CHAPTER 11
Evolutionary Conservation under Climate Change

Jason P. Sexton and Alden B. Griffith
Abstract. Faced with rapid climatic change, we can lower extinction probabilities and maximize the potential for species to track rapid climate change by facilitating evolutionary adaptation. Evolutionary-management alternatives range from intensive interventions in extreme cases (e.g., rescuing a species by breeding it with another species) to more subtle alternatives (e.g., conserving land to act as gene-flow corridors among populations). Resource managers may improve conservation results by applying evolutionary theory in their assessments of climate change risk, taking steps to maximize the adaptive potential of species of concern, and intervening when necessary, followed by careful monitoring of intervention outcomes. We discuss a variety of “evolutionary risk assessment” approaches, including identifying barriers to biological adaptation and evaluating the adaptive potential of populations and species, and make recommendations for operating within an evolutionarily minded framework in conservation biology.
Key Points
• Evolutionary adaptation is an important way living things cope with new or changing environments and is one means by which some species may avoid extinction under rapid climate change.
• Genetic variation among individuals is the means or currency by which evolutionary adaptation occurs and it can be measured, but it can also be in limited supply.
• It is critical that resource managers and conservationists maximize the adaptive capacity of species by incorporating evolutionarily minded approaches into their plans and actions.
• There is a wide variety of actions to help maximize evolutionary potential, including estimating genetic variation within populations, protecting gene-flow corridors, including assisted breeding or assisted-immigration actions, identifying and removing human-made gene-flow barriers, and limiting actions that reduce population size and genetic variation.
INTRODUCTION
How humans can positively influence evolutionary processes to maintain diverse ecosystems is a relatively new scientific and management topic, yet it is a vital one. Evolution has produced the tremendous biodiversity that we now see rapidly disappearing through human-caused global change. Although humans cannot control evolution on a grand scale, we can take actions to protect and promote the evolutionary processes that create and maintain biodiversity; that is to say, we can manage to maximize adaptive genetic variation, which is the backbone of biological adaptation, and ultimately, biodiversity.
We know that species are already responding to the effects of human-caused climate change in several ways: Changing phenologies, shifting geographic ranges, and changing abundances (Root et al. 2003, Parmesan 2006, Anderegg and Root, this volume). Many of these shifts will be necessary for species to adjust to a rapidly changing climate, but may not always be fully realized due to constraints imposed by habitat availability and biological limits to migration and dispersal (Geber and Dawson 1993, Sexton et al. 2009). For those species that cannot move to suitable habitat, and have reached their limits of environmental tolerance, adaptation via evolutionary processes (see Rice and Emery 2003), or extinction, is the only option in the absence of human intervention (Ackerly 2003).
Rapid evolution to changing conditions, including human-induced conditions, is not a new concept (Hendry et al. 2010). In the 19th century, Charles Darwin observed rapid evolution in moths in response to industrial air pollution. Agriculturalists use breeding and genetic technologies to increase yields under various growth environments by changing the traits of domesticated plants and animals. For example, modern-day corn is the product of human-induced evolution from thousands of years of domestication, with rapid evolution occurring during the last half-century through intensive breeding and hybridization research (Moeller and Wang 2008). The theoretical and quantitative underpinnings of evolutionary processes are strong and often well understood (more so than many important ecological processes) due to the rich history of genetics research and recent advances (e.g., genomics). Nevertheless, many questions remain about which naturally occurring species can adapt quickly enough to the environmental changes imposed by climate change (Gienapp et al. 2008).
In general, a species tolerates multiple environments within its geographic range through population-based local adaptation, broad environmental tolerance, or both (Bradshaw 1965). Thus, if a species has an overall negative response to increasing temperatures, for example, the fate of that species may lie within the adaptive capacity of individual populations that are more tolerant of increased temperatures than other populations. If these populations are well connected, gene flow can assist populations in adapting to the new temperature regime throughout the species range (Geber and Dawson 1993, Aitken and Whitlock 2013).
The idea that evolutionary and ecological events can occur on similar timescales has gradually gained traction (Hendry et al. 2011, Sgrò et al. 2011). Adaptations to climate on a yearly basis have been observed through long-term studies (Grant and Grant 2002), and the past few decades have revealed a slew of studies illustrating rapid responses (Kinnison and Hairston 2007). Additionally, many cases of rapid evolution are observed from studying the evolutionary ecology of invasive species (Box 11.1), and these cases illustrate key factors that enable rapid evolution during climate change, namely high levels of adaptive genetic variation (Skelly et al. 2007). However, although much can and has been learned from the adaptive potential of invasive species (and the role of humans in influencing adaptive potentials), there are many unknowns about how to move forward to protect native species from climate change and how to effectively and ethically maximize adaptive potential. Resource managers can facilitate adaptive processes (Ashley et al. 2003, Sgrò et al. 2011), indirectly or extrinsically (see Klausmeyer and Shaw 2009), by maximizing the potential for species to adapt (e.g., by protecting or providing restored habitats that are likely to be viable under future climate condition) or, directly, through efforts to improve the intrinsic adaptive potential of species, whether by intentional breeding or by movement of individuals to increase genetic variation to respond to natural selection. In both cases, species can and should be viewed as potential sets of populations that can respond differently to different climatic conditions, and not singly, as entities that either fit or do not fit within particular climate envelopes (see Holmstrom et al. 2010, for an example of the consequences of not viewing species as a set of distinct populations). Ultimately, it is the fitness of individuals in particular environments that will affect population growth rates and the success of a given species across its range (Kinnison and Hairston 2007). Evolutionarily minded management can increase the likelihood that populations are able to evolve and adapt to rapidly changing environments, and can minimize the risk of unintended consequences (e.g., Holmstrom et al. 2010).
In this chapter, we review the basic information necessary for resource managers and conservation biologists to apply evolutionary theory and principles to rapid climate change and other human-induced disturbances. We focus here on single-species or single-taxon conservation responses, but evolutionary management can and should be used in community and ecosystem contexts as well (see Mace and Purvis 2008). Evolutionary management that forestalls extinctions under climate change and habitat degradation is a new field of research combining conservation genetics and evolutionary biology. We review the following: (1) Constraints on and barriers to evolutionary adaptation to climate change, (2) how to evaluate the adaptive potential of populations and species of concern, (3) possible actions to maximize adaptation, and (4) management implications and tools. Climate change involves complex changes to a variety of environmental variables (e.g., temperature, precipitation, atmospheric carbon dioxide concentrations). For simplicity, we will discuss adaptive examples in the context of warming climates, but it is important to recognize that changes in response to different climate variables in different ecosystems will have diverse evolutionary consequences.
BOX 11.1 • Rapid Evolution and Adaptive Lessons from Invasive Species
Invasive species give us clues as to how management can maximize evolutionary responses in native species (e.g. Griffith et al. 1989). The key is genetic variation, which can be enhanced through maintaining large populations, maintaining adaptive gene flow among populations, and perhaps through interpopulation (and interspecies) breeding to produce new, adaptive genetic combinations (Box Figure 11.1.1). Rapidly changing environments are creating new opportunities for biological invasions (including diseases) (Harvell et al. 2002). Whereas some biological invasions have colonized multiple environments through broad phenotypic plasticity (e.g., Williams et al. 1995), others have benefited from population-based differences, multiple introductions, and rapid evolution. Nowhere is the adaptive potential of biological invasions more apparent than when we attempt to control or eradicate them (Carroll 2011). Many organisms have evolved resistance to chemical control (e.g., herbicides and vaccines), and resistance often evolves in less than 20 years (Palumbi 2001).
Genetic variation available for rapid evolution has been documented in many invasive species (Reznick and Ghalambor 2001, Maron et al. 2004). For example, Sexton et al. (2002) found potentially adaptive differences in root investment and plasticity between invaded northern and southern populations of saltcedar (Tamarix spp.) in the United States, and there appears to be an adaptive cline in wood tissue resistance to frost damage (Friedman et al. 2008). Saltcedar has had multiple introductions, and its high genetic variation has been enhanced through hybridization between at least two species in its invaded range (Gaskin and Schaal 2002). Leger and Rice (2007) found that invasive populations of California poppies (Eschscholzia californica) in Chile have evolved trait differences along climate gradients in a similar fashion to native California populations since their introduction 150 years ago. The fly, Drosophila subobscura, has evolved a strikingly similar cline in wing size within 20 years in introduced populations in North America—but in different parts of the wing—to clines found in the Old World (Huey et al. 2000). Additionally, several invasions have experienced increased genetic variability within their introduced ranges (likely due to multiple introductions and genetic reshuffling), which may have strong implications for adaptive potential (Dlugosch and Parker 2008). A worst-case evolutionary scenario under climate change would involve decreasing adaptive potentials in native species and increasing them in biological invasions.
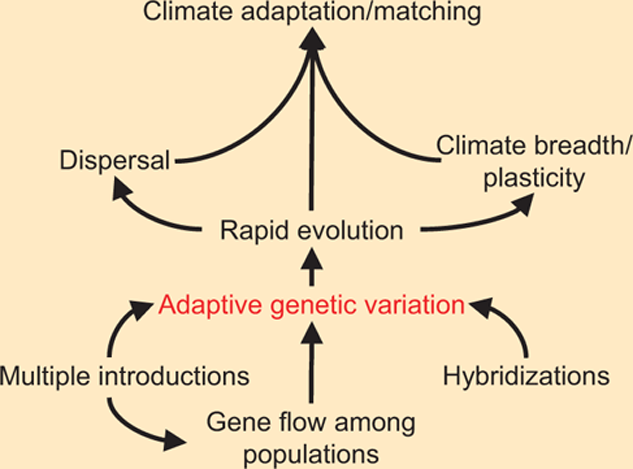
BOX FIGURE 11.1.1: Illustration of factors that can increase adaptive genetic variation, which can then increase climate adaptation through rapid evolution, increased dispersal ability, and increased climate tolerance (breadth or plasticity).
Constraints to Evolutionary Adaptations to Climate Change
Demographic constraints to evolution arise mostly from small population sizes among other risk factors (Holt 2003) (see Box 11.2). Small populations tend to be more limited in their genetic variation and thus have fewer genetic resources upon which natural selection may act (e.g., Kelly et al. 2011). For small populations to persist in the face of climate change, they may need to either be moved to appropriate climates or be rescued by other populations through the process of migration or gene flow (Sexton et al. 2011, Aitken and whitlock 2013).
The adaptive potential to respond to climate change may be present within the species as a whole, but it may be distributed across many populations that vary in isolation from one another. Ironically, this isolation among populations may have historically allowed the genetic variation in climate-related traits to sort and build over the species’ history. For example, a species with populations distributed across a wide range of environments may have adaptations to cold tolerance at one end of its geographic range, and adaptations to heat tolerance at the other. If individuals from different environments were to breed, the species as a whole might utilize a greater amount of their adaptive potential in response to climate. Yet there is also the danger of increasing the genetic load (i.e., maladaptation) when mixing populations (Aitken and Whitlock 2013). Therefore, increasing adaptive potential by using populations better adapted to predicted future environments (discussed below) is ideal. Thinking holistically in this vein about how species’ populations are distributed along climatic gradients can help in designing adaptive, climate change strategies.
Even when the genetic variation required to adapt rapidly is present within a population, the arrangement of that variation may preclude rapid evolution. The trait combinations we see in individuals (e.g., delayed flowering and slow leaf development) can often be considered as “packages” that have proven successful and been selected for over time in certain environments. Owing to genetic correlations among traits, it may be very difficult to produce individuals with the most favorable combinations of trait values in time to track climate evolutionarily (Davis et al. 2005). Etterson and Shaw (2001) found that populations of the prairie annual plant Chamaecrista fasciculata had the genetic variation to produce individuals capable of tracking potential future climates, but that correlations among important traits could forestall this process. For example, a positive correlation between leaf number and leaf thickness suggests that natural selection cannot easily respond to higher temperatures, which favor individuals with both more and thinner leaves.
Mating system (asexual, selfing, mixed mating, etc.) and life history (short-lived vs. long-lived) may affect a population’s adaptive potential over short timescales. The type of mating system is generally fixed within a species, but it can also vary so that some populations differ from one another. When individuals only rarely interbreed, it can take longer for adaptations to arise because recombination is occurring less widely, and thus producing novel combinations at a slower rate. Hence, less outcrossing populations or species may have slower evolutionary rates of response to selection and may require higher levels of human intervention (e.g., assisted breeding) to adapt. Similarly, organisms that reproduce less often and in fewer numbers may have fewer opportunities to produce large numbers of genetic variants that may be successful in novel or rapidly changing environments.
Evolution may also be constrained if behavioral responses become maladaptive due to a decoupling of perceived cues and actual environmental conditions / fitness. Climate change and other human-induced environmental impacts are likely increasing the prevalence of such “evolutionary traps,” which may limit or alter the evolutionary potential of certain species (Robertson et al. 2013).
The variety of constraints to evolutionary processes does not necessarily represent constraints for management actions (Lankau et al. 2011). On the contrary, recognizing those constraints may often be the impetus for action. Knowledge of how adaptive potential is con-strained increases the chance for successful management and reduces the risk of unintended consequences. The examples of evolutionary constraints discussed above—small population sizes, distribution of genetic variation, trait correlations, mating system, life history, behavioral responses—all inform our ability to incorporate evolutionary processes in management and serve as guides for applied research.
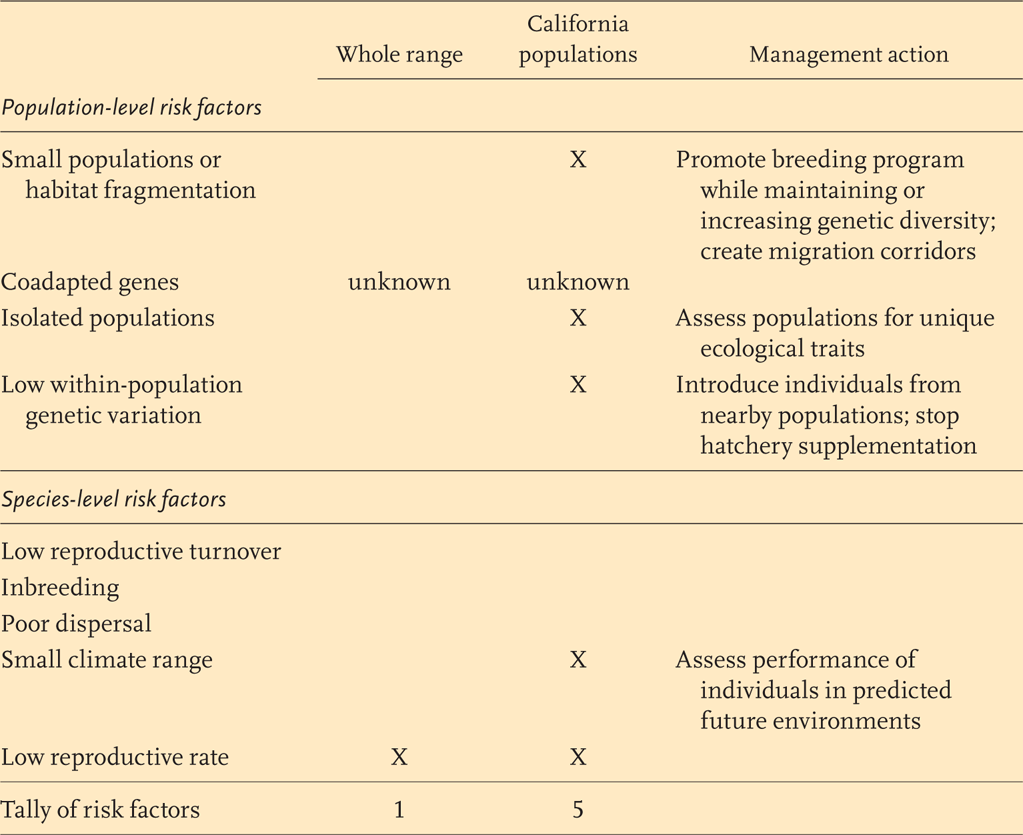
BOX 11.2 • Assessing Risk Factors to Climate Change Adaptation
Many factors can reduce or bring “risk” to the process of climate adaptation. Box Table 11.2.1 lists some population- and species-level factors that can forestall climate adaptation and uses California populations of Chinook salmon (Oncorhynchus tshawytscha) as an example. Presence of a risk factor (represented by “X”) is determined from an assessment of average population estimates based on biological opinion or from available data. As a species, Chinook salmon may have few evolutionary risk factors and may be able to adapt to climate change with monitoring and intervention. On the other hand, California populations of Chinook salmon are a small subset of Chinook populations and occur at the southern range boundary of the species range. Rapid adaptation in California populations may not be possible without genetic rescue or other management actions. We note that genetic rescue is a complex issue (Tallmon et al. 2004) and is not equivalent to hatchery supplementation, which may actually decrease genetic diversity and reproductive potential of supplemented populations (Dylan 2008).
Such coarse tools can be used to begin a dialogue with multiple stakeholders and can lead to more sophisticated models predicting population responses. This cursory assessment assumes that the species must adapt in place and cannot migrate to suitable habitats in future climates. Such tools can be catered to specific systems to incorporate a wide range of factors, including ecological, geographic, and human impacts.
BOX TABLE 11.2.1
Risk factors for the Chinook salmon (Oncorhynchus tshawytscha) at both the population and species level.
Evaluating Future Responses and Adaptive Potential
The vulnerability to, and hence consequences of, climate change will differ among species. Given life history information, one can assess the feasibility of migration to, and the likely quality of, potentially appropriate habitats. For example, as climatic conditions change over time, the most favorable climate for a population or species could occur within human-developed areas, which otherwise could be inhospitable to a species. When these preferred conditions are projected to occur in future locations that are too far away for individuals to migrate to (or too degraded to be habitable), the potential for climate tolerance and adaptation in current locations should be estimated. This process begins with identifying which traits and trait values will be most critical as the climate changes, and then determining adaptive potentials of these traits in various populations.
The adaptive potential to respond to climate change must be evaluated on two levels: Population-level factors (e.g., demographic and genetic constraints discussed above, connectedness, etc.) and species-level factors (e.g., life history characteristics such as mating system and generation time). These are not mutually exclusive. Many factors vary by species and population (e.g., morphological traits, type of mating system) and we suggest that taxa be evaluated from both of the following viewpoints: What is the potential of a species to adapt to climate change given its populations’ characteristics? What is the potential of a population to evolve rapidly given its species-level life history?
However, how does one actually evaluate the adaptive potential of a species or population? Adaptation is the product of natural selection acting on heritable genetic variation. Therefore, in order to fully evaluate adaptive potential, we must determine which traits are important under climate change, measure the amount of genetic variation distributed within and among populations, and estimate the strength of selection due to climate change (how much of an advantage to survival and reproduction certain trait values convey) while recognizing the constraints and barriers discussed above. Although this can be time-consuming, we hope to demonstrate that even a partial or basic knowledge of a species’ or population’s adaptive potential can be very informative from a management standpoint. Additionally, information from each of the above steps can help guide or necessitate investigation of other steps.
Identifying Climate-Related Trait Variation
Variation in climate-related traits is often best explored across environmental gradients. Ecotypes, and adaptations to associated climates, have long been demonstrated in plants (Turesson 1925, Clausen et al. 1940) and animals (Mayr 1947), and the process of ecotypic differentiation can have major effects on life history evolution (Rezende et al. 2004, Bradshaw and Holzapfel 2007). Climate-related trait variation across environmental gradients is often queued by photoperiod differences related to latitude (Bradshaw and Holzapfel 2007). For this reason, gene flow from warmer latitudes may not realize immediate benefits in rapidly warming climates if the newly derived population is maladapted to the location’s photoperiod (Bradshaw and Holzapfel 2006, Visser 2008). Therefore, one should strike a balance between combining climate-related genes in new, adaptive ways and minimizing a maladaptive genetic load.
Flexibility is a big advantage in rapidly changing climates, and individuals that can successfully inhabit a wide range of habitats will be favored. For simplicity, we refer to individuals possessing broad environmental tolerance or high phenotypic plasticity (Bradshaw 1965, Ghalambor et al. 2007) as “generalists.” Generalists are favored in environments that shift from year to year (Levins 1968). Thus, some populations of the same species may have more generalist characteristics than others, and there can be a clear genetic basis to these differences (Via et al. 1995, Sultan 2001). In fact, generalist performance is not fixed, but can evolve as a trait itself, and genetic variation for plasticity among important climate traits can and should be evaluated when possible (Via et al. 1995), especially with respect to its role in climate change responses (Hendry et al. 2008). Richards et al. (2006) discuss phenotypic plasticity in an invasion biology context and suggest ways to evaluate its role in colonizing new environments. A relatively quick assay for generalist genotypes is to raise individuals in two environments that approach both ends of a species’ range for a particular environmental variable (e.g., temperature, precipitation). Generalist genotypes will be those that perform relatively well in both environments.
Similar to the advantages conferred by a generalist genotype, an organism’s dispersal ability, which can involve many heritable traits, can be of high importance in variable or shifting environments (Johnson and Gaines 1990). Darling et al. (2008) observed greater dispersal ability—via larger-winged fruit—in peripheral populations of coastal dune sand verbena (Abronia umbellata) in California and Oregon. They posit that since range margins can shift back and forth from year to year, heightened dispersal is favored in these populations. The legs of cane toad (Bufo marinus) individuals at the invasive front of Queensland populations were found to be longer, giving them greater dispersal ability (Phillips et al. 2006). Dispersal ability can also be important in the context of disturbances such as fire and dieback from disease, which are anticipated to increase due to climate change.
Estimating Adaptive Potential
There are two main ways to assess the genetic variation of a population relevant to climate change. One approach is to quantify and monitor the amount of overall genetic variation within or among populations using variation in DNA, without respect to any particular trait (this is often referred to as “neutral genetic variation”) (see Schwartz et al. 2007). This approach has a long history in conservation biology and roughly represents a bet-hedging strategy. In essence, it is assumed that the more overall genetic variability within a population, the more likely the population will avoid problems associated with detrimental genes and inbreeding depression, and the more likely it will be able to respond to natural selection. Additionally, if some populations are known to contain high levels of overall genetic diversity, they may represent good candidates for sources of genetic variation in managed breeding programs. Grivet et al. (2008) present a good example of this approach, where molecular variation is coupled with spatial data to identify potentially important regions (i.e., highly diverse areas with little protection) of the species range of California valley oak. Table 11.1 lists case studies where molecular data have been used to infer population processes that can help inform conservation decisions. Genetic-marker data may also be used to estimate the connectedness and conservation status of natural populations in the face of global change (Schwartz et al. 2007, Lawrence et al. 2008). Although neutral genetic divergence (based on molecular techniques) does tend to positively correlate with variation in actual phenotypic traits, it is also important to note that for many species, the relationship is weak or absent and thus may grant little predictive power (McKay and Latta 2002, Leinonen et al. 2008). Nevertheless, genetic diversity of molecular markers is often used to assess the importance of populations for economic value (crop plants, etc.), and Jump et al. (2009) argue that this “option value” should also be applied for its potential conservation value in wild species.
A second approach, and a more direct one, is to examine genetically based variability in important climate-related phenotypic traits (i.e., physical traits like body size, time to development, and leaf shape). In order to maximize adaptive potential in the successful management of populations, there must be sufficient genetic variation in traits of interest. Controlled conditions (e.g., common garden experiments in plants and laboratory trials in animals), in which individuals from different locations are grown together within similar conditions, have long been used to minimize environmentally caused variation in phenotypes to expose underlying heritable variation. [See Kellermann et al. 2009 for an example of estimating genetic variation in climate-related traits among many species of fruit flies (Drosophila spp.).] Genetic variation can also be measured in the field for organisms not suited to labs or gardens (see Conner and Hartl 2004). If these experiments are conducted in more than one environment, they can also help determine the degree of phenotypic plasticity and reveal generalist genotypes. This combined approach assesses both genetic variation and the potential need for management in the first place; that is to say, perhaps high plasticity will be sufficient to tolerate environmental change for some species. Nevertheless, a recent experiment revealed population responses to climate change are lagging in the mouse-ear cress, Arabidopsis thaliana (Wilczek et al. 2014).
Beyond estimates of genetic variability, it is important to understand how this variability responds to selection. There are two main techniques for estimating evolutionary responses (or response rate) of populations to predicted future environments: (1) Retrospective temporal analyses, where changes in populations are correlated with observed changes in the environment, and (2) selection experiments, where experimental populations are exposed to changing or target conditions for several generations and the resulting changes to the population are measured (see Box 1 in Reusch and Wood 2007). Recent concern over the evolutionary consequences of climate change has driven many such studies. For example, evolved differences in reproductive timing were detected in field mustard (Brassica rapa) grown from seeds collected before and after a five-year drought in southern California (Franks et al. 2007). Similarly, recent adaptive genetic shifts in the timing of reproduction and development in response to rapid climate change have been detected in animals, including insects, birds, and mammals (Bradshaw and Holzapfel 2006). To maximize predictive power of population-response estimates, the future environment should be estimated and many individuals should be sampled, observed, or manipulated for several generations. Nevertheless, much can also be learned from single-generation observations of natural selection in rapidly changing environments. A simple understanding of a species’ life history (e.g., generation times, self-fertilization rates) or the results from studies on analogous organisms may inform the likelihood of a response to selection.
TABLE 11.1
Case studies where molecular marker data have been used to infer population processes relevant to climate change
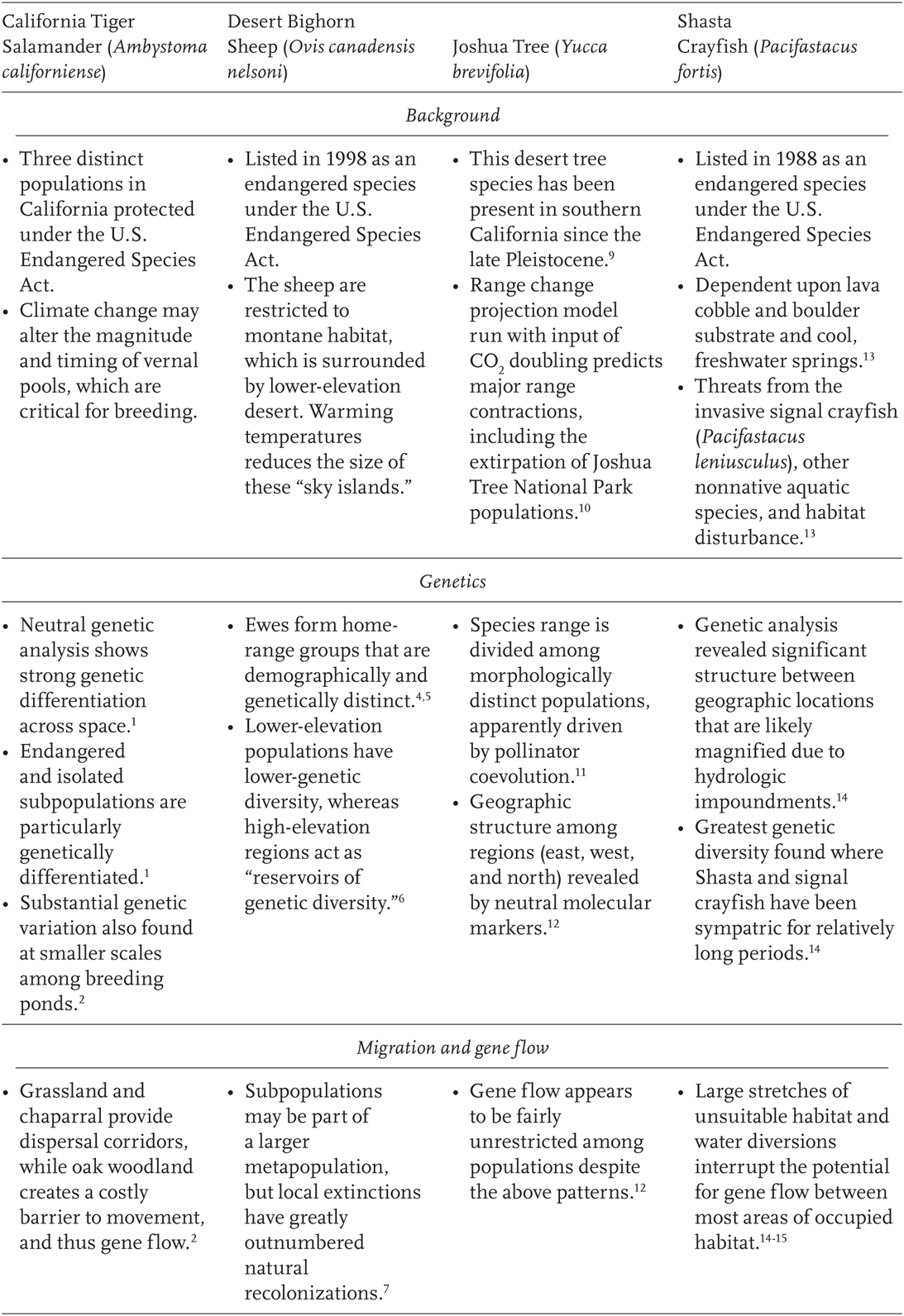
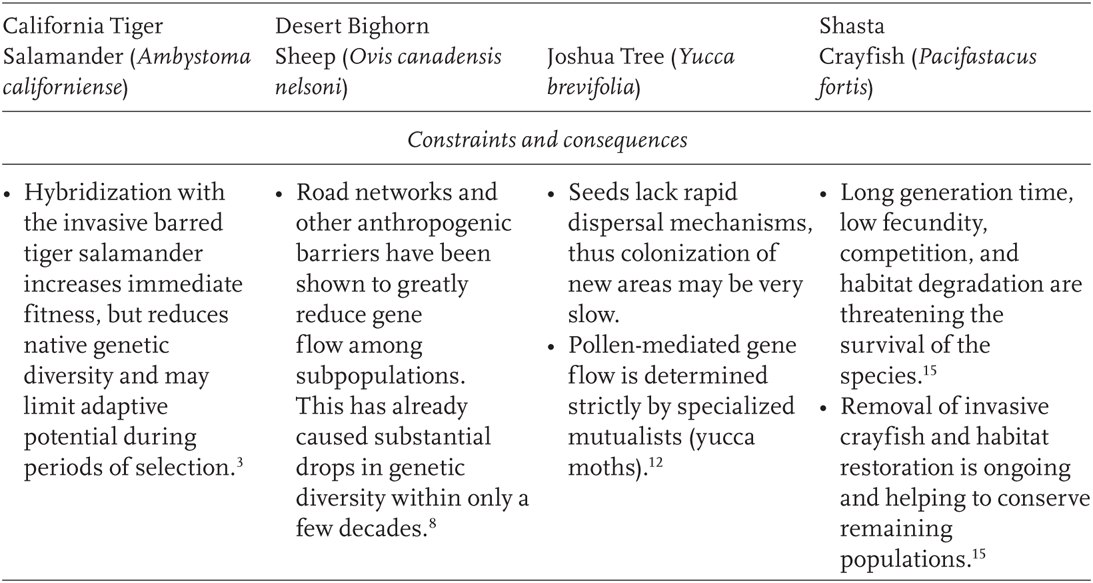
1Shaffer et al. 2004; 2Wang et al. 2009; 3Fitzpatrick and Shaffer 2007; 4Rubin et al. 1998; 5Boyce et al. 1999; 6Epps et al. 2006; 7Epps et al. 2004; 8Epps et al. 2005; 9Holmgren and Betancourt 2008; 10Cole et al. 2011 ; 11Godsoe et al. 2008; 12Smith et al. 2008; 13USFWS 1998; 14Petersen et al., unpublished data; 15Spring Rivers 2009.
Assessing gene flow potential across climatic gradients is useful for understanding adaptive potential, especially if there are populations assumed to be favored in future climates. Historical and contemporary gene flow can be estimated through the use of molecular genetic markers (see examples in Excoffier and Heckel 2006). Alternatively, estimates of gene flow potential can be made with basic observations of seasonal timing of reproduction coupled with life history information. For example, populations that are well connected spatially, overlap in reproductive timing, and can disperse easily have potentially high gene flow.
Utilizing Evolutionary Potential—Goals and Potential Actions
Adaptive potential often means the availability of adaptive genetic variation within a species. In the worst-case scenario, we are forced to attempt to manipulate evolutionary outcomes to save a species. In the best-case scenario, we are preserving evolutionary processes and allowing for adaptive change to occur on its own (Latta 2008). In this section, we progress from evolutionarily minded practices that vary between low and high levels of intervention. These measures vary from regional, ecosystem-level responses to population- and individual-level actions. To maximize chances for success, researchers should initiate a working relationship with resource managers, and managers should seek input from researchers (Richardson et al. 2009).
Prioritizing Populations
Management plans focused on preserving evolutionary processes would target large, genetically diverse populations as adaptive sources, but would also consider habitat quality and availability (Lande 1988). These may be useful in safeguarding unique populations at risk of going extinct, such as populations that have been historically isolated. Populations showing evidence of having unique properties (either morphological or from molecular markers) may require prioritization and special protections (Lesica and Allendorf 1995, Crandall et al. 2000). These should also include portions of the species range that best represent future climates, such as relatively warm areas (lower latitudes and elevations). Warm-adapted populations may have useful genetic attributes for adapting to future climates, but they may also be the first to be extirpated (as they may already be living near their physiological limits) depending on the ability of individuals to migrate (Figure 11.1; but see Crimmins et al. 2011 for how distributions along gradients may shift counterintuitively in response to climate change). Thus, it may be important to conserve seeds, eggs, or individuals from ecologically extreme areas of the range to ensure their availability (see Havens et al. 2006 for seed-banking examples). Maintaining natural habitat linkages between neighboring populations could also be prioritized, especially across ecological and geophysical gradients and transitions that support and have promoted diversification (Anderson and Ferree 2010). If contemporary and historical genetic and ecological data are available, populations can be defined in a more holistic context (see Crandall et al. 2000). Overall, it is likely that trade-offs between preserving unique biological units (e.g., populations) and maximizing climate adaptive potential will exist. See Carroll (2011) for a perspective on incorporating eco-evolutionary management in rapidly changing ecosystems.
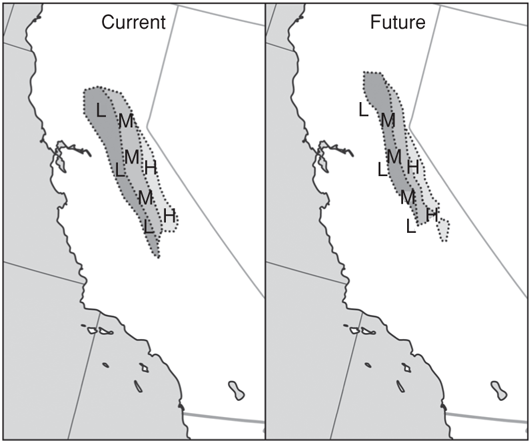
FIGURE 11.1: Hypothetical example of how climate zones with locally adapted genotypes may shift in the future. In the current scenario, there are three climate belts with three adapted genotypes (occupying differing elevations of the Sierra Nevada mountains), such that individuals with the “L” (low-elevation) genotype are favored in the warmest climate zone and individuals with the “H” (high-elevation) genotype are favored in the coolest zone. Without migration, genotypes and climate zones are no longer matched in the future scenario. L can withstand the warmest temperatures and could lend useful genes to “M” (middle-elevation), but many of the L populations might go extinct because they are beyond their physiological limits in future scenarios. New areas are potentially suitable for the species in future climates, but colonization to these areas may be slower than rapid climate change permits. Some new climate zones are effectively islands that cannot be easily reached through natural migration.
Assisting Gene Flow and Migration
During climate shifts, selection pressures will result in local differences in individual survival and reproduction, but these same pressures can also cause a replacement of local individuals with migrant ones (Davis et al. 2005). This process is not all-or-nothing. Complete replacement of local individuals by immigrating ones is one possible outcome, while the alternative is a mixing of genotypes. The latter case may be more favorable since climate, while important, is not the only driver of biological distributions. Local individuals may perform poorly in a rapidly shifting climate, but they may possess special adaptations key to their existence in a particular place. For example, individuals may be locally adapted to a particular soil type or predation regime. Immigrants may have a favored climate tolerance, but may be unable to perform well with other novel conditions. Hence, new combinations—produced through sexual reproduction—may allow for successful climate matching while retaining local advantages (adaptations) (Kinnison and Hairston 2007). Sgrò et al. (2011) present an example for how one might stock or seed individuals from varying distances with the goal of maximizing adaptive potential under climate change (Figure 11.2).
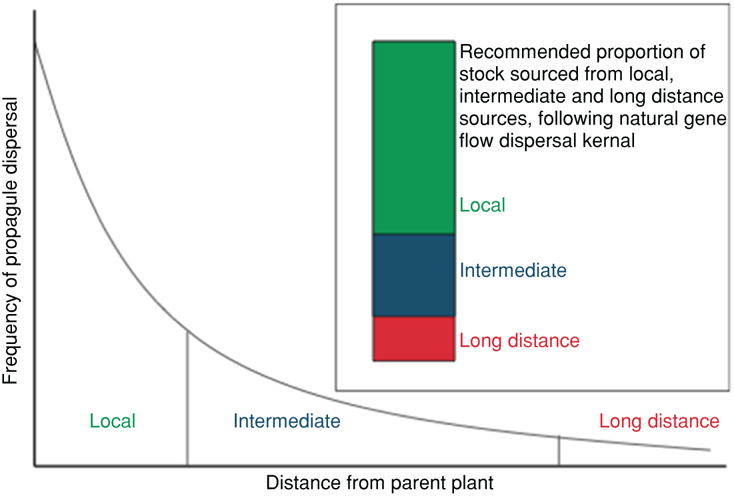
FIGURE 11.2: Potential stocking or seeding scheme to maximize adaptive potential at a given population under environmental or habitat change. The stocking proportions are intended to mimic the natural gene flow proportions a well-connected population might encounter and could be used to restore a population, to bolster depauperate populations, or for managed translocation outside the current range. Source: Figure taken from Sgrò et al. (2011).
Given the highly modified nature of many modern landscapes, management actions that promote dispersal of individuals will often be necessary to help species respond to environmental stress. These actions can be indirect / passive (e.g., removing barriers to movement, creating corridors) or direct / active (e.g., managed relocation). Using corridors to encourage movement is a widely accepted practice in conservation biology, but it is undervalued for its potential to facilitate rapid adaptation within species by increasing gene flow (although see Loss et al. 2011, Groves et al. 2012). The theory is the same: Both individuals and their genes need to be able to freely move to preferred sites. In the context of climate change and evolutionary adaptation, this means maximizing connectivity among populations and across climatic gradients. In this vein, evolutionary processes are being conserved, not just individuals or populations (Crandall et al. 2000, Latta 2008).
When it is clear that there is no gene flow among populations and population declines can be attributed to shifting climates, managed relocation may be considered (see Richardson et al. 2009). We are unaware of programs that have yet used managed relocation to address climate change concerns, but there are examples of matings between populations being used for conservation purposes. Tallmon et al. (2004) highlight several examples where genetic rescue using a small number of immigrants has proven successful in the conservation of small populations (e.g., prairie chicken, Florida panther). Sexton et al. (2011) showed that experimental gene flow between isolated populations occurring along the same warm, low-elevation range limit of the California Sierra Nevada endemic plant, Mimulus laciniatus, increased fitness of individuals at the range limit. In another example, individual tidepool copepod, Tigriopus californicus, populations have limited potential for adaptation to heat stress (Kelly et al. 2011); however, experimental crosses between heat-tolerant populations further increased tolerance to heat stress (Pereira et al. 2014). Thus, gene flow among populations occupying similarly stressful environments may provide benefits in rapidly changing environments while simultaneously reducing genetic load. Highly endangered populations of the mountain pygmy possum (Burramys parvus) in alpine Australia (Mitrovski et al. 2008) have recently produced interbred individuals from introductions from distant mountains, which may improve the target population’s adaptive capacity (A.R. Weeks, pers. comm.). Important questions remain: How many individuals are necessary to assist adaptive evolution and where should they come from? What is the balance between the benefits of novel gene introductions and the cost of altering local adaptive genetic combinations? As discussed above, source populations should consist of individuals with traits conferring an advantage in a new climate regime. Nevertheless, as the rate of evolution depends on many factors (e.g., strength of selection, genetic diversity of locals plus migrants), it is difficult to know how many migrants will be required. When managed gene flow or relocation seems appropriate, we suggest using initially low numbers of individuals followed by long-term monitoring.
Population Restoration
If local populations are extirpated due to climate change, there is little chance that restoring populations using original, local individuals (if they are somehow available from seed storage or captive populations, etc.) will be successful. Climate-matching (as close as possible) candidate populations with the geographic area to be restored is a sensible strategy, as well as introducing a mix of individuals from varying ecological or “adaptive” distances to ensure adequate levels of genetic variation (see Sgrò et al. 2011). Montalvo and Ellstrand (2000) found that greater fitness of the plant Lotus scoparius in restored southern Californian coastal sage scrub populations was most highly correlated with greater genetic and environmental similarity to source populations. However, molecular markers may not be reliable for estimating the suitability of populations for restoration. Knapp and Rice (1998) found that the source environment of populations was the best predictor of successful matching of phenotypic trait values with restoration sites. Managers should seek as many sources of information as possible to inform restoration transplant decisions, but there is no substitute for performance-based information.
Captive Breeding and Species Hybridization
Interventions such as captive breeding can be used to save species or populations, but should only be employed when there is a reasonable chance for success and when there is no other alternative (e.g., captive breeding in the California condor, a species that had reached a population low of 22 individuals). A few important general points are worth considering. Microevolution (genetic changes within populations) is always occurring. Hence, populations will respond evolutionarily (and possibly behaviorally) to the captive environment, often to the detriment of reintroduced wild populations. Frankham (2008) describes how this process can happen and advises that population-based, captivity-program replicates be used and that individuals be kept in captivity for as few generations as possible to avoid the accumulation of harmful genes (deleterious alleles).
Species hybridization is a means of acquiring new adaptations (see Box 11.1 on rapid evolution in invasive species) and is a natural mechanism for speciation in certain groups (Rieseberg 2006), yet it has not to our knowledge been employed as a tool for evolutionary adaptation to climate change, but see Tallmon et al. (2004). While we do not advocate this as a mainstream conservation strategy, it is worth noting that hybridization could be a preferred, last-ditch alternative to the extinction of endemic species that cannot survive rapid climate change (see Fitzpatrick and Shaffer 2007). Hybridization with closely related, climate-tolerant species might be possible for certain species; however, as is true for managed relocation, philosophical and ethical factors must certainly be considered (Richardson et al. 2009).
Incorporating New Management Perspectives and Tools
For which cases do we need to make evolutionary management a priority in response to climate change? Until recently, management actions that explicitly consider evolutionary processes have typically been reserved for extreme cases, such as captive breeding programs or the introduction of individuals from distant populations (Tallmon et al. 2004). For such cases, genetic diversity and / or structure have become critically impaired due to anthropogenic influences, and any attempt to conserve or restore healthy populations depends on our knowledge of evolutionary biology. In the case of anthropogenic climate change, we can anticipate widespread changes to selection pressures and evolutionary processes, and we have the chance to incorporate evolutionary concerns at an early management stage. As a first step, surveys of the genetic diversity of populations across broad ranges would help prioritize the need for action and provide baseline data from which to draw in the future. While this “snapshot” approach has limitations (e.g., neutral genetic diversity not necessarily corresponding to diversity of climate-related traits), it provides a coarse view of which species and populations may be limited in their evolutionary response. This type of dataset, combined with knowledge of life histories and population sizes, could be very powerful from a management standpoint. Furthermore, resources for incorporating such evolutionary concepts in policy and management are becoming more widely available (Hendry et al. 2010).
The magnitude and duration of human-caused climate change depends largely on our current and future behaviors (greenhouse gas emissions, land use decisions, etc.), which can only be estimated as scenarios. Hence, we do not know how long or large of a climate shift to manage for. Millar et al. (2007) have suggested that management strategies targeting the ecological effects of climate change can be categorized as either resistance (managing systems to avoid change), resilience (managing systems for an eventual return to previous states), or response (managing systems to accommodate change). For many species, maximizing adaptive potential may improve the efficacy of all three strategies. From a management standpoint, evolution is an extremely powerful and pervasive tool by which populations cope with environmental change. However, the process of natural selection is entirely reactive and never proactive. We have the unique position of affecting evolutionary processes in order to anticipate future selection regimes. A rapid evolutionary response requires strong selection pressure and enough genetic variability to confer advantageous (or at least tolerant) phenotypes—climate change will provide the former and humans should manage for the latter.
One central question that remains is how much do we need to know in order to incorporate evolutionary processes into climate change management? The degree to which we can positively influence evolutionary responses is a new scientific and management frontier, but there is growing recognition for the need to incorporate evolutionary understanding into management plans designed to accommodate change (Hoffmann and Sgrò 2011, Groves et al. 2012, Stein et al. 2013). At the very least, we should incorporate knowledge of evolutionary processes into decision-making, and at most directly influence evolutionary trajectories for positive outcomes. Most importantly, well-intended management actions could fail or have long-lasting negative evolutionary consequences if evolutionary concerns are not taken into account (Ashley et al. 2003, Mace and Purvis 2008). Through climate change, humans are imposing a tremendous force in altering natural selection pressures, and the composition of the earth’s biotas will likely shift dramatically if current trends continue. In trying to preserve biodiversity, we must be sure not to lose sight of the evolutionary processes that created it in the first place.
ACKNOWLEDGMENTS
We thank David Ackerly, Jessica Blois, Kim Hall, Mark Herzog, Rebecca Quinones, Kevin Rice, Terry Root, and Blake Suttle for helpful comments. Brad Shaffer, Jessica Petersen, and Ian Wang generously provided information about the population genetics of their respective study systems. J.P.S. was supported by the NSF Responding to Rapid Environmental Change (REACH) Integrative Graduate Education and Research Traineeship (IGERT) at UC Davis (NSF-DGE 0801430) and NSF award no. DEB 1003009 during the writing of this chapter.
Manager Comments
Jason P. Sexton and Alden B. Griffith
in conversation with
Rob Klinger
Sexton and Griffith: Rob, how much attention do you see being paid to evolutionary concerns, and what are the constraints to greater consideration?
Klinger: As your chapter points out, the idea that evolutionary considerations need to be integral to management planning as proactive rather than just reactive strategies is not new. But your discussion also underscores some of the potential conflicts and challenges evolutionary arguments will have to effectively address to become significant components of management programs. Ultimately, what will determine the degree to which evolutionary perspectives become major components of management programs will depend on three factors: (1) How well evolutionary considerations dovetail with broader management strategies, especially in relation to the timescales at which most management programs are typically implemented and evaluated; (2) the institutional capacity of agencies to manage climate change-related issues; and (3) the degree to which our perceptions and conservation values change in such an uncertain environmental and management setting.
Sexton and Griffith: Are there principles or mandates within the current framework for management that can help promote greater consideration of evolutionary factors and adaptive potential? Similarly, are current paradigms in conflict with this goal?
Klinger: Key to incorporating evolutionary considerations into climate change management will be the ability to answer the question “In practical terms, what can we do about that?” This takes the discussion from the abstract to the concrete, and it is where your point about focusing on first principles (e.g., maintaining connectivity) becomes extremely important. This would be especially so in highly threatened and fragmented ecosystems with large numbers of endemic and rare species (e.g., vernal pool systems in the Central Valley and coastal scrub in southern California), or for one or a few high-profile species in ecosystems where changes from climatic shifts are expected to be rapid and pronounced (e.g., fishers and spotted owls in the Sierra Nevada / Cascade range). Some agencies such as the U. S. Fish & Wildlife Service (USFWS) and the California Department of Fish & Game (CDFG) will be focusing on particular species, but it will be critical for these agencies to develop approaches to decide on which species they devote resources to. Though originally proposed in the context of invasive species management, Brooks and Klinger (2009) described a process for integrating prioritization with predictive modeling for a suite of potential target species. Not only could this approach be modified for groups of species threatened by climate shifts, but it would also provide management agencies with a systematic and concrete set of options and be a way of directly incorporating evolutionary principles into a prioritization effort.
Incorporating evolutionary considerations into climate change management programs will be something most managers and scientists have no problem with conceptually or in principle. However, the reality of actually having them as primary components of direct management action is another matter. Over the last 30 years, management of natural resources in the United States has evolved from a focus on particular species or groups of species such as game animals, timber, charismatic, or flagship species, and threatened or endangered species, to a far greater emphasis on ecosystem management conducted within an adaptive management framework. Most resource management agencies, whether governmental or nongovernmental, adopted the ecosystem management philosophy at least a decade ago and proposals to base management on evolutionary considerations could, in many ways, be perceived as a return to the era of single-species management. Regardless of whether it is a charismatic species such as a bighorn sheep or a more obscure species such as a salamander or herbaceous plant, management programs based on individual species are, by definition, very limited in scope and often very expensive. One of the basic ideas behind ecosystem management is that large-scale management actions will benefit many species, including those that are rare. The notion of ecosystem management being based on one or a few flagship rare species is certainly not new (although these tend to be large, charismatic species); nevertheless, the strategy of most agencies is to focus on ecosystem and ecological processes, and the stressors on those processes, and there is little evidence indicating this will change. Evolutionary considerations are only one of many issues that will need to be taken into account in management programs developed around climate change, and it may be difficult for agencies to justify devoting resources to projects unless they have outcomes that can be measured within a decade (at the outside) and have management implications beyond just one or a few species.
Sexton and Griffith: In your experience, how would you describe the current capacity of agencies to consider and incorporate the many important management issues stemming from climate change, including evolutionary considerations?
Klinger: Governmental and nongovernmental resource agencies are faced with an overwhelming number of issues related to climate shifts, but the rate and magnitude of environmental changes have caught most of them unprepared to effectively deal with them. Consequently, the institutional capacity of many of these agencies for managing climate-induced changes is in a nascent state. Most are rapidly trying to develop integrated climate change strategies and programs, but because agency capacity is limited, many potentially useful studies and projects are competing for a limited pool of financial and logistical resources. And of course, this pool of funds depends on congressional support, which we have all observed can wax and wane. So, not only is this pool of funds limited, but it is also unpredictable. Nevertheless, it is apparent that some resources will continue to be devoted to management strategies and programs focused on climatic shifts. For example, the U. S. Forest Service and National Park Service have been developing national climate change programs structured around adaptation and mitigation of change. Each program has pilot projects in California, but neither of them is directly addressing evolutionary issues. It is during the developmental stage of such programs that evolutionary perspectives may have the best chance to become integrated into programmatic goals and strategies. This would increase the likelihood that agencies have the capacity to support studies and programs with a significant evolutionary / genetic focus. However, as noted above, the key to effectively including evolutionary perspectives in climate change programs will be dovetailing these perspectives into existing management strategies and demonstrating concretely how this directly benefits management programs.
Sexton and Griffith: Are there ways in which you see evolutionary perspectives in management naturally dovetailing with future shifts in management priorities, perspectives, and practices as the climate continues to change?
Klinger: There is often an underlying assumption that management is absolutely necessary to try and maintain ecosystems in some familiar configuration, which in the context of climate change has become especially manifest with notions such as managing ecosystems for resistance and resilience, and managed relocation. But climate change, especially rapid change, has made it apparent that the development of novel ecosystems is almost ensured, and even those that keep some semblance of their structure and processes will likely have different geographic boundaries.
Attempting to manage resources for desired conditions based on the past will often be ill-fated, and this is forcing a reevaluation of not just what conditions we find desirable, but also whether it is feasible to attain those conditions. As your chapter implies, there is little sense in managing for resistance to change when the agent of change cannot be altered in any meaningful way, and managing for resilience does not make much sense when ecosystems will not be persisting in an equilibrium or historical state. Given the level of ecological uncertainty that already exists with the direction and extent of climate-associated changes, the added controversy, uncertainty, and expense associated with moving species means that evolutionary, as well as ecological, arguments would have to be extremely compelling to justify such systematic efforts.
What complicates the evolutionary perspective on climate change is that adaptations are individual traits that become translated at the population level, but entire ecosystems are undergoing transformations now (for examples in California, see Cayan et al. 2008). It is not unreasonable to think that many species have already begun to adapt to these changes, but identifying which species these are and then translating individual responses into entire communities poses tremendous challenges. Millar et al. (2007) described a framework on how to think in terms of ecosystem responses in the future, but what is needed is a similar framework to help scientists and managers think through the transformations that are happening now. This becomes particularly important when considering calls for fairly rapid and controversial management actions, such as managed relocation (McLachlan et al. 2007). There are abundant examples of unintended consequences from well-intentioned management actions, so a sad irony would be undertaking costly climate response programs that were unnecessary or even counterproductive. At this point, one of the best strategies may be simple patience combined with good monitoring, particularly when thinking in evolutionary terms.
LITERATURE CITED
Brooks, M. L. and R. C. Klinger. 2009. Practical considerations for early detection monitoring of plant invasions. Chapter 2. In Inderjit (ed.), Management of Invasive Weeds. Springer, New York, NY. 9–33.
Cayan, D. R., E. P. Maurer, M. D. Dettinger, M. Tyree, and K. Hayhoe. 2008. Climate change scenarios for the California region. Climatic Change 87:S21–S42.
McLachlan, J. S., J. J. Hellman, and M. W. Schwartz. 2007. A framework for debate of assisted migration in an era of climate change. Conservation Biology 21:297–302.
Millar, C. I., N. L. Stephenson, and S. L. Stephens. 2007. Climate change and forests of the future: Managing in the face of uncertainty. Ecological Applications 17:2145–2151.
LITERATURE CITED
Ackerly, D. D. 2003. Community assembly, niche conservatism, and adaptive evolution in changing environments. International Journal of Plant Sciences 164:S165–S184.
Aitken, S. N. and M. C. Whitlock. 2013. Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology and Systematics 44:367–388.
Anderson, M. G. and C. E. Ferree. 2010. Conserving the stage: Climate change and the geophysical underpinnings of species diversity. PLOS ONE 5:e11554.
Ashley, M. V., M. F. Willson, O. R. W. Pergams, D. J. O’Dowd, S. M. Gende, and J. S. Brown. 2003. Evolutionarily enlightened management. Biological Conservation 111:115–123.
Boyce, W. M., R. R. Ramey, T. C. Rodwell, E. S. Rubin, and R. S. Singer. 1999. Population subdivision among desert bighorn sheep (Ovis canadensis) ewes revealed by mitochondrial DNA analysis. Molecular Ecology 8: 99–106.
Bradshaw, A. D. 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13:115–155.
Bradshaw, W. E. and C. M. Holzapfel. 2006. Evolutionary response to rapid climate change. Science 312:1477–1488.
Bradshaw, W. E. and C. M. Holzapfel. 2007. Genetic response to rapid climate change: It’s seasonal timing that matters. Molecular Ecology 17:157–166.
Carroll, S. P. 2011. Conciliation biology: The eco-evolutionary management of permanently invaded biotic systems. Evolutionary Applications 4:184–199.
Clausen, J., D. D. Keck, and W. M. Hiesey. 1940. Experimental Studies on the Nature of species. I. Effect of Varied Environments on western North American Plants. Carnegie Institution of Washington Publication 520, Washington, DC.
Cole, K. L., K. Ironside, J. Eischeid, G. Garfin, P. B. Duffy, and C. Toney. 2011. Past and ongoing shifts in Joshua tree distribution support future modeled range contraction. Ecological Applications 21:137–149.
Conner, J. K. and D. L. Hartl. 2004. A Primer of Ecological Genetics. Sinauer Associates, Sunderland, MA.
Crandall, K., O. Bininda-Emonds, G. Mace, and R. Wayne. 2000. Considering evolutionary processes in conservation biology. Trends in Ecology and Evolution 15:290–295.
Crimmins, S. M., S. Z. Dobrowski, J. A. Greenberg, J. T. Abatzoglou, and A. R. Mynsberge. 2011. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331:324–327.
Darling, E., K. E. Samis, and C. G. Eckert. 2008. Increased seed dispersal potential towards geographic range limits in a Pacific coast dune plant. New Phytologist 178:424–435.
Davis, M. B., R. G. Shaw, and J. R. Etterson. 2005. Evolutionary responses to changing climate. Ecology 86:1704–1714.
Dlugosch, K. M. and I. M. Parker. 2008. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology 17:431–449.
Dylan, J. F. 2008. How well can captive breeding programs conserve biodiversity? A review of salmonids. Evolutionary Applications 1:535–586.
Epps, C. W., D. R. McCullough, J. D. Wehausen, V. C. Bleich, and J. L. Rechel. 2004. Effects of climate change on population persistence of desert-dwelling mountain sheep in California. Conservation Biology 18:102–113.
Epps, C. W., P. Palsboll, J. D. Wehausen, G. K. Roderick, and D. R. McCullough. 2006. Elevation and connectivity define genetic refugia for mountain sheep as climate warms. Molecular Ecology 15:4295–4302.
Epps, C. W., P. J. Palsboll, J. D. Wehausen, G. K. Roderick, R. R. Ramey, and D. R. McCullough. 2005. Highways block gene flow and cause a rapid decline in genetic diversity of desert bighorn sheep. Ecology Letters 8:1029–1038.
Etterson, J. R. and R. G. Shaw. 2001. Constraint to adaptive evolution in response to global warming. Science 294:151–154.
Excoffier, L. and G. Heckel. 2006. Computer programs for population genetics data analysis: A survival guide. Nature Reviews Genetics 7:745–758.
Fitzpatrick, B. M. and H. B. Shaffer. 2007. Hybrid vigor between native and introduced salamanders raises new challenges for conservation. Proceedings of the National Academy of Sciences of the United States of America 104:15793–15798.
Frankham, R. 2008. Genetic adaptation to captivity in species conservation programs. Molecular Ecology 17:325–333.
Franks, S. J., S. Sim, and A. E. Weis. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences of the United States of America 104:1278–1282.
Friedman, J. M., J. E. Roelle, J. F. Gaskin, A. E. Pepper, and J. R. Manhart. 2008. Latitudinal variation in cold hardiness in introduced Tamarix and native Populus. Evolutionary Applications 1:598–607.
Gaskin, J. F. and B. A. Schaal. 2002. Hybrid Tamarix widespread in U.S. invasion and undetected in native Asian range. Proceedings of the National Academy of Sciences of the United States of America 99:11256–11259.
Geber, M. A. and T. E. Dawson. 1993. Evolutionary responses of plants to global change. In P. M. Kareiva, J. G. Kingsolver, and R. B. Huey (eds), Biotic Interactions and Global Change. Sinauer, Sunderland, MA. 179–197.
Ghalambor, C. K., J. K. McKay, S. P. Carroll, and D. N. Reznick. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21:394–407.
Gienapp, P., C. Teplitsky, J. S. Alho, J. A. Mills, and J. Merila. 2008. Climate change and evolution: Disentangling environmental and genetic responses. Molecular Ecology 17:167–178.
Godsoe, W., J. B. Yoder, C. I. Smith, and O. Pellmyr. 2008. Coevolution and divergence in the Joshua tree / yucca moth mutualism. American Naturalist 171:816–823.
Grant, P. R. and B. R. Grant. 2002. Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711.
Grivet, D., V. L. Sork, R. D. Westfall, and F. W. Davis. 2008. Conserving the evolutionary potential of California valley oak (Quercus lobata Née): A multivariate genetic approach to conservation planning. Molecular Ecology 17:139–156.
Griffith, B., J. M. Scott, J. W. Carpenter, and C. Reed. 1989. Translocation as a species conservation tool: status and strategy. Science 245:477–480.
Groves, C. R., E. T. Game, M. G. Anderson, M. Cross, C. Enquist, Z. Ferdana, E. Girvetz, A. Gondor, K. R. Hall, J. Higgins et al. 2012. Incorporating climate change into systematic conservation planning. Biodiversity and Conservation 21:1651–1671.
Harvell, C. D., C. E. Mitchell, J. R. Ward, S. Altizer, A. P. Dobson, R. S. Ostfeld, and M. D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162.
Havens, K., P. Vitt, M. Maunder, E. O. Guerrant Jr., and K. Dixon. 2006. Ex situ plant conservation and beyond. Bioscience 56:525–531.
Hendry, A. P., T. J. Farrugia, and M. T. Kinnison. 2008. Human influences on rates of phenotypic change in wild animal populations. Molecular Ecology 17:20–29.
Hendry, A. P., M. T. Kinnison, M. Heino, T. Day, T. B. Smith, G. Fitt, C. T. Bergstrom, J. Oakeshott, P. S. Jørgensen, M. P. Zalucki et al. 2011. Evolutionary principles and their practical application. Evolutionary Applications 4:159–183.
Hendry, A. P., L. G. Lohmann, E. Conti, J. Cracraft, K. A. Crandall, D. P. Faith, C. Haeuser, C. A. Joly, K. Kogure, A. Larigauderie et al. 2010. Evolutionary biology in biodiversity science, conservation, and policy: A call to action. Evolution 64:1517–1528.
Hoffmann, A. A. and C. M. Sgrò. 2011. Climate change and evolutionary adaptation. Nature 470:479–485.
Holmgren, C. A. and J. L. Betancourt. 2008. A Long-Term Vegetation History of the Mojave-Colorado Desert Ecotone at Joshua Tree National Park. Final Report Prepared for Joshua Tree National Park Association.
Holmstrom, R. M., J. R. Etterson, and D. J. Schimpf. 2010. Dune restoration introduces genetically distinct American beachgrass, Ammophila breviligulata, into a threatened local population. Restoration Ecology 18:426–437.
Holt, R. D. 2003. On the evolutionary ecology of species’ ranges. Evolutionary Ecology Research 5:159–178.
Huey, R. B., G. W. Gilchrist, M. L. Carlson, D. Berrigan, and L. Serra. 2000. Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309.
Johnson, M. L. and M. S. Gaines. 1990. Evolution of dispersal: Theoretical models and empirical tests using birds and mammals. Annual Reviews in Ecology and Systematics 21:449–480.
Jump, A. S., R. Marchant, and J. Peñuelas. 2009. Environmental change and the option value of genetic diversity. Trends in Plant Science 14:51–58.
Kelly, M. W., E. Sanford, and R. K. Grosberg. 2011. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proceedings of the Royal Society B: Biological Sciences 279:349–356. doi: 10.1098 / rspb.2011.0542.
Kellermann, V., B. van Heerwaarden, C. M. Sgro, and A. A. Hoffmann. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325:1244–1246.
Kinnison, M. and N. Hairston. 2007. Eco-evolutionary conservation biology: Contemporary evolution and the dynamics of persistence. Functional Ecology 21:444–454.
Klausmeyer, K. R. and M. R. Shaw. 2009. Climate change, habitat loss, protected areas and the climate adaptation potential of species in mediterranean ecosystems worldwide. PLOS ONE 4:e6392.
Knapp, E. E. and K. J. Rice. 1998. Comparison of isozymes and quantitative traits for evaluating patterns of genetic variation in purple needlegrass (Nassella pulchra). Conservation Biology 12:1031–1041.
Lande, R. 1988. Genetics and demography in biological conservation. Science 241:1455–1460.
Lankau, R., P. S. Jørgensen, D. J. Harris, and A. Sih. 2011. Incorporating evolutionary principles into environmental management and policy. Evolutionary Applications 4:315–325.
Latta, R. G. 2008. Conservation genetics as applied evolution: From genetic pattern to evolutionary process. Evolutionary Applications 1:84–94.
Lawrence, H. A., G. A. Taylor, D. E. Crockett, C. D. Millar, and D. M. Lambert. 2008. New genetic approach to detecting individuals of rare and endangered species. Conservation Biology 22:1267–1276.
Leinonen, T., R. B. O’Hara, J. M. Cano, and J. Merila. 2008. Comparative studies of quantitative trait and neutral marker divergence: A meta-analysis. Journal of Evolutionary Biology 21:1–17.
Lesica, P. and F. W. Allendorf. 1995. When are peripheral populations valuable for conservation? Conservation Biology 9:753–760.
Levins, R. 1968. Evolution in Changing Environments. Princeton University Press, Princeton, NJ.
Leger, E. A. and K. J. Rice. 2007. Assessing the speed and predictability of local adaptation in invasive California poppies (Eschscholzia californica). Journal of Evolutionary Biology 20:1090.
Loss, S. R., L. A. Terwilliger, and A. C. Peterson. 2011. Assisted colonization: Integrating conservation strategies in the face of climate change. Biological Conservation 144:92–100.
Mace, G. M. and A. Purvis. 2008. Evolutionary biology and practical conservation: Bridging a widening gap. Molecular Ecology 17:9–19.
Maron, J. L., V. Montserrat, R. Bommarco, S. Elmendorf, and P. Beardsley. 2004. Rapid evolution of an invasive plant. Ecological Monographs 74:261–280.
Mayr, E. 1947. Ecological factors in speciation. Evolution 1:263–288.
McKay, J. K. and R. G. Latta. 2002. Adaptive population divergence: Markers, QTL and traits. Trends in Ecology and Evolution 17:285–291.
Millar, C. I., N. L. Stephenson, and S. L. Stephens. 2007. Climate change and forests of the future: Managing in the face of uncertainty. Ecological Applications 17:2145–2151.
Mitrovski, P., A. A. Hoffmann, D. A. Heinze, and A. R. Weeks. 2008. Rapid loss of genetic variation in an endangered possum. Biology Letters 4:134–138.
Moeller, L. and K. Wang. 2008. Engineering with precision: Tools for the new generation of transgenic crops. Bioscience 58:391–401.
Montalvo, A. M. and N. C. Ellstrand. 2000. Transplantation of the subshrub Lotus scoparius: Testing the home-site advantage hypothesis. Conservation Biology 14:1034–1045.
Palumbi, S. R. 2001. Humans as the world’s greatest evolutionary force. Science 293:1786–1790.
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37:637–669.
Pearce, C. M. and D. G. Smith. 2001. Plains cottonwood’s last stand: Can it survive invasion of Russian olive onto the Milk River, Montana floodplain? Environmental Management 28:623–637.
Pereira, R. J., F. S. Barreto, and Burton, R. S. 2014. Ecological novelty by hybridization: Experimental evidence for increased thermal tolerance by transgressive segregation in Tigriopus californicus. Evolution 68:204–215.
Petersen, J. L., M. J. Ellis, and B. P. May. Conservation genetics of California’s endangered Shasta crayfish (Pacifastacus fortis). Unpublished data. Phillips, B., G. Brown, J. Webb, and R. Shine. 2006. Invasion and the evolution of speed in toads. Nature 439:803–803.
Reusch, T. B. H. and T. E. Wood. 2007. Molecular ecology of global change. Molecular Ecology 16:3973–3992.
Rezende, E. L., F. Bozinovic, and T. Garland. 2004. Climatic adaptation and the evolution of basal and maximum rates of metabolism in rodents. Evolution 58:1361–1374.
Reznick, D. N. and C. K. Ghalambor. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113:183–198.
Rice, K. J. and N. C. Emery. 2003. Managing microevolution: Restoration in the face of global change. Frontiers in Ecology and the Environment 1:469–478.
Richards, C. L., O. Bossdorf, N. Z. Muth, J. Gurevitch, and M. Pigliucci. 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters 9:981–993.
Richardson, D. M., J. J. Hellmann, J. S. McLachlan, D. F. Sax, M. W. Schwartz, P. Gonzalez, E. J. Brennan, A. Camacho, T. L. Root, O. E. Sala et al. 2009. Multidimensional evaluation of managed relocation. Proceedings of the National Academy of Sciences of the United States of America 106:9721–9724.
Rieseberg, L. H. 2006. Hybrid speciation in wild sunflowers. Annals of the Missouri Botanical Garden 93:34–48.
Robertson, B. A., J. S. Rehage, and A. Sih. 2013. Ecological novelty and the emergence of evolutionary traps. Trends in Ecology and Evolution 28:552–560.
Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig, and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421:57–60.
Rubin, E. S., W. M. Boyce, M. C. Jorgensen, S. G. Torres, C. L. Hayes, C. S. O’Brien, and D. A. Jessup. 1998. Distribution and abundance of bighorn sheep in the peninsular ranges, California. Wildlife Society Bulletin 26:539–551.
Schwartz, M., G. Luikart, and R. Waples. 2007. Genetic monitoring as a promising tool for conservation and management. Trends in Ecology and Evolution 22:25–33.
Sexton, J. P., P. J. Mcintyre, A. L. Angert, and K. J. Rice. 2009. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics 40:415–436.
Sexton, J. P., S. Y. Strauss, and K. J. Rice. 2011. Gene flow increases fitness at the warm edge of a species’ range. Proceedings of the National Academy of Sciences of the United States of America 108:11704–11709. doi: 10.1073 / pnas.1100404108.
Sexton, J. P., J. K. McKay, and A. Sala. 2002. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecological Applications 12:1652–1660.
Sgrò, C. M., A. J. Lowe, and A. A. Hoffmann. 2011. Building evolutionary resilience for conserving biodiversity under climate change. Evolutionary Applications 4:326–337.
Shaffer, H. B., G. B. Pauly, J. C. Oliver, and P. C. Trenham. 2004. The molecular phylogenetics of endangerment: Cryptic variation and historical phylogeography of the California tiger salamander, Ambystoma californiense. Molecular Ecology 13:3033–3049.
Skelly, D. K., L. N. Joseph, H. P. Possingham, L. K. Freidenburg, T. J. Farrugia, M. T. Kinnison, and A. P. Hendry. 2007. Evolutionary responses to climate change. Conservation Biology 21:1353–1355.
Smith, C. I., W. K. W. Godsoe, S. Tank, J. B. Yoder, and O. Pellmyr. 2008. Distinguishing coevolution from covicariance in an obligate pollination mutualism: Asynchronous divergence in Joshua tree and its pollinators. Evolution 62:2676–2687.
Spring Rivers. 2009. Shasta Crayfish Technical Review Committee Summary Report. Prepared for Pacific Gas and Electric Company, Environmental Services by Spring Rivers Ecological Sciences LLC of Cassel, California. Pacific Gas and Electric Company, San Francisco, CA.
Stein, B. A., A. Staudt, M. S. Cross, N. S. Dubois, C. Enquist, R. Griffis, L. J. Hansen, J. J. Hellmann, J. J. Lawler, E. J. Nelson et al. 2013. Preparing for and managing change: Climate adaptation for biodiversity and ecosystems. Frontiers in Ecology and the Environment 11:502–510.
Sultan, S. E. 2001. Phenotypic plasticity and ecological breadth in plants. American Zoologist 41:1599.
Tallmon, D. A., G. Luikart, and R. S. Waples. 2004. The alluring simplicity and complex reality of genetic rescue. Trends in Ecology and Evolution 19:489–496.
Turesson, G. 1925. The plant species in relation to habitat and climate. Hereditas 6:147–236.
USFWS. 1998. Shasta Crayfish Recovery Plan. United States Fish and Wildlife Service, Portland, OR, USA.
Via, S., R. Gomulkiewicz, G. De Jong, S. M. Scheiner, C. D. Schlichting, and P. H. Van Tienderen. 1995. Adaptive phenotypic plasticity: Consensus and controversy. Trends in Ecology and Evolution 5:212–217.
Visser, M. E. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proceedings of the Royal Society B: Biological Sciences 275:649–659.
Wang, I. J., W. K. Savage, and H. B. Shaffer. 2009. Landscape genetics and least cost path analysis reveal unexpected dispersal routes in the California tiger salamander (Ambystoma californiense). Molecular Ecology 18:1365–1374.
Wilczek, A. M., M. D. Cooper, T. M. Korves, and J. Schmitt. 2014. Lagging adaptation to warming climate in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 111:7906–7913.
Williams, D. G., R. N. Mack, and R. A. Black. 1995. Ecophysiology of introduced Pennisetum setaceum on Hawaii - the role of phenotypic plasticity. Ecology 76:1569–1580.