CHAPTER 12
Fossils Predict Biological Responses to Future Climate Change

Jessica L. Blois and Elizabeth A. Hadly
Abstract. The next few centuries will most likely see rapid and severe climate change. The legacy of climate change will unfold in biological systems over both short and long timescales. While policy often focuses on the effects of climate change over the next decades and century, a longer-term perspective allows better understanding of how species and communities will most likely respond fully to changing climates of the future. The fossil record provides such a long-term perspective, and illustrates a variety of impacts associated with climate change. Moreover, the fossil record can show whether the estimates of current and future climate change and the corresponding biological responses are within the range of historic variability. In addition to playing a role in extinction events, changes in past climates affected species abundances and geographic ranges, population demographics, genetic diversity, and morphological features of individual species such as body size. Over longer time periods, climate affected the macroevolution and species diversity of mammals by influencing rates and magnitudes of immigration, emigration, extinction and speciation. Because rates and magnitudes of climate change over the next few centuries are mostly predicted to be greater than past climate changes and will interact with other human impacts, a wide variety of biological processes are likely to show greater changes than they have at any time in the past. These processes include geographic range shifts, microevolutionary changes in populations (e.g., loss or gain of adaptive genotypes), and biotic turnover more typical of longer temporal scales. Overall, California is likely to experience increasing abundances of some highly adaptable species, but an overall loss of diversity; the sum of all these processes will likely result in novel communities in California.
Key Points
• Climate influences mammals and other species in many different ways, with ample evidence in the fossil record supporting effects on abundance, genetics, morphology, geographic distributions, extinction, and speciation.
• The effects of climate change are primarily on population processes, such as abundance, genetic, and morphological change, and on range shifts. These interacting processes can affect larger-scale processes such as speciation and extinction.
• The fossil record highlights the potential for unexpected and significant changes within future mammalian communities—communities of the future are likely to be very different than those on the landscape today.
• One of the primary ways managers can positively influence outcomes in the face of climate change is by increasing the overall resilience of biological systems, but as climate change accelerates beyond the capacity of species to naturally respond and adapt, more active management of populations may be necessary.
INTRODUCTION
Environments of the future are likely to be substantially different from past or present environments due to rapid and large-magnitude climate change (Williams et al. 2007, IPCC 2013). The predicted magnitude and rate of climate change, in the context of other human impacts, has serious implications for the persistence of California’s native ecosystems. A factor that complicates society’s ability to address these changes is that biological responses to climate change will unfold over both short (10–100 years) and long (1000–1,000,000 years) timescales, much longer than the annual to decadal timescales over which policy decisions are made. While studies of responses to recent (e.g., past 100 years) and ongoing climate change are highly useful (see review by Anderegg and Root, Chapter 3), the fossil record provides additional insight into how the changes we are observing today may translate into longer-term ecological and evolutionary trends.
The fossil record also helps constrain uncertainty about the effects of climate change by providing benchmarks to assess whether current and projected climate change and biological responses are within the range of historical variability. Throughout paleohistory, change has been the normal state for both the climate (Figure 12.1) and biological systems, though these changes primarily occurred without the added stressors of human impacts such as habitat destruction, hunting, and species introductions. Paleontological data can help determine which species are most vulnerable to climate change, which traits are associated with vulnerability, and which types of biological changes are most likely (Flessa and Jackson 2005, Hadly and Barnosky 2009, Dietl and Flessa 2011).
In this chapter, we highlight the influence of climate on multiple levels of the biological hierarchy, from genes to communities, using the mammalian fossil record (Table 12.1; Blois and Hadly 2009). Mammals are dominant elements of modern ecosystems and anthropogenic climate change has already affected many of them (Beever et al. 2003, Schwartz and Armitage 2004). Ecologically, mammals help structure biological communities since they function as predators, herbivores, seed dispersers, and scavengers (Huntley and Reichman 1994, Dirzo et al. 2014). California contains several important fossil sites to study the influence of climate change on mammals. In particular, fossil deposits from two caves in northern California (Samwell Cave and Potter Creek Cave) contain a rich mammalian assemblage from the late Pleistocene (see Figure 12.1 for timeline and climatic context; Sinclair 1904; Furlong 1906). We recently excavated a new deposit from Samwell Cave that encompasses both Holocene and late Pleistocene mammalian communities (Samwell Cave Popcorn Dome; Blois 2009, Blois et al. 2010), completing a time series of the mammalian community in this region over the past 18,000 years. Fossils from these sites show many changes through time within both individual species and the overall mammalian community.
BIOTIC RESPONSES TO PAST CLIMATE CHANGE
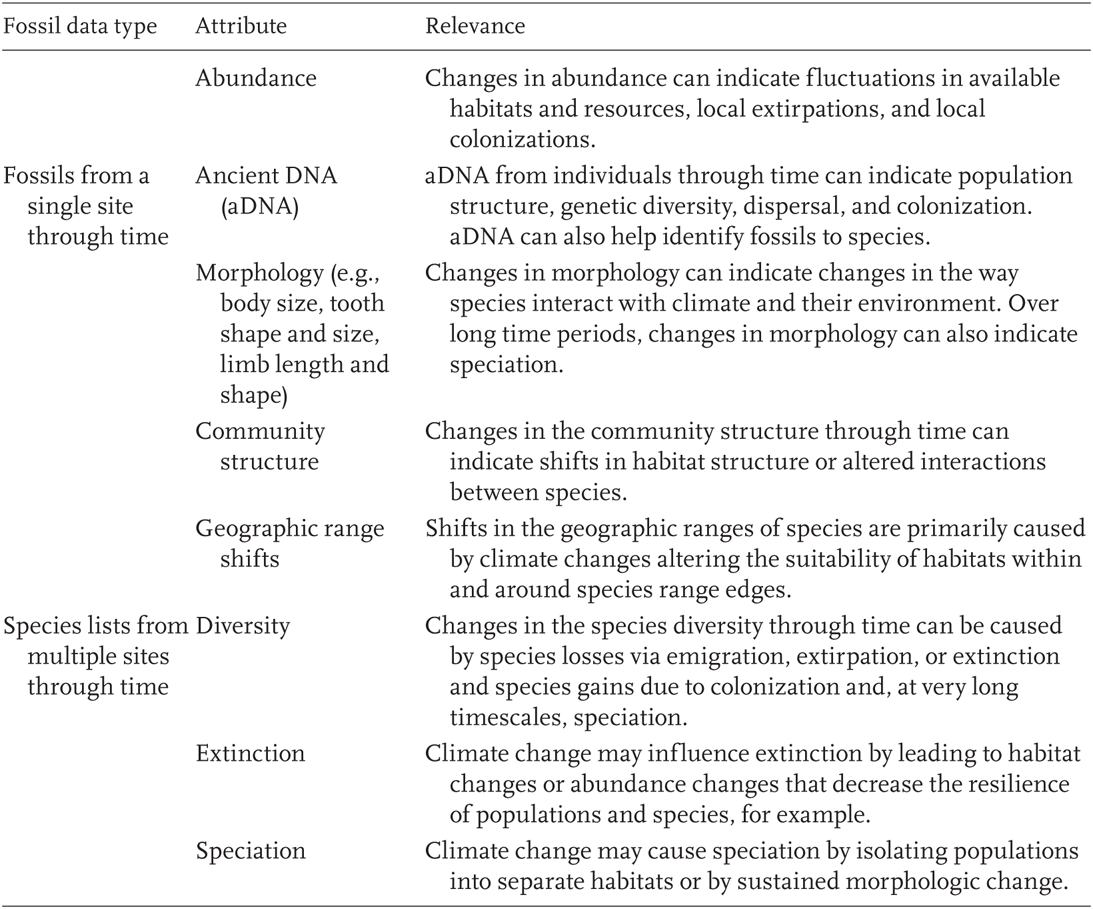
The biotic responses to paleoclimate change reviewed here fall into six categories spanning the biological hierarchy: Abundance change, genetic change, morphologic change, range shifts, speciation, and extinction (Blois and Hadly 2009).
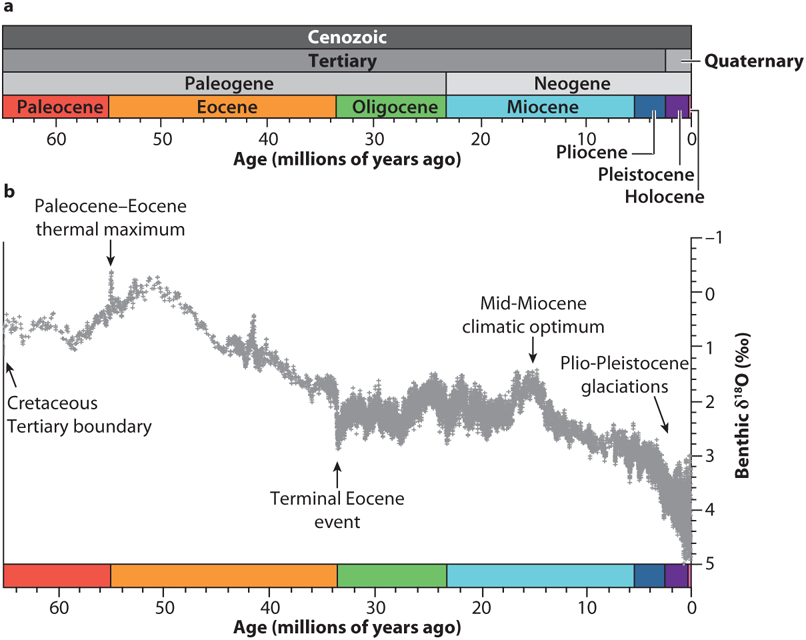
FIGURE 12.1: (a) Cenozoic timeline and (b) climate reconstruction, modified from Zachos et al. 2008. Source: Reprinted from Blois and Hadly (2009).
Abundance change
At the most fundamental level, climate may influence population size and / or density (here referred to as “abundance”) (Table 12.1). The abundance of a population is ultimately determined by the carrying capacity of the environment (Andrewartha and Birch 1954), which may change as climate changes influence the mammal’s preferred habitat (del Monte-Luna et al. 2004). Overall population connectivity and the resilience of a species are influenced by the distribution of the abundance of individuals and populations across a species range (Lundberg et al. 2000). Thus, abundance changes influence other aspects of populations and species, such as genetic diversity, morphology, and geographic ranges.
In northern California, small mammals have shown significant abundance changes over the past 18,000 years (Figure 12.2; Blois et al. 2010). The greatest changes occurred during the Pleistocene–Holocene transition around 11 thousand years ago (Kya) (Figures 12.1 and 12.2). During this time period, northern California transitioned from cold and mesic conditions with low fire frequency to very warm and dry conditions (Daniels et al. 2005). In the Holocene, deer mice (Peromyscus) started to dominate the small mammal community, concurrent with decreases in the relative abundance of wood rats (Neotoma), ground squirrels (Spermophilus), some gophers (Thomomys), and many other species (Figure 12.2).
Change in the abundance of two animals in particular stand out, both likely occurring in response to climate changes during the Pleistocene–Holocene transition. First, the mountain beaver (Aplodontia rufa) became locally extirpated from the region around the caves (Figure 12.2f). Remains of this animal are found in northern California cave deposits that date to the Last Glacial Maximum (LGM) at 18 Kya, but disappear from the deposits by 13 Kya. A series of warm and dry periods within the late Pleistocene and early and middle Holocene (Daniels et al. 2005) may have caused local extirpation of mountain beaver populations as the landscape became drier. Today, the mountain beaver is found only in cooler, mesic locations in the region, generally at higher elevations. These animals are tied to mesic habitats because their kidneys are very inefficient, requiring that they constantly drink water and consume water-rich plant material (Carraway and Verts 1993). Second, the gopher Thomomys cf. mazama decreased in abundance and was completely replaced by a different gopher (Thomomys bottae) during the Pleistocene–Holocene transition (Figures 12.2b; Blois et al. 2010). The climate changes at this transition and the environmental niches occupied currently by the different types of gophers suggest that climate influenced the local extirpation of Thomomys cf. mazama (Blois 2009, Blois et al. 2010) because this gopher is confined to generally higher elevations in the region today.
TABLE 12.1
Methods to examine relationships between different attributes of fossil data and climate.
The outcome of abundance changes within individual species, particularly the decline of formerly common species, was a net loss of local diversity as measured both by a decline in species richness (the number of species present) and a reduction in evenness (similarity in the abundance of different species) (Blois et al. 2010). Today’s mammal community is very different from the community present during the Pleistocene. These examples illustrate that biotic change is dynamic and adjustments to population abundance can cause local extirpation, range shifts, and biodiversity loss, up to global extinction of species (Table 12.1).

FIGURE 12.2: Abundance of key small mammal taxa in the Samwell Cave Popcorn Dome deposit. Source: Modified from Blois et al. (2010); supplementary information).
Genetic change
Genetic change is a result of population-level processes such as recombination, mutation, selection, random genetic drift, and gene flow (Charlesworth et al. 2003), all of which take place in mammals over tens to thousands of years. Most of these processes may be influenced by climate, either directly such as when climate selects for or against key traits or indirectly through abundance or habitat change, which influences gene flow and genetic drift. Ancient DNA (aDNA) provides the opportunity to study the impact of climate change on the evolutionary dynamics within past populations and species (Table 12.1). aDNA is DNA that has survived in the remains of ancient organisms. Under optimal preservation conditions such as in permafrost settings, aDNA from mammals can be as old as 50,000–65,000 years or older (Willerslev et al. 2003). Few examples of aDNA studies exist for California populations of terrestrial mammals (Blois 2009), though increasing attention is being paid to museum specimens that extend DNA samples back to the early 1900s (e.g., Perrine et al. 2007). Our work on ancient genetic data from Samwell Cave confirmed the replacement event among gophers (described above) at the Pleistocene–Holocene transition (Blois et al. 2010). We are also using aDNA to investigate causes of the expansion of T. bottae in northern California (Figure 12.2b). In North America and elsewhere, aDNA has been used to identify species that are morphologically indistinguishable from one another in the fossil record and thus help describe past geographic ranges of species (Willerslev et al. 2003, Gilbert et al. 2008). It has also been used to identify all organisms (plant, animal, microbial, etc.) present at a particular time and place from the DNA preserved in ancient sediments (Kuch et al. 2002, Willerslev et al. 2003, Willerslev et al. 2014).
aDNA can also be used to characterize the structure and genetic diversity of fossil animal populations (e.g., Hadly et al. 2004, Hofreiter et al. 2004). For example, aDNA combined with population genetic models has been used to discriminate if populations were connected or isolated during periods of climate change (Hadly et al. 2004, Hofreiter et al. 2004), the probable size of ancient populations (Chan et al. 2006), survival of clades across periods of climate change (Brace et al. 2012, Foote et al. 2013, Fulton et al. 2013), and whether changes in population size are consistent with what is known about the population biology of the species and the climatic history of the region (Hadly et al. 2004, Lorenzen et al. 2011). Genetic change may become increasingly important for species to successfully adapt to anthropogenic climate change (e.g., Jump and Penuelas 2005; Sexton and Griffith, Chapter 11).
Morphologic change
Trends in the average size or shape of mammals are among the most commonly documented responses to climate in the fossil record (Table 12.1; Blois and Hadly 2009). Changes in climate may affect the morphology of individuals directly or indirectly. For example, mammals are generally larger in cold or high latitude climates (Ashton et al. 2000), presumably because larger bodies are more efficient at maintaining high internal body temperatures. Climate may also act directly by changing the amount of time available to forage during favorable weather or by influencing the fasting endurance of individuals (Millar and Hickling 1990, Sinervo et al. 2010).
Climate change also may influence animals indirectly via effects on vegetation and habitat. For example, tooth size, shape, or structure may change due to changing type and quality of food resources (Patton and Brylski 1987). Additionally, as resources become more or less abundant due to climate change, body size should change accordingly (Lomolino 2005). Finally, changes in habitat structure (e.g., transitions from a forested habitat to grassland) may cause changes in limb morphology, such as the increase in body size and lengthening of limbs (facilitating greater running ability) that coincided with the expansion of more open grasslands in North America and elsewhere (MacFadden 1992, Janis 2008).
Often, trends in morphology across space, such as the pattern of mammals being larger at higher latitudes, are used to predict changes through time (e.g., Smith and Betancourt 1998, McGuire 2010). These patterns do not always hold, though, as demonstrated by time-series data from the fossil record. For example, mammals should be smaller today than during the LGM because it is warmer today. However, the body size of California ground squirrels (Spermophilus beecheyi) in the region around Samwell and Potter Creek Caves increased between the LGM (cold and dry) and today (warm and relatively mesic) (Blois et al. 2008). Contrary to expectations based on temperature gradients across space, Blois et al. (2008) found that body size variation across both time and space was primarily explained by differences in precipitation. Space–time comparisons such as this are useful for narrowing down which element of climate change will have the greatest impact on particular species and communities.
Range shifts
Fossil data are useful for detecting range shifts because they allow researchers to track species ranges over thousands of years (Table 12.1). These data have contributed to evidence that range shifts are one of the strongest responses a species can have to climate change, and show that while substantial range adjustments can happen within decades (Parmesan and Yohe 2003, Root et al. 2003), in some cases these responses occur over millennia (Graham et al. 1996, Lyons 2003).
While a single site such as the Samwell Cave Popcorn Dome deposit is not sufficient to detail the full geographic range shifts of California mammals since the LGM (Table 12.1), it does provide evidence for local range adjustments through time. For example, neither A. rufa nor Thomomys cf. mazama is found locally in the Samwell Cave area today, but both were there in the past, indicating that the ranges of these animals adjusted to their present-day ranges by moving northward and upward in elevation through time (Blois et al. 2010). Similarly, McGuire (2011) documented the past occurrence of the long-tailed vole (Microtus longicaudus) at two late Quaternary sites in the San Francisco Bay Area, substantially removed from their present range limits, indicating range shift or contraction through time. Integration of the fossil deposits from northern California with data from other localities throughout California and North America can increase the power to observe range changes through time (Graham et al. 1996, McGuire and Davis 2013). For example, range shifts in response to Late Pleistocene warming occurred in most North American mammals (Graham et al. 1996, Lyons 2003), with over approximately 30% of the mammals experiencing substantial range shifts between the LGM and the Holocene (Lyons 2003). The range shifts between the LGM and the Holocene showed strong directionality during periods of significant climate change (Graham et al. 1996, Lyons 2003). Additionally, poleward range limits, which are located in areas that typically experience greater magnitude climate changes than other areas in the range, shifted more than the limits facing the equator, on average, during periods of more significant warming such as the transition from the LGM to the Holocene (Lyons 2003). These findings are consistent with observations that the majority of species showing range shifts over the past century have moved poleward (Parmesan and Yohe 2003, Root et al. 2003, Hickling et al. 2006). Modern studies have also shown strong links with climate, presumably temperature, along the poleward edge of ranges, at least in many birds (Root 1988).
Speciation
Prolonged and unidirectional periods of climate change can result in speciation and / or lineage diversification (Table 12.1). Climate change may contribute to speciation by creating patchy habitats within the geographic range of a species (Vrba 1992, Gavrilets et al. 1998, Barnosky 2001), each supporting an isolated population that may proceed down independent evolutionary trajectories. Prolonged environmental change may also cause morphologic change leading to speciation (Gingerich 1985, Martin 1993). Both types of speciation are common and often simultaneously observed in the fossil record.
In California, several sites have long enough fossil records to investigate speciation, particularly the Barstow Formation in southeastern California. Pagnac (2006) surmised that speciation within the Miocene horse lineage Scaphohippus may have been related to climate. Rodents within the Barstow Formation are also a good example of morphologic evolution and speciation (Lindsay 1972), though no thorough investigations of the links with climate have been made using these deposits. Revisiting these deposits may be a fruitful area of future research on the effects of climate on speciation within California mammals.
Extinction
Extinction is the certain outcome for all species; in fact, most of the mammals that have inhabited the earth have already become extinct (Avise et al. 1998, Alroy 2000). The fossil record can be used to identify factors associated with extinction risk (Table 12.1) such as properties of mammals themselves (e.g., body size, degree of specialization, and rarity) as well as other factors such as habitat reduction, disease, and human hunting (e.g., Thomas et al. 2004a, Harrison et al. 2008, Nogués-Bravo et al. 2008), and determine how these factors interact with climate change. Species that are highly specialized to particular climate regimes and / or habitat types will be most vulnerable to extinction (e.g., Menendez et al. 2006), but with even greater amounts of climate change, the same mechanisms may affect less specialized species as well.
Many California fossil localities contain records of extinct animals (e.g., Sinclair 1904, Furlong 1906, Springer et al. 2010). For example, 24% of the mammals at Potter Creek Cave and Samwell Cave went globally extinct at the end of the Pleistocene, all but two of which were >44 kg (these very large animals are termed “megafauna”). A similar picture emerges from fossil deposits in southern California: 43% of the mammals went extinct, with a distinct bias toward larger body sizes (Stock and Harris 1992). The Pleistocene megafaunal extinctions in California and the rest of North America were likely due to the effects of climate change and human hunting, as well as other impacts such as disease (Barnosky et al. 2004, Nogués-Bravo et al. 2008). These same cascading factors are also likely to lead to future extinctions (Thomas et al. 2004b, Rosenzweig et al. 2008, Davidson et al. 2009, Barnosky et al. 2011).
Overall biotic change
In mammals, several episodes of significant, community-wide biological change seen in the past 65 million years occurred during periods when the rate of climate change was high (Figure 12.3) (Barnosky et al. 2003). Given that current and future rates of average global climate change are much higher than anything recorded by the fossil record (Figure 12.3), a large amount of ecological change should be expected in the future, possibly at a level not encountered before in the fossil record.
SYNERGIES AND SOLUTIONS
Overall, the California fossil record highlights the many ways climate may influence mammals and points to the potential for unexpected and significant changes within future mammalian communities. The effects of climate change are on population processes, such as abundance, genetic, and morphological change, and range shifts. These processes interact with one another during times of significant climate change and can affect larger-scale processes such as speciation and extinction. Ultimately, all of these processes combine to affect the overall structure of mammalian diversity. Thus, changes to the population processes can be early signals of the effects of anthropogenic climate change on the biological system (e.g., Ceballos and Ehrlich 2002, Post et al. 2009, Drake and Griffen 2010). Some of these signals are already being seen today (e.g., Parmesan and Yohe 2003, Root et al. 2003, Parmesan 2006, Rosenzweig et al. 2008) and the fossil record offers several concrete lessons to biologists, managers, and policy-makers to address the changes ahead (Hadly and Barnosky 2009).
As this review has demonstrated, species and communities will not remain static in the future. Greater amounts of climate change will likely lead to greater ecological changes (Figure 12.3; Barnosky et al. 2003), including population losses, range shifts, and extinction. Given the dominance of range shifts as a major response to climate change, significant abundance declines within populations, population losses within species, the prevalence of introduced species, and the threat of widespread extinctions, many communities of the future will be very different than those of today (Williams and Jackson 2007, Stralberg et al. 2009).
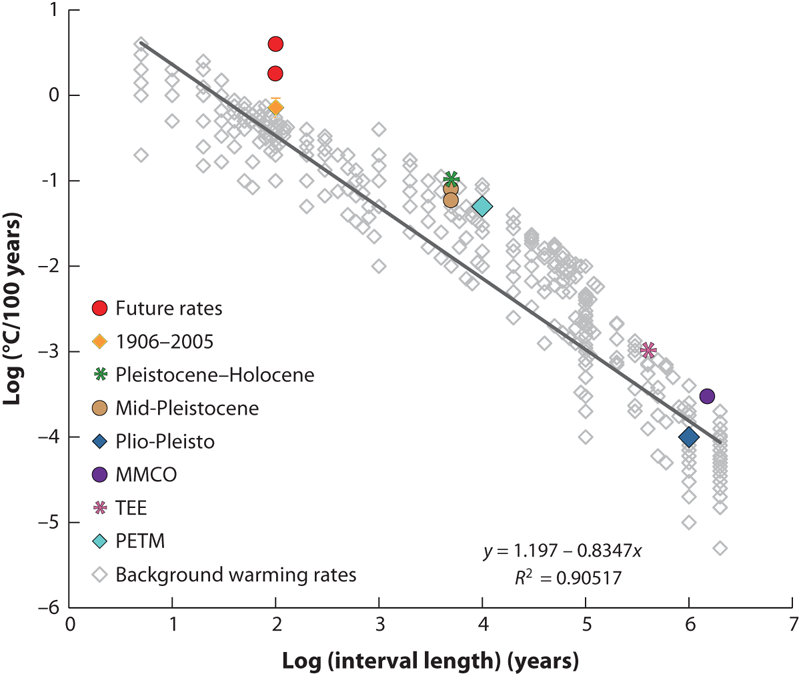
FIGURE 12.3: Per-100-year temperature change rates for different intervals throughout the Cenozoic, on a log–log scale. The best-fit line is shown, as well as the fit of the line (R2). All rates plotted are rates of warming, except for the Terminal Eocene Event (TEE), which is rate of cooling. The 1906–2005 per-100-year warming rate is estimated at 0.74 ± 0.18 based on the IPCC 2007 assessment; the low and high 95% bounds are plotted. Future rates are based on the IPCC 2007 assessment of “best estimate” of future temperature in 2090–2099 for different emission scenarios. The low estimate is based on the B1 emissions scenario and the high estimate is based on the A1F1 emissions scenario. Past and future 100-year data are from Solomon et al. (2007), TEE data are from Zachos et al. (2001), and Paleocene–Eocene Thermal Maximum (PETM) data are from Zachos et al. (2008). All other data are as in Figure 2 (top) from Barnosky et al. (2003). MMCO: Mid-Miocene Climatic Optimum. Source: Modified from Blois and Hadly (2009).
Proactive management will need to balance needs of current species with the goal of allowing communities to adapt and change over time. For example, if particular species are identified as high priority for conservation, detailed knowledge of the life history of those species and how life history is influenced by climate is very helpful because species are influenced by climate in different ways (e.g., McCain and King 2014) and there are many possible pathways to extinction (e.g., Davidson et al. 2009). However, we will still lose some species due to climate change. It will be useful to decide ahead of time which species are most important for conservation purposes and what amount of species loss is tolerable. Can key ecosystem functions be maintained, but with a reduced or different set of species?
The effects of climate change have and will continue to interact with other impacts such as invasion of nonnative species, habitat degradation, and habitat loss (Vermeij 1991, D’Antonia and Vitousek 1992). One of the primary ways managers can positively influence outcomes in the face of climate change is by increasing the overall resilience of biological systems. This can be done by preserving habitats, creating or expanding corridors between habitats, and preventing the invasion or establishment of particularly detrimental nonnative species. More active management of populations themselves may also be necessary. For example, if forestalling the loss of a particular species is the goal, the evolutionary timescale may need to be “sped up,” perhaps by transplanting individuals to increase genetic diversity or maintain genetic connectivity between populations. Additionally, if natural range shifts become impossible due to the pace of climate change or barriers to dispersal, transplanting populations into new habitats where they can be managed may be necessary (e.g., McLachlan et al. 2007, Richardson et al. 2009).
CONCLUSION
Mammals respond to climate change at all levels of biological organization. Climate affects population size and density, as well as habitat connectivity, which interact with the strength of climate change on individuals themselves to influence genetic and morphologic processes. Climate change also forces species to shift their geographic range or adapt to the new climate conditions if climates become intolerable. Finally, all of these processes interact to influence longer-term processes such as speciation and extinction, and ultimately how diversity across the globe is shaped through time.
All of these changes should be expected in the future; indeed, many of them are already observed in response to climate change over the past century. Given the diverse impacts of climate and humans over the past few centuries, we may already be nearing the sixth mass extinction event in earth’s history (Barnosky et al. 2011). The fossil record demonstrates that communities are very resilient to climate change, but also that surprises are likely and many species will respond in unknown ways. Life has persisted on earth for over 5 billion years and will persist for many more years to come. However, the shape of modern-day communities and ecosystems may be vastly different than those of the past, leading to unknown effects on human societies dependent on those ecosystems. It is up to human society to decide what our global biotic systems will be.
ACKNOWLEDGMENTS
The authors would like to thank C. Millar, J. Sexton, J. Dorman, K. Hall, T. Root, C. Howell, and M. Herzog for comments that greatly improved the manuscript. This research was supported by a BICCCA-PIREA fellowship to JLB and NSF grants EAR-0545648 and EAR- 0719429 to EAH.
LITERATURE CITED
Alroy, J. 2000. New methods for quantifying macroevolutionary patterns and processes. Paleobiology 26:707–733.
Andrewartha, H. G. and L. C. Birch. 1954. The Distribution and Abundance of Animals. University of Chicago Press, Chicago, IL.
Ashton, K. G., M. C. Tracy, and A. de Queiroz. 2000. Is Bergmann’s rule valid for mammals? The American Naturalist 156:390–415.
Avise, J. C., D. Walker, and G. C. Johns. 1998. Speciation durations and Pleistocene effects on vertebrate phylogeography. Proceedings of the Royal Society of London, Series B: Biological Sciences 265:1707–1712.
Barnosky, A. D. 2001. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. Journal of Vertebrate Paleontology 21:172–185.
Barnosky, A. D., E. A. Hadly, and C. J. Bell. 2003. Mammalian response to global warming on varied temporal scales. Journal of Mammalogy 84:354–368.
Barnosky, A. D., P. L. Koch, R. S. Feranec, S. L. Wing, and A. B. Shabel. 2004. Assessing the causes of late Pleistocene extinctions on the continents. Science 306:70–75.
Barnosky, A. D., N. Matzke, S. Tomiya, G. O. U. Wogan, B. Swartz, T. B. Quental, C. R. Marshall, J. L. McGuire, E. L. Lindsey, K. C. Maguire et al. 2011. Has the earth’s sixth mass extinction already arrived? Nature 471:51–57.
Beever, E. A., P. E. Brussard, and J. Berger. 2003. Patterns of apparent extirpation among isolated populations of pikas (Ochotona princeps) in the Great Basin. Journal of Mammalogy 84:37–54.
Blois, J. L. 2009. Ecological Responses to Paleoclimatic Change: Insights from Mammalian Populations, Species and Communities. Stanford University, Stanford, CA.
Blois, J. L. and E. A. Hadly. 2009. Mammalian response to Cenozoic climatic change. Annual Review of Earth and Planetary Sciences 37:181–208.
Blois, J. L., R. Feranec, and E. A. Hadly. 2008. Environmental influences on spatial and temporal patterns of body-size variation in California ground squirrels (Spermophilus beecheyi). Journal of Biogeography 35:602–613.
Blois, J. L., J. L. McGuire, and E. A. Hadly. 2010. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature 465:771–774.
Brace, S., E. Palkopoulou, L. Dalén, A. M. Lister, R. Miller, M. Otte, M. Germonpre, S. P. E. Blockley, J. R. Stewart, and I. Barnes. 2012. Serial population extinctions in a small mammal indicate Late Pleistocene ecosystem instability. Proceedings of the National Academy of Sciences of the United States of America 109:20532–20536.
Carraway, L. N. and B. J. Verts. 1993. Aplodontia rufa. Mammalian Species 431:1–10.
Ceballos, G. and P. Ehrlich. 2002. Mammal population losses and the extinction crisis. Science 296:904–907.
Chan, Y. L., C. N. K. Anderson, and E. A. Hadly. 2006. Bayesian estimation of the timing and severity of a population bottleneck from ancient DNA. PlOS Genetics 2:451–460.
Charlesworth, B., D. Charlesworth, and N. H. Barton. 2003. The effects of genetic and geographic structure on neutral variation. Annual Review of Ecology, Evolution, and Systematics 34:99–125.
Daniels, M. L., R. S. Anderson, and C. Whitlock. 2005. Vegetation and fire history since the Late Pleistocene from the Trinity Mountains, northwestern California, USA. Holocene 15:1062–1071.
D’Antonia, C. M. and P. M. Vitousek. 1992. Biological invasions by exotic grasses, the grass / fire cycle, and global change. Annual Review of Ecology and Systematics 23:63–87.
Davidson, A. D., M. J. Hamilton, A. G. Boyer, J. H. Brown, and G. Ceballos. 2009. Multiple ecological pathways to extinction in mammals. Proceedings of the National Academy of Sciences of the United States of America 106:10702–10705.
del Monte-Luna, P., B. W. Brook, M. J. Zetina-Rejon, and V. Cruz-Escalona. 2004. The carrying capacity of ecosystems. Global Ecology and Biogeography 13:485–495.
Dietl, G. P. and K. W. Flessa. 2011. Conservation paleobiology: Putting the dead to work. Trends in Ecology & Evolution 26:30–37.
Dirzo, R., H.S. Young, M. Galetti, G. Ceballos, N.J.B. Isaac, and B. Collen. 2014. Defaunation in the Anthropocene. Science 345:401–406.
Drake, J. M. and B. D. Griffen. 2010. Early warning signals of extinction in deteriorating environments. Nature 467:456–459.
Flessa, K. W. and S. T. Jackson. 2005. The Geological Record of Ecological Dynamics: Understanding the Biotic Effects of Future Environmental Change. National Academy Press, Washington, DC.
Foote, D., K. Kaschner, S. E. Schultze, C. Garilao, S. Y. W. Ho, K. Post, T. F. G. Higham, C. Stokowska, H. van der Es, C. B. Embling et al. 2013. Ancient DNA reveals that bowhead whale lineages survived Late Pleistocene climate change and habitat shifts. Nature Communications 4:1677.
Fulton, T. L., R. W. Norris, and R. W. Graham. 2013. Ancient DNA supports southern survival of Richardson’s collared lemming (Dicrostonyx richardsoni) during the last glacial maximum. Molecular Ecology 22:2540–2548.
Furlong, E. L. 1906. The exploration of Samwel Cave. American Journal of Science 172:235–247.
Gavrilets, S., H. Li, and M. D. Vose. 1998. Rapid parapatric speciation on holey adaptive landscapes. Proceedings of the Royal Society of London, Series B: Biological Sciences 265:1483–1489.
Gilbert, M. T. P., D. L. Jenkins, A. Gotherstrom, and N. Naveran. 2008. DNA from pre-Clovis human coprolites in Oregon, North America. Science 320:786–789.
Gingerich, P. D. 1985. Species in the fossil record: Concepts, trends, and transitions. Paleobiology 11:27–41.
Graham, R. W., E. L. Lundelius Jr., M. A. Graham, E. K. Schroeder, R. S. Toomey III, E. Anderson, A. D. Barnosky, J. A. Burns, C. S. Churcher, D. K. Grayson et al. 1996. Spatial response of mammals to Late Quaternary environmental fluctuations. Science 272:1601–1606.
Hadly, E. A. and A. D. Barnosky. 2009. Vertebrate fossils and the future of conservation biology. In G. P. Dietl and K. W. Flessa (eds), Conservation Paleobiology: Using the Past to Manage for the Future. The Paleontological Society Papers. The Paleontological Society, Boulder, CO. 39–59.
Hadly, E. A., U. Ramakrishnan, Y. L. Chan, M. van Tuinen, K. O’Keefe, P. A. Spaeth, and C. J. Conroy. 2004. Genetic response to climatic change: Insights from ancient DNA and phylochronology. PLOS Biology 2:e290.
Harrison, S., J. H. Viers, J. L. Thorne, and J. B. Grace. 2008. Favorable environments and the persistence of naturally rare species. Conservation Letters 1:65–74.
Hickling, R., D. B. Roy, J. K. Hill, R. Fox, and C. D. Thomas. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology 12:450–455.
Hofreiter, M., G. Rabeder, V. Jaenicke-Després, G. Withalm, D. Nagel, M. Paunovic, G. Jambresic, and S. Pääbo. 2004. Evidence for reproductive isolation between cave bear populations. Current Biology 14:40–43.
Huntly, N. and O.J. Reichman. 1994. Effects of subterranean mammalian herbivores on vegetation. Journal of Mammalogy 75:852–859.
Intergovernmental Panel on Climate Change (IPCC). 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Janis, C. M. 2008. An evolutionary history of browsing and grazing ungulates. In I. J. Gordon and H. H. T. Prins (eds), The Ecology of Browsing and Grazing. Springer, Berlin. 21–45.
Jump, A. and J. Penuelas. 2005. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecology Letters 8:1010–1020.
Kuch, M., N. Rohland, J. L. Betancourt, and C. Latorre. 2002. Molecular analysis of a 11 700-year-old rodent midden from the Atacama Desert, Chile. Molecular Ecology 11:913–924.
Lindsay, E. H. 1972. Small Mammal Fossils from the Barstow Formation, California. University of California Publications in Geological Sciences, Berkeley, CA.
Lomolino, M. V. 2005. Body size evolution in insular vertebrates: Generality of the island rule. Journal of Biogeography 32:1683–1699.
Lorenzen, E. D., D. Nógues-Bravo, L. Orlando, J. Weinstock, J. Binladen, K. A. Marske, A. Ugan, M. K. Borregaard, M. T. P. Gilbert, R. Nielsen et al. 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479:359–364.
Lundberg, P., E. Ranta, J. Ripa, and V. Kaitala. 2000. Population variability in space and time. Trends in Ecology & Evolution 15:460–464.
Lyons, S. 2003. A quantitative assessment of the range shifts of Pleistocene mammals. Journal of Mammalogy 84:385–402.
MacFadden, B. J. 1992. Fossil Horses: Systematics, Paleobiology and Evolution of the Family Equidae. Cambridge University Press, New York.
Martin, R. A. 1993. Patterns of variation and speciation in Quaternary rodents. In R. A. Martin and A. D. Barnosky (eds), Morphological Change in Quaternary Mammals of North America. Cambridge University Press, New York. 226–280.
McCain, C. M. and S. R. B. King. 2014. Body size and activity times mediate mammalian responses to climate change. Global Change Biology 20:1760–1769.
McGuire, J. 2010. Geometric morphometrics of vole (Microtus californicus) dentition as a new paleoclimate proxy: Shape change along geographic and climatic clines. Quaternary International 212:198–205.
McGuire, J. L. 2011. Identifying California Microtus species using geometric morphometrics documents Quaternary geographic range contractions. Journal of Mammalogy 92:1383–1394.
McGuire, J. L. and E. B. Davis. 2013. Using the palaeontological record of Microtus to test species distribution models and reveal responses to climate change. Journal of Biogeography 40:1490–1500.
McLachlan, J. S., J. J. Hellmann, and M. W. Schwartz. 2007. A framework for debate of assisted migration in an era of climate change. Conservation Biology 21:297–302.
Menendez, R, A. G. Megias, J. K. Hill, B. Braschler, S. G. Willis, Y. Collingham, R. Fox, D. B. Roy, and C. D. Thomas. 2006. Species richness changes lag behind climate change. Proceedings of the Royal Society of London, Series B: Biological Sciences 273:1465–1470.
Millar, J. S. and G. J. Hickling. 1990. Fasting endurance and the evolution of mammalian body size. Functional Ecology 4:5–12.
Nogués-Bravo, D., J. Rodríguez, J. Hortal, P. Batra, and M. B. Araújo. 2008. Climate change, humans, and the extinction of the woolly mammoth. PLOS Biology 6:e79.
Pagnac, D. 2006. Scaphohippus, a new genus of horse (Mammalia: Equidae) from the Barstow Formation of California. Journal of Mammalian Evolution 13:37–61.
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics 37:637–669.
Parmesan, C. and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42.
Patton, J. L. and P. V. Brylski. 1987. Pocket gophers in alfalfa fields: Causes and consequences of habitat-related body size variation. American Naturalist 130:493–506.
Perrine, J., J. Pollinger, B. Sacks, R. Barrett, and R. Wayne. 2007. Genetic evidence for the persistence of the critically endangered Sierra Nevada red fox in California. Conservation Genetics 8:1083–1095.
Post, E., J. Brodie, M. Hebblewhite, A. D. Anders, J. A. K. Maier, and C. C. Wilmers. 2009. Global population dynamics and hot spots of response to climate change. BioScience 59:489–497.
Richardson, D. M., J. J. Hellmann, J. S. McLachlan, D. F. Sax, M. W. Schwartz, P. Gonzalez, E. J. Brennan, A. Camacho, T. L. Root, O. E. Sala et al. 2009. Multidimensional evaluation of managed relocation. Proceedings of the National Academy of Sciences of the United States of America 106:9721–9724.
Root, T. 1988. Environmental factors associated with avian distributional boundaries. Journal of Biogeography 15:489–505.
Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig, and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421:57–60.
Rosenzweig, C., D. Karoly, M. Vicarelli, P. Neofotis, Q. Wu, G. Casassa, A. Menzel, T. L. Root, N. Estrella, B. Seguin et al. 2008. Attributing physical and biological impacts to anthropogenic climate change. Nature 453:353–357.
Schwartz, O. A. and K. B. Armitage. 2004. Weather influences on demography of the yellow-bellied marmot (Marmota flaviventris). Journal of Zoology 265:73–79.
Sinclair, W. J. 1904. The exploration of the Potter Creek Cave. American Archaeology and Ethnology 2:1–27.
Sinervo, B., F. Méndez-de-la-Cruz, D. B. Miles, B. Heulin, E. Bastiaans, M. Villagrán-Santa Cruz, R. Lara-Resendiz, N. Martinez-Mendez, M. L. Calderon-Espinosa, R. N. Meza-Lazaro et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899.
Smith, F. A. and J. L. Betancourt. 1998. Response of bushy-tailed woodrats (Neotoma cinerea) to late Quaternary climatic change in the Colorado Plateau. Quaternary Research 50:1–11.
Solomon, S., D. Qin, M. Manning, R.B. Alley, T. Berntsen, N.L. Bindoff, Z. Chen, A. Chidthaisong, J.M. Gregory, G.C. Hegerl et al. 2007.
Technical summary. In S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor, and H.L. Miller (eds), Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York, NY.
Springer, K., E. Scott, J. C. Sagebiel, and L. K. Murray. 2010. Late Pleistocene large mammal faunal dynamics from inland southern California: The Diamond Valley Lake local fauna. Quaternary International 217:256–265.
Stock, C. and J. M. Harris. 1992. Rancho La Brea: A Record of Pleistocene Life in California. Natural History Museum of Los Angeles County Science Series No. 37. The Natural History Museum of Los Angeles County, Los Angeles, CA. 113 pp.
Stralberg, D., D. Jongsomjit, C. Howell, M. Snyder, J. Alexander, J. Wiens, and T. L. Root. 2009. Re-shuffling of species with climate disruption: A no-analog future for California birds. PLOS ONE 4:e6825.
Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B. F. N. Erasmus, M. F. de Siqueira, A. Grainger, L. Hannah et al. 2004a. Extinction risk from climate change. Nature 427:145–148.
Thomas, J. A., M. G. Telfer, D. B. Roy, C. D. Preston, J. J. D. Greenwood, J. Asher, R. Fox, R. T. Clarke, and J. H. Lawton. 2004b. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303:1879–1881.
Vermeij, G. J. 1991. When biotas meet: Understanding biotic interchange. Science 253:1099–1104.
Vrba, E. S. 1992. Mammals as a key to evolutionary theory. Journal of Mammalogy 73:1–28.
Willerslev, E., A. J. Hansen, J. Binladen, and T. B. Brand. 2003. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science 300:791–795.
Willerslev, E., J. Davison, M. Moora, M. Zobel, E. Coissac, M. E. Edwards, E. D. Lorenzen, M. Vestergård, G. Gussarova, J. Haile et al. 2014. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506:47–51.
Williams, J. and S. T. Jackson. 2007. Novel climates, no-analog communities, and ecological surprises. Frontiers in Ecology and the Environment 5:475–482.
Williams, J. W., S. T. Jackson, and J. E. Kutzbach. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences of the United States of America 104:5738–5742.
Zachos, J., M. Pagani, L. Sloan, E. Thomas, and K. Billups. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292:686–693.
Zachos, J. C., G. R. Dickens, and R. E. Zeebe. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451:279–283.