rhizomatous grass. Error bars represent ±1 standard error.
HYPOTHESIS 3: DIFFERENCES IN AMOUNTS OF SOIL RESPIRATION Differences in soil respiration rates explain differences in soil carbon storage at both of these sites because net carbon storage is the difference between annual carbon inputs into the soil (annual productivity) and outputs from the soil (soil respiration) (Figure 10.1). At both the Tennessee Valley and Bolinas sites, we found no consistent pattern in soil respiration differences between native and nonnative grass communities that held across all years (Table 10.1). These results would seem to disprove the hypothesis that soil respiration contributes to the drop in soil carbon storage in nonnative grasslands. In each site comparison, for each individual year, however, soil carbon fluxes into the soil due to plant growth, and senescence exceeds or equals carbon fluxes out of the soil from soil respiration in the native grass types, and the reverse is true for the nonnative grass types (Figure 10.5).
Soil respiration rates were positively correlated with (1) the timing of organic plant fluxes into the soil, (2) the soil carbon pool size, (3) the product of soil temperature and moisture, and (4) soil moisture as the dominant factor (i. e., soil fluxes from the soil were highest during the months that the decomposing plant tissues were actively entering the soil) (Koteen et al. 2011). We also found that soil moisture differs with depth along the soil profile among grass types, and with respect to the location of soil carbon, which is highest at the top of the soil profile and declines with soil depth (Figures 10.4a and 10.4b). At both sites, soil moisture was higher at the top of the soil profile in native grasses relative to nonnative grasses and lower at the bottom of the soil profile (Figures 10.6 and 10.7). Soil temperature was consistently negatively correlated with soil moisture (data not shown).

FIGURE 10.5: Annual productivity and soil respiration for native perennial and nonnative annual grasses, Tennessee Valley and the Bolinas Lagoon Preserve. Annual production of biomass carbon and annual soil respiration for the water years extending from October through September for 2003–2004 (a), 2004–2005 (b), and 2005–2006 (c), for the Tennessee Valley and Bolinas Lagoon Preserve Field sites (d and e). Agrostis is the native rhizomatous grass and Festuca is the native bunch grass. Error bars represent ±1 standard deviation. Different letters represent significant differences at p < 0.05 between annual productivity and soil respiration within individual grass communities.
We further examined the possibility that differences in soil properties (i. e., soil texture, soil pH, soil rock content) might explain differences in soil carbon storage. In general, we found small differences in these properties among soils of different grass types, with the exception of soil texture. Small but significant differences in soil texture may have contributed to, but do not explain, the differences we found in soil carbon storage.
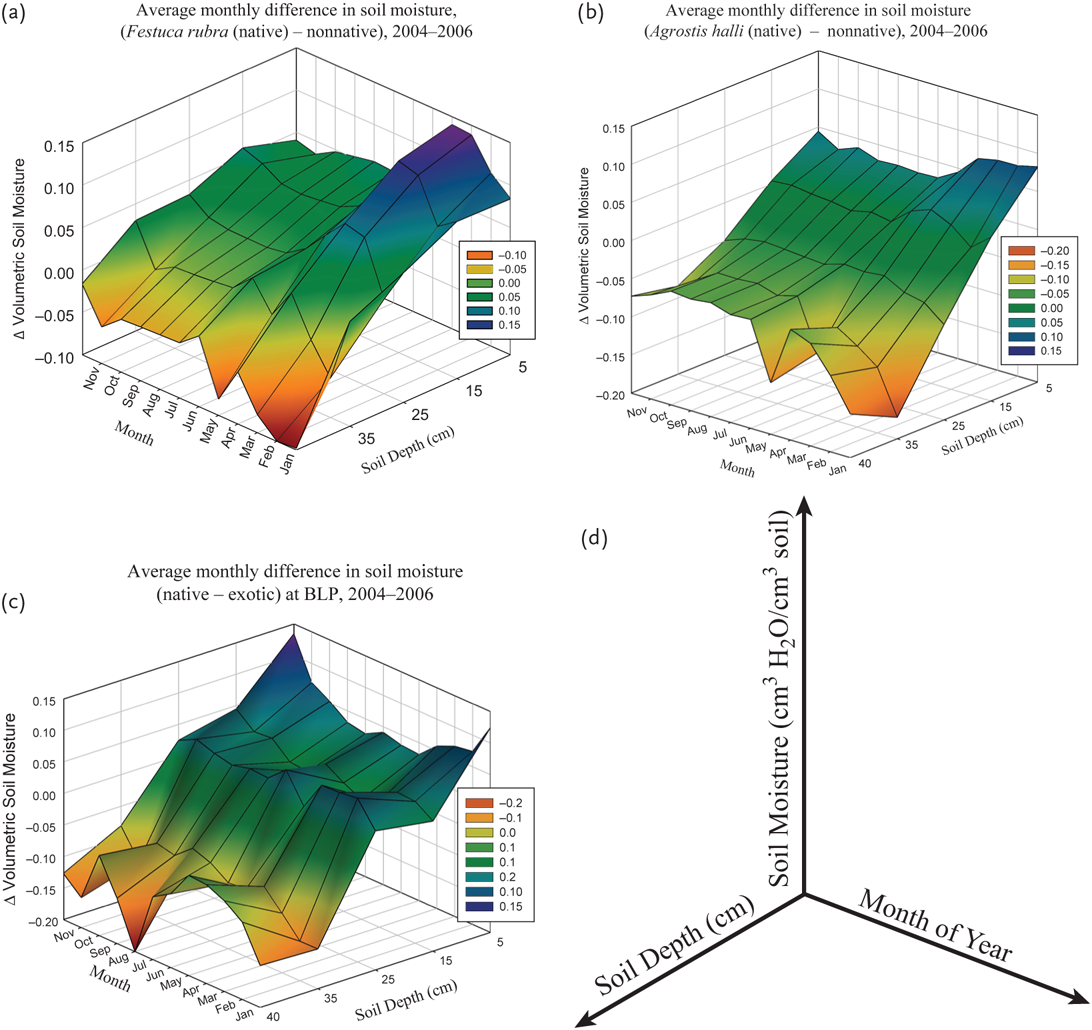
FIGURE 10.6: The difference between grass types in volumetric soil moisture along the soil profile. (a) Differences between the Festuca (native bunch grass) community and the nonnative community at Tennessee Valley (b) between the Agrostis (native rhizomatous grass) community and the nonnative community at Tennessee Valley, and (c) the native bunch grasses and nonnative communities at the Bolinas Lagoon Preserve, and (d) a diagram of axes defining the variables represented on each axis.
DISCUSSION
Our findings indicate that the invasion of California’s grasslands by nonnative grasses from Mediterranean Europe has caused a substantial drop in soil carbon storage equaling from 30 to 60 Mg per hectare at the sites examined. We attribute the drop in soil carbon to the difference in the cumulative annual net carbon flux between native and nonnative grass types since the time of annual grass invasion.
The loss of soil carbon appears to stem from key differences in annual and perennial plant traits that evolved in response to periods of seasonal water scarcity. Native perennials possess deep roots, and a dense aboveground structure that inhibits soil evaporation. Nonnative annuals, in contrast, have shallow roots, a short life cycle, and a sparse plant canopy. In this case, the same strategies employed to survive the summer drought also explain the differences we found between grass types in carbon cycling and storage.
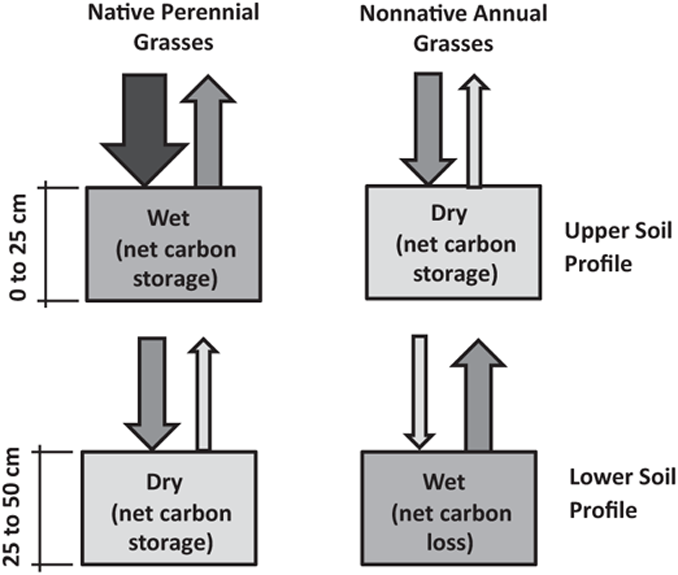
FIGURE 10.7: Contrasting carbon cycling dynamics in coastal native perennial and nonnative annual grass communities within individual research sites. Diagram of theorized annual carbon inputs and outputs to and from the soil. The inputs and outputs (indicated by arrows) are relative to each other in the same depth profile and indicate average conditions over the course of the annual cycle. Soil depth divisions between the upper and lower soil profiles are approximate and vary by species. The size of the soil respiration term is highly correlated with the soil moisture content. See Discussion text for a detailed explanation of the contrasting plant strategies that produce differences in soil moisture content and biomass inputs.
In addition to differences in plant traits, the difference in growing-season length also helps explain the loss of soil carbon following nonnative grass invasion through its effect on the balance between Net Primary Production (NPP) and soil respiration in each grass type, especially in years of high rainfall (2004–2006). Whereas both productivity and soil respiration in Tennessee Valley vary positively with water availability in native perennial grasses (Figure 10.5), other studies have shown soil respiration varies more than NPP as a function of water availability, in soils dominated by nonnative annuals (Ma et al. 2007, Zhang et al. 2010). The timing of annual grass senescence is, at least in part, internally set by the need for reproduction to occur every year regardless of environmental conditions, and senescence closely follows flowering in this grass type (Jackson and Roy 1986, Jackson et al. 1988). Therefore, productivity is capped in the annual grass type by constraints on growing-season length, whereas soil respiration is not. Our findings indicate that this imbalance in soil fluxes into and out of soil accounts for the loss in soil carbon in the annual grass type. The timing of summer dormancy in native perennial grasses varies to a lesser degree than in annual grasses (Laude 1953). And the higher NPP in years of high rainfall in most cases supersedes the loss of soil carbon due to higher soil respiration, leading to net soil carbon accumulation where native perennial grasses are found.
The differences in plant strategies with regard to water use also help explain the pattern of soil carbon differences between grass types in the upper and lower soil profiles. Near the soil surface, soil carbon storage is greater in the perennial grass community. However, the difference is small given the large differences in total productivity between native and nonnative grass communities, and significant in most, but not all, comparisons (Figures 10.4a and 10.4b). This outcome is consistent with the interpretation that both biomass inputs and soil respiration are high in the upper soil profile of the perennial grass type and relatively low in the nonnative grass type (Figure 10.7). We attribute differences in soil-respiration rates to differences in soil moisture in upper soil layers between grass types. We found that the dense aboveground cover and deeper root system of perennial grasses lead to a relatively even drawdown of soil moisture along the depth of the soil profile. In contrast, the relatively sparse aboveground cover of nonnative grasses produces a soil moisture profile that is dry for much of the year near the soil surface, suppressing soil respiration. Deeper in the soil, the carbon pool sizes diverge between grass types with significantly greater carbon storage present where native perennial grasses are found. At these depths, perennial grasses have both a greater biomass flux into soil and lower respiratory losses (Figures 10.4c, 10.4d, and 10.7). In contrast, very low inputs from shallow-rooted annual grasses, coupled with high soil respiration rates associated with untapped soil moisture beneath the rooting zone, have caused a loss in soil carbon at these depths.
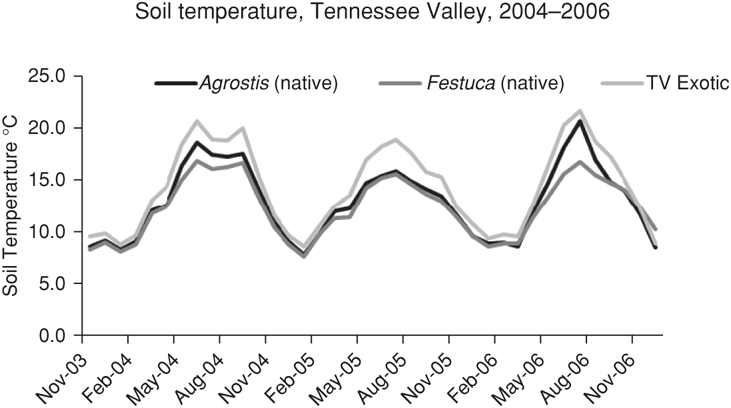
FIGURE 10.8: Soil temperature near the top of the soil profile for native and nonnative grass communities at the Tennessee Valley site.
These findings are largely consistent with other studies investigating nonnative grass invasions into grasslands. Generally, similar research has found that invading species possess higher aboveground and lower belowground productivity, greater seed production, lower belowground allocation, and a shallower rooting depth in comparison to the native grasses they displace (Christian and Wilson 1999, Wilsey and Polley 2006, Adair and Burke 2010). These findings differ in that we also found consistently higher aboveground productivity in the native grasses at the Tennessee Valley site.
In addition to promoting higher carbon storage, we found that native perennial grasses also influence the microclimate in ways that may increase their resilience to continued increases in regional temperature. Specifically, we found that the denser structure and more complete cover of perennial grasses lead to a moister, cooler soil surface environment when compared to soil surfaces under nonnative grasses, particularly at the Tennessee Valley site (Figure 10.8). Thus, perennial grasses may promote the persistence of associated plant and animal species that are close to the upper limits of species-specific temperature or drought tolerances. And where natives remain in small patches in largely annual dominated grasslands, they may serve as refugia to associated species during periods in which climatic conditions in nonnative patches are unfavorable.
Lastly, we note that the changes we document in the grasslands of northern coastal California are similar to changes that have occurred elsewhere in the state and in many temperate grasslands worldwide where deep-rooted vegetation has been replaced by shallow-rooted crop species, such as cereal crops. Therefore, in documenting the change in soil carbon storage within coastal California’s grasslands, we are focusing on a phenomenon that may be much more widespread (Houghton 1999).
CONCLUSION
With concerns that global climate change will reconfigure our land surface with ecosystems of novel character and uncertain function, much ecological research is devoted to understanding how climate change alters the composition of terrestrial ecosystems and instigates carbon feedbacks. Although most changes in community composition in California’s grasslands are not the result of a changing climate, they are an excellent example of the types of changes climate change may bring about, and therefore warrant further study. Moreover, because these vegetation shifts occurred long ago, these systems are advanced in their progression toward a new equilibrium with respect to resident species and carbon cycle changes. As such, they offer insights into ecosystem-level processes and changes that may not yet be apparent in ecosystems currently in a state of transition. Further, California’s altered grasslands may be representative of ecosystem-level changes that may increase in the future. They are composed largely of invasive species and support lower species diversity (Stromberg et al. 2001, Coleman and Levine 2007). As the climate changes, many species will experience heightened physiological stress, disease, and disturbance (Koteen 2002, Roy et al. 2004, Evans et al. 2008), causing land areas to open up (Westerling and Bryant 2006). By definition, invasive species are less hampered by competitive pressures than native species and may therefore have an advantage in colonizing areas of recent disturbance.
LESSONS FOR GRASSLAND RESTORATION FROM EXPERIMENTAL AND OBSERVATIONAL STUDIES
The primary solution to soil carbon loss with nonnative grass invasion is restoration of native perennial grass communities (Box 10.1). An informed approach would consider the soil types, future climate, and species for which restoration efforts might have the maximum effect in increasing soil carbon storage. Although the changes documented here represent a modest contribution to atmospheric CO2, native perennial grasses do store significantly more carbon than nonnative annuals, and their loss represents the near disappearance of a unique and iconic ecosystem that once populated large areas of California. Therefore, the restoration of native perennial grasses to some of the locations they once occupied would simultaneously sequester carbon and restore native biodiversity. However, if one’s goal is to simply sequester the maximum amount of carbon without regard for benefits to biodiversity, prevented emissions, reforestation, or afforestation would be superior options (Hudiburg et al. 2009, Battles et al. 2014). Other land-based options for increasing soil carbon storage in California, where nonnative annual grasslands are likely to persist, include applying organic amendments to soil grazed pastures and other grasslands (DeLonge and Ryals 2013, Ryals et al. 2014).
When designing restoration protocols for California’s native grasses, several considerations should be instituted in order to ensure restoration success. Restoration efforts should focus on locations where reconversion to exotic annual grasses is least likely, should draw from seed sources local to the area where restoration is taking place, and should focus on species native to the region. Because remnant native perennial grasslands are found primarily along California’s coast, and especially along the northern coast, presumably restoration efforts would be most successful in these regions. Perennial-grass persistence along the coast is thought to be aided by the existence of moisture inputs from the summer fog cycle (Corbin et al. 2005). In light of climatic changes, regions that possess landscape features that mitigate projected temperature increases and enhanced drought (i. e., northerly aspect) or which are projected to receive summer precipitation or an extended rainy season may also be good locations for restoration efforts. Any climatic changes that increase soil water availability after nonnative annual grass senescence would uniquely benefit natives. Regions other than those considered strictly coastal may be good candidates for native grass restoration as well. Beyond geographic location, the absence of prior cultivation is strongly associated with the persistence of native perennial grasses (Stromberg and Griffin 1996).
A second measure that will increase grassland restoration success is the maintenance of management protocols until perennial grasses become well established (Stromberg et al. 2007). Methods that seek to overcome the relative seed limitation of perennial grasses during establishment, as well as space and light limitations, may aid in producing self-sustaining native grass communities (DiVittorio et al. 2007). Several recent experiments have found that multiple species of native perennial grasses were superior competitors to exotic annuals, or achieved a state of persistent coexistence with nonnative annuals under a range of climatic conditions and treatments when steps were taken to overcome barriers to establishment (Seabloom et al. 2003, Corbin and D’Antonio 2004, Suttle and Thomsen 2007).
In a number of earlier studies, nonnative annual grasses proved to be strong competitors against the native grass, N. pulchra, in a range of experiments in California’s Central Valley (Dyer and Rice 1997, Dyer and Rice 1999). Overall, it appears unlikely that annual grasses are superior competitors to many well-established native perennial grasses, and that at least in some locations, the reverse may be true.
Overall, the results of this comparative study indicate that a native perennial grass restoration effort would yield multiple benefits for the state of California. Native perennial grasses are found to positively impact global climate relative to annual grasses by sequestering more carbon in the soil. They also support more diverse ecosystems than nonnative annuals, and may provide associated taxa with microclimatic conditions more favorable to their persistence during hotter and drier portions of the year. Therefore, efforts that seek to restore native grasses would serve both climate and biodiversity goals. In general, the expectation that perennial grass communities function in ways that promote climate change objectives relative to nonnative annuals are in good agreement with this study’s findings and with an understanding of their fundamental differences in their perennial or annual life cycles.
BOX 10.1 • Native Grass Restoration as a Carbon Offset
If we assume the site-averaged difference in soil carbon storage between native and nonnative grasslands from this study (45 Mg ha−1) can be applied more generally to grasslands in California, the potential opens up for grassland restoration activities to gain financial support through state or federal carbon offset programs. According to the 2007 EPA estimates, a vehicle of average fuel efficiency emits approximately 5 metric tons of CO2 per year (EPA 2009). Therefore, in carbon currency, we estimate that the restoration of 500 ha of grassland to native species would be equivalent to removing more than 4000 cars from the road for a year. In practice, however, the total gain in grassland soil carbon storage would accrue over many years. Therefore, in this context, and other biological settings, the actual funds to support such efforts would require established rules for reimbursement over time, and a carbon market interested in supporting projects that support both climate and conservation goals.
Manager Comments
Laura Koteen
in conversation with
Mark Stromberg
Koteen: In my work, I identify restoration of native grasslands as an important component of responding to climate change in California. Can you comment on what you see as the main impediments to restoration of native grasslands? Ecological? Social? Economic?
Stromberg: Ecological impediments include climatic factors. If one is using seed, native grasses have the greatest chance of successful establishment, and are most competitive in a good year, which means a warm, wet year: a good warm, wet fall to get them well germinated and with sufficient growth, and a good wet spring to solidify their establishment. However, these years are kind of rare. Their chances of successful establishment and survival are also best along the coast regions, where the fog belt is active in summer, because it is wetter and cooler. It is unclear how climate change may affect restoration efforts vis-à-vis the establishment phase: whether it will increase the frequency of warm, wet years that enhance restoration success, or whether it will alter rainfall patterns in ways that hinder native grass establishment. It is also possible that climatic conditions will shift so that exotic grasses and / or other community actors are favored. For example, exotic perennial grasses have already greatly increased their presence in grass communities along the coast, and brush encroachment of both native and nonnative shrubs has increased in recent decades.
The biggest social impediment is the loss of a connection with nature. People don’t have a good knowledge of California’s natural history. They don’t know that perennial bunch grasses were once the norm in many places (e. g., Berkeley) and that our grasslands are composed of largely nonnative grasses. They are not able to identify native from nonnative grasses by sight. People do notice that the flowers have been lost over time. However, they are not aware that spring flower shows are gone because the native grass community that supports them is gone, as well as the native processes, such as grazing or fire that maintain native grassland by reducing the pressure of invasive species.
From an economic perspective, grass restoration is expensive, especially over large areas. To keep it affordable, grass restoration should be confined to those areas where grasses are self-sustaining once well established (i. e., the coastal terraces, interior areas with deep soil, often subirrigated).
Koteen: In addition to possible carbon storage / climate benefits, what additional benefits do you see to native grass restoration?
Stromberg: I see many benefits to native grassland restoration, including connecting people back to nature and to California’s natural history if the restoration builds in an educational component. I also see the potential for better water quality through reduced erosion, and wildlife benefits. In learning of the carbon / climate benefits of restoring grasslands from this research, I see the potential for using native grassland restoration to obtain funding through carbon offsets. There is a lot of root tissue stored below ground in native grasses. In dry years, a lot of that root carbon will become incorporated into the soil.
Koteen: Are there particular locations you would prioritize in terms of promoting native grassland restoration?
Stromberg: Different areas are important for different reasons. I would include the fog zone all along the undeveloped coastline of California, and in the near coastal region in protected natural areas, where there is a marine influence and inputs of moisture from fog. Not only does the climate support grasses in these locations, if the plant communities within the coastal terraces are restored, but they also have the potential to become really spectacularly diverse native areas. Of course, it is also unclear if climate change may impact the summer fog cycle in California, which provides the moisture inputs which allow native grass communities to flourish. The native coastal terrace grassland communities of California are among the top 1% of diverse grassland communities worldwide. Good restoration could occur from San Diego County where protected areas remain and north from Santa Barbara up through Mendocino County.
I would also prioritize the San Francisco Bay Area, because there, one-third of the coastal areas are in protected lands, generally open spaces supported by the local communities. There is also a knowledge base and a cultural base that values preserving these open spaces and restoring native vegetation. Moreover, it’s an urban area. In addition to having ecological value, grasses there have an educational value. If they are in the Bay Area, people will see them and learn about them, and may then appreciate that they are a rich part of California’s natural heritage. Similarly, rooftop restorations, such as the California Academy of Science, are also good areas to prioritize, again because of the educational value of having them in such high impact places. Restoration efforts on rooftops and in heavily frequented areas also provide opportunities for people to learn about the carbon storage benefits associated with native grass restoration. The restoration of Crissy Field, near the Golden Gate Bridge, is another good example of an area where lots of people see and experience native grasses and are thus educated about them.
Agricultural areas can also be good candidates for restoration, such as areas of the Central Valley where the soil remains damp. This includes low-level swampy areas, locations of natural springs as well as areas that receive subsurface flows from irrigation runoff. At the farm scale, good places include strips of grassland in between crops. If placed between crops, such hedgerows could provide important habitat for native pollinators, and serve as pollination sources for crops. Roadsides, ditches, and slopes are also good locations for native grasses. The deep roots of native grasses hold the soil together and reduce erosion and reduce or eliminate annual road and ditch grading. Particularly in light of possible increases in storm intensity, which may accompany global climate change, the value of erosion reduction will increase.
Koteen: Coming back to carbon, to what extent do your management objectives incorporate carbon storage? Do you see opportunities to highlight the value of these functions and their dependence on management actions that promote native species with state agencies or funding sources?
Stromberg: The University of California Natural Reserve System, where I work, has not previously highlighted these functions, and the natural reserve system has primarily research and education functions. However, I do see the value in promoting carbon storage through native grassland restoration and have seen landowners in Arizona and California tout these benefits in other restoration efforts. There is also a project in the Dakotas for native grassland preservation supported by a grant obtained from Ducks Unlimited and conservation partners based on carbon storage potential of Midwestern native grasslands. In addition, I have been involved with efforts that explicitly recognize the carbon storage potential of oak woodlands and which attempt to educate private landowners about the potential revenue for management activities to increase carbon storage in oak woodlands through the Climate Action Reserve, a state program. Such revenues could potentially be available for grassland restoration as well.
Koteen: Has this chapter led you to consider any changes in objectives or methods for monitoring at the University of California Reserves?
Stromberg: I think it would be useful to set up long-term monitoring of carbon accumulation in soils that have been restored to native grassland. UC Reserves can be good locations for such monitoring and can be used to highlight ecosystem services provided by plant communities.
Koteen: Are there other ways you can point to that we should be thinking about climate change in reference to grassland management than the considerations suggested by this study?
Stromberg: I think carbon credits could be used for purchasing some of the areas of native grassland that remain and preserving them. I think any environmental impact review that is considering restoration of native grassland should have enhanced carbon storage as part of the standard text.
LITERATURE CITED
Adair, E. C. and I. C. Burke. 2010. Plant phenology and life span influence soil pool dynamics: Bromus tectorum invasion of perennial C3–C4 grass communities. Plant and Soil 335(1–2):255–269.
Battles, J. J., P. Gonzalez, T. Robards, B. M. Collins, and D. S. Saah. 2014. California Forest and Rangeland Greenhouse Gas Inventory Development, CA Air Resources Board Agreement 10–778. California Air Resources Board, Sacramento, CA.
Chou, W. W., W. L. Silver, R. D. Jackson, A. W. Thompson, and B. Allen-Diaz. 2008. The sensitivity of annual grassland carbon cycling to the quantity and timing of rainfall. Global Change Biology 14(6):1382–1394.
Christian, J. M. and S. D. Wilson. 1999. Long-term ecosystem impacts of an introduced grass in the northern Great Plains. Ecology 80(7):2397–2407.
Coleman, H. M. and J. M. Levine. 2007. Mechanisms underlying the impacts of exotic annual grasses in a coastal California meadow. Biological Invasions 9(1):65–71.
Corbin, J. D. and C. M. D’Antonio. 2004. Competition between native perennial and exotic annual grasses: Implications for an historical invasion. Ecology 85(5):1273–1283.
Corbin, J. D. and C. M. D’Antonio. 2010. Not novel, just better: Competition between native and non-native plants in California grasslands that share species traits. Plant Ecology 209(1):71–81.
Corbin, J. D., M. A. Thomsen, T. E. Dawson, and C. M. D’Antonio. 2005. Summer water use by California coastal prairie grasses: Fog, drought, and community composition. Oecologia 145(4):511–521.
DeLonge, M. S., R. Ryals, and W. L. Silver. 2013. A lifecycle model to evaluate carbon sequestration potential and greenhouse gas dynamics of managed grasslands. Ecosystems 16:962–979.
DiVittorio, C. T., J. D. Corbin, and C. M. D’Antonio. 2007. Spatial and temporal patterns of seed dispersal: An important determinant of grassland invasion. Ecological Applications 17(2):311–316.
Dukes, J. S., N. R. Chiariello, E. E. Cleland, L. A Moore, M. R. Shaw, S. Thayer, T. Tobeck, H. A. Mooney, and C. B. Field. 2005. Responses of grassland production to single and multiple global environmental changes. PLOS Biology 3(10):1829–1837.
Dyer, A. R. and K. J. Rice. 1997. Intraspecific and diffuse competition: The response of Nassella pulchra in a California grassland. Ecological Applications 7(2):484–492.
Dyer, A. R. and K. J. Rice. 1999. Effects of competition on resource availability and growth of a California bunchgrass. Ecology 80(8):2697–2710.
Ehrenfeld, J. G. 2003. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6(6):503–523.
EPA. 2009. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2007. US EPA, Washington, DC.
Evans, N., A. Baierl, M. A. Semenov, P. Gladders, and B. D. L. Fitt. 2008. Range and severity of a plant disease increased by global warming. Journal of the Royal Society Interface 5(22):525–531.
Houghton, R. A. 1999. The annual net flux of carbon to the atmosphere from changes in land use 1850–1990. Tellus Series B: Chemical and Physical Meteorology 51(2):298–313.
House, J., V. Brovkin, R. Betts, R. Costanza, M. A. Silva Dias, E. Holland, C. Le Quéré, N. K. Phat, U. Riebesell, M. Scholes et al. 2006. Climate and air quality. In P. Kabat and S. Nishioka (eds), Millennium Ecosystem Assessment 2006 – Current State and Trends. Findings of the Condition and Trends Working Group (Ecosystems and Human Well-Being) 1. Island Press, Washington, DC. 355–390.
Hudiburg, T., B. Law, D. P. Turner, J. Campbell, D. Donato, and M. Duane. 2009. Carbon dynamics of Oregon and Northern California forests and potential land-based carbon storage. Ecological Applications 19(1):163–180.
Hudson, B. D. 1994. Soil organic matter and available water capacity. Journal of Soil and Water Conservation 49(2):189–194.
Jackson, L. E. and J. Roy. 1986. Growth patterns of Mediterranean annual and perennial grasses under simulated rainfall regimes of southern France and California. Acta Oecologica 7(2):191–212.
Jackson, L. E., R. B. Strauss, M. K. Firestone, and J. W. Bartolome. 1988. Plant and soil nitrogen dynamics in California annual grassland. Plant and Soil 110(1):9–17.
Koteen, L. 2002. Climate change, whitebark pine, and grizzly bears in the Greater Yellowstone Ecosystem. In S. H. Schneider and T. L. Root (eds), Wildlife Responses to Climate Change: North American Case Studies. Island Press, Washington, DC. 343–414.
Koteen, L. E., D. D. Baldocchi, and J. Harte. 2011. Invasion of non-native grasses causes a drop in soil carbon storage in California grasslands. Environmental Research Letters 6(4):044001.
Laude, H. M. 1953. The nature of summer dormancy in perennial grasses. Botanical Gazette 114(3):284–292.
Lauenroth, W. K., A. A. Wade, M. A. Williamson, B. E. Rose, S. Kumar, and D. P. Cariveau. 2006. Uncertainty in calculations of net primary production for grasslands. Ecosystems 9(5):843–851.
Liao, C. Z., R. H. Peng, Y. Luo, X. H. Zhou, X. W. Wu, C. M. Fang, J. K. Chen, and B. Li. 2008. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytologist 177(3):706–714.
Ma, S., D. D. Baldocchi, L. Xu, and T. Hehn. 2007. Interannual variability in carbon dioxide exchange of an oak / grass savanna and open grassland in California. Agricultural and Forest Meteorology 147(3–4):157–171.
McClaugherty, C. A., J. Pastor, J. D. Aber, and J. M. Melillo. 1985. Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66(1):266–275.
Pumpanen, J., H. Ilvesniemi, and P. Hari. 2003. A process-based model for predicting soil carbon dioxide efflux and concentration. Soil Science Society of America Journal 67(2):402–413.
Roy, B. A., S. Gusewell, and J. Harte. 2004. Response of plant pathogens and herbivores to a warming experiment. Ecology 85(9):2570–2581.
Ryals, R., M. Kaiser, M. S. Torn, A. A. Berheb, and W. L. Silvera. 2014. Impacts of organic matter amendments on carbon and nitrogen dynamics in grassland soils. Soil Biology and Biochemistry 68:52–61.
Ryan, M. G., J. M. Melillo, and A. Ricca. 1990. A comparison of methods for determining proximate carbon fractions of forest litter. Canadian Journal of Forest Research 20(2):166–171.
Scurlock, J. M. O., K. Johnson, and R. J. Olson. 2002. Estimating net primary productivity from grassland biomass dynamics measurements. Global Change Biology 8(8):736–753.
Schlesinger, W. H. 1997. Biogeochemistry, 2nd ed. Academic Press, San Diego, CA. 588 pp.
Seabloom, E. W., E. T. Borer, V. L. Boucher, R. S. Burton, K. L. Cottingham, L. Goldwasser, W. K. Gram, B. E. Kendall, and F. Micheli. 2003a. Competition, seed limitation, disturbance, and reestablishment of California native annual forbs. Ecological Applications 13(3):575–592.
Seabloom, E. W., W. S. Harpole, O. J. Reichman, and D. Tilman. 2003b. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proceedings of the National Academy of Sciences of the United States of America 100(23):13384–13389.
Soil Conservation Service. 1985. Soil Survey of Marin County, California. USDA, Sacramento, CA. 229 pp.
Stromberg, M. R. and J. R. Griffin. 1996. Long-term patterns in coastal California grasslands in relation to cultivation, gophers, and grazing. Ecological Applications 6(4):1189–1211.
Stromberg, M. R., P. Kephart, and V. Yadon. 2001. Composition, invasibility, and diversity in coastal California grasslands. Madrono 48(4):236–252.
Stromberg, M. R., C. M. D’Antonio, T. P. Young, J. Wirka, and P. R. Kephart. 2007. California grassland restoration. In M. R. Stromberg, J. D. Corbin, and C. M. D’Antonio (eds), California Grasslands: Ecology and Management. University of California Press, Berkeley, CA. 254–280.
Suttle, K. B. and M. A. Thomsen. 2007. Climate change and grassland restoration in California: Lessons from six years of rainfall manipulation in a north coast grassland. Madrono 54(3):225–233.
Verbeeck, H., R. Samson, F. Verdonck, and R. Lemeur. 2006. Parameter sensitivity and uncertainty of the forest carbon flux model FORUG: A Monte Carlo analysis. Tree Physiology 26(6):807–817.
Westerling, A. and B. Bryant. 2006. Climate Change and Wildfire in and Around California: Fire Modeling and Loss Modeling. California Climate Change Center, Sacramento, CA. 28 pp.
Wieder, R. K. and G. E. Lang. 1982. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–1642.
Wilsey, B. J. and H. W. Polley. 2006. Aboveground productivity and root-shoot allocation differ between native and introduced grass species. Oecologia 150(2):300–309.
Zhang, L., B. K. Wylie, L. Ji, T. G. Gilmanov, and L. L. Tieszen. 2010. Climate-driven interannual variability in net ecosystem exchange in the Northern Great Plains grasslands. Rangeland Ecology & Management 63(1):40–50.