3
The Brain on Placebos
Perception of an object costs
Precise the Object’s loss—
Perception in itself a Gain
Replying to its Price—
The Object Absolute—is nought—
Perception sets it fair
And then upbraids a Perfectness
That situates so far—
—Emily Dickinson, “Perception of an Object Costs,” 1866
At the start of the new millennium, a series of groundbreaking neuroimaging studies moved placebo effects from the domain of behavior to the business of the brain. As we saw in the last chapter, expectations and associative learning powerfully influence placebo effects. But how do these behavioral influences coupled with an inert pill or sham intervention induce brain signaling to produce placebo effects?
The brain during pain is a particularly useful model for studying placebo effects because pain is easily manipulated in laboratory settings, and the neural process of nociception (the perception of pain from a noxious stimulus like heat or mechanical pressure) is experimentally reliable and well studied. Thus most placebo neuroscience research has focused on pain and placebo-induced pain relief or analgesia in healthy controls.
With elaborate ruses designed to manipulate expectations or induce associative learning, placebo neuroscientists have used neuroimaging to examine the brain before, during, and after a painful stimulus plus placebo treatment. Over the last two decades, these elegant and ingenious experiments linked distinct brain regions with responses to a placebo. The findings of these studies demonstrated that our brains form predictions of how an incoming stimulus will be experienced based on contextual cues, prior experiences, and expectations. These predictions are encoded in neurological activity driving mechanisms that override the information carried in incoming pain signals to shape our perception. So thermal heat at the same temperature can be experienced as more or less painful depending on what we think about the nature of the pain we are about to experience.
In this chapter, I will concentrate first on neuroimaging studies of placebo analgesia and a recent working model used to explain what is happening to the brain on placebo. I will then discuss changes in the brain related to placebo-induced symptom relief in conditions like Parkinson’s disease or depression. Let’s start with a little background.
The Brain in Pain
The human brain is a uniquely complex organ that on the surface, appears not to be doing a whole lot. On closer inspection, however, by recording from single nerve cells or using neuroimaging techniques like functional magnetic resonance imaging (fMRI) or positron emission tomography (PET), we see that the eighty-six billion nerves or neurons, neatly organized by function, are in constant communication. Therefore neuroimaging allows us to objectively examine the mechanisms involved in generating placebo effects.
Information moves within as well as between neurons by electric signaling and neurotransmitters. Neurotransmitters are small molecules or chemicals produced in the body that are passed from one neuron to the next, exciting or inhibiting downstream signaling by binding to proteins on the surface of neurons called receptors. The two main neurotransmitters implicated in placebo effects are opioids and dopamine. Opioids bind to opioid receptors and are involved in the transmission of pain relief signals. The mu-opioid receptor is the most widely studied type of opioid receptor, and is responsible for binding to endogenous (made in the body) opioids and painkillers like morphine. Dopamine binds to dopamine receptors, and is involved in signaling related to reward and physical movement. Though dopamine and opioids are the most commonly studied in placebos, other molecules that have effects in the brain, like the hormone vasopressin, have recently caught the interest of neurobiologists and been shown to have effects on placebo responsiveness.1
Damage to bodily tissues causes pain or nociception. This type of pain is sensed by specialized nerve cells or neurons called nociceptors. Nociceptors carry pain signals up from the peripheral regions of the body to the central nervous system, which consists of the spinal cord and brain. Pain signals that enter the spinal cord at the dorsal horn are routed up to the base of the brain or brain stem. From there they are directed to the “pain matrix,” a group of brain regions involved in the emotional, cognitive, and motor aspects of pain experience.2 The regions of the pain matrix are linked by neural circuits and include the thalamus, which relays motor and sensory pain signals; hypothalamus, a major control center connecting hormones to the nervous systems that regulates body temperature and blood pressure; amygdala, involved in emotional responses, particularly feelings of anxiety, fear, pleasure, and response to threat; insula, which mediates emotion, empathy, and interpersonal experience; somatosensory cortex, which receives and processes pain; and the anterior cingulate cortex (ACC), involved in emotion, empathy, and decision-making. As we will soon see, regions of the pain matrix are also recruited in the processing of placebo-induced pain relief or analgesia.
Opioids and Pain
Pain is an almost universal human experience. Though it manifests uniquely in individuals, pain is one of the most common reasons patients seek medical attention. The most enduring and popular of pain treatments comes to us from the poppy plant, which contains opium, a highly addictive pain-relieving narcotic. The opiates morphine, codeine, and heroin are derived from the resin of the poppy seed capsule. While opiates are naturally occurring, the endogenous opioids enkephalin and endorphin are made in the human brain, and opioids like OxyContin, hydrocodone, fentanyl, and remifentanil are synthetic. Like opiates and synthetic opioids, endogenous opioids modify pain signals by binding to opioid receptors, proteins located on the surface of neurons. Many of the brain regions associated with pain-signal processing have high concentrations of opioid receptors, making them natural targets for pain relievers and placebo effects.
Placebo Analgesia in Neuroimaging Studies
To carry out placebo studies in the laboratory setting, neuroscientists developed a working model of pain stimulus and placebo analgesia.3 The general format of these studies starts with a scan of the brain to determine baseline activity. This is followed by a training phase in which a painful stimulus (e.g., thermal heat) is delivered to a readily accessible region on the body of the subject (e.g., the forearm) while they are in a neuroimaging scanner. The subject is asked to rate their pain on a visual analog scale from zero, the equivalent of “no pain,” to a hundred, indicating the “highest imaginable pain” intensity. The brain is scanned to determine activity with pain exposure. In expectation experiments, the subject is then given a placebo cream or injection with verbal suggestions that create an expectation of benefit, or reduction in the experience of pain from the intervention. Unbeknownst to the subject, the painful stimulus is then reduced so the subject begins to think that the placebo is actually effective at reducing the experience of pain. This step is sometimes repeated to reinforce the association between the placebo intervention and reduction in pain. After this associative learning phase, there is a testing phase in which the subject is given the original level of pain along with the now deemed “effective” placebo intervention. The brain is scanned several times throughout the experiment to determine differences in brain activity with and without expectation of protection from the painful stimulus. The difference between the level of pain experienced before and after the placebo intervention is considered a measure of the placebo effect.
In the over three hundred placebo neuroimaging studies, there have been numerous iterations of the experimental model of pain and placebo analgesia. There have been variations in types of pain (e.g., pressure, heat, or electric shock), instructions (e.g., positive or negative), and conditioning cues (e.g., syringe, cream, or pill). Some studies use associative learning with a powerful painkiller like morphine or remifentanil to maximize the placebo effects or a drug that blocks opioid signaling like naloxone to block the placebo effects. Today, neuroimaging studies coupled with increasingly sophisticated and powerful computational models are revealing the inner workings of the brain during placebo analgesia.
Brain Activity during Placebo Analgesia
Placebo neuroscientists are able to take advantage of the binding of neurotransmitters to their receptors by using radioactively labeled versions of neurotransmitters to track the receptor-binding activity as a proxy for receptor activity. Radioactive carfentanil is a radiopharmaceutical that is molecularly similar to the synthetic opioid fentanyl and can be detected using PET.4 In PET brain imaging, subjects are infused with a radiopharmaceutical that emits gamma rays detected by gamma cameras to create a three-dimensional image of the brain. After over twenty years of neuroimaging studies using PET and other modalities, the specific regions of the brain involved in placebo-induced analgesia have been revealed.5
An early clue that endogenous opioids were involved in placebo analgesia came from studies in the late 1970s that found that pretreatment with the drug naloxone could abolish placebo effects.6 Naloxone is a drug that works by blocking opioid binding to mu-opioid receptors and is used today under the trade name Narcan to reverse opioid overdoses. The finding that naloxone can also reverse placebo responses has been replicated numerous times. We now know that placebo effects induced by both expectation and conditioning with the opioid morphine can be at least partially blocked by naloxone.7
In one of the earliest neuroimaging studies, brain activation in response to a placebo was compared to that seen with remifentanil, an opioid with analgesic potency comparable to fentanyl.8 In this study, thermal pain administered a few seconds before a PET scan reliably demonstrated increased activity in regions of the pain matrix, including the thalamus, insula, and ACC. Before the administration of subsequent painful stimuli, subjects were told they would receive an injection of one of two powerful painkillers. In actuality, the painkillers were remifentanil or placebo saline. Both remifentanil and the placebo effectively reduced the pain experienced by the subjects. Remifentanil and the placebo also induced similar increases in brain activity in the rostral anterior cingulate cortex (rACC, a subsection of the ACC that has a high density of opioid receptors and is activated with opioid treatment) and periaqueductal gray (PAG, an area in the brain stem that plays a role in motivated behavior and response to threatening stimuli, and is important in descending pain modulation). The observation that the activation of the rACC covaried with the activation of PAG provided early evidence that placebo effects could lead to the interception of incoming pain signals and exert “top-down” control over the pain. In top-down processing, our brain’s expectations and prior knowledge reinterpret or change the perception of an incoming sensory signal. Put simply, placebos allow our brains to experience sensory signals for pain as less painful.
Further placebo analgesia experiments revealed that the coactivation of the rACC and PAG was correlated with multiple other factors: it was proportional to the magnitude of the placebo response, correlated with reduced signaling in the pain matrix somatosensory cortex, could be blocked by naloxone, and was associated with reductions in activity in the rostral ventromedial medulla (RVM), a key structure located in the spinal cord that is also involved in the top-down pain modulation system.9
The Very Major Placebo Frontal Commander
Tor Wager is among the placebo neuroscientists who have generated substantial evidence that the ventromedial prefrontal cortex (vmPFC), located proximal to the rACC, is a key region in integrating memory, prior experience, context, expectations, and incoming sensory information to generate placebo effects. As a main change center, it appears that the vmPFC uses this integration of information to override sensory inputs, changing the way we think about our surroundings and experiences. In this top-down manner, the vmPFC redirects our interpretation of the moment. Thus it is not surprising that the vmPFC, or what I call the “very major placebo frontal commander,” has emerged as a central region of interest in placebo research (figure 9).
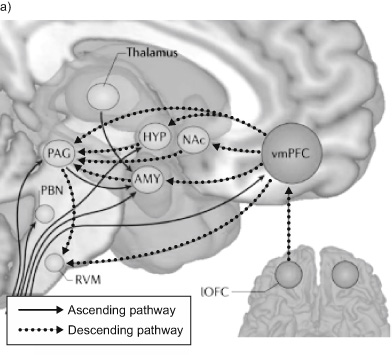
Figure 9a Converging ascending and descending pathways. NAc is nucleus accumbens, HYP is hypothalamus, AMY is amygdala, PAG is periacqueductal gray, RVM is rostral ventral medulla, PBN is parabrachial nucleus, and lOFC is lateral orbitofrontal cortex, part of the prefrontal cortex. Source: T. D. Wager and L. Y. Atlas, “The Neuroscience of Placebo Effects: Connecting Context, Learning and Health,” Nature Reviews Neuroscience 16, no. 7 (July 2015): 403–418, https://doi.org/10.1038/nrn3976.
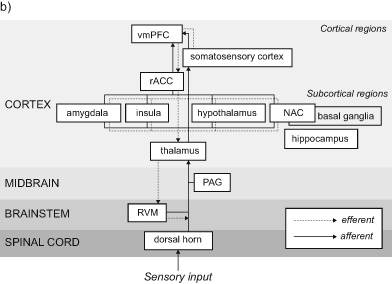
Figure 9b A simplified map of the brain regions active in a putative placebo effect network. In pain, incoming nociceptive signals (red lines) ascend to pain-processing brain regions that include the amygdala, thalamus, insula, and somatosenory cortex. The top-down modulation of the incoming pain signals is influenced by the vmPFC and rACC cortex, which project to pain-processing centers, which in turn project to PAG, RVM, and ultimately the dorsal horn of the spinal cord.
One way to invoke top-down pain modulation is through cognitive reappraisal. An example of using cognitive reappraisal in medical settings is encouraging subjects or patients to think about a painful stimulus or symptomatic pain as a “warm blanket on a cold day” rather than as a sharp pain. In trials of cognitive reappraisal across disease models, the vmPFC is consistently activated. Further, the vmPFC is associated with the control of emotional responses, self-control, and decision-making, and is highly connected to the rest of the brain.10 Increasing supportive care and touch are two factors of the therapeutic relationship demonstrated to activate the vmPFC.11 Interestingly, structural differences in the vmPFC have been observed in multiple conditions known to have a high placebo response in clinical trials, including depression, attention deficit hyperactivity, substance misuse or addiction, schizophrenia, and dementia.12 Outside psychiatric challenges, the vmPFC has been shown to be activated during meaning-related tasks like the formation of therapeutic relationships.
Top-Down Processing and Placebo Effects
In top-down processing, perception is driven by cognition; we leverage what we already know or have experienced to build a model or expectation that shapes what we experience. To get a better sense of how top-down processing works, take a look at the two boxes below.

We read the context of the top box as a sequence of letters and might perceive the middle character to be the letter B in the sequence A, B, and C. In the lower box, we see a sequence of numbers and read the same character in the first box, now displayed in the middle of a string of numbers, as the number 13.

What does top-down processing have to do with placebo effects? As we will see in the upcoming sections, behavioral and neuroimaging experiments provide compelling evidence that placebo analgesia is a special case of a top-down modulation of pain signals. This evidence dates back to work done by Howard Fields in the 1960s in which he demonstrated that analgesia could be induced by direct electrophysiological stimulation of the PAG. The PAG is the same region in the brain stem that years later was found to have increased opioid signaling in concert with the rACC during placebo-induced pain relief.13 Fields proposed that incoming pain signals traveling up through the spinal cord could be perturbed in a top-down fashion. More recent neuroimaging studies showed that this top-down pain modulation could, like placebo analgesia, be blocked by naloxone. To examine how top-down modulation works, placebo neuroscientists like Christian Buchel turned to a framework or model called predictive coding.
Predictive Coding toward a Model of Precision and Placebo Analgesia
It is intriguing to consider where Emily Dickinson (1830–1886) in her “Perception of an Object Costs” derived the inspiration for what arguably is the most succinct and elegant explanation of predictive coding. Perhaps it was through her close relationship with the mathematician Susan Gilbert that Dickinson learned of the theories of Hermann von Helmholtz (1821–1894). Helmholtz was a German physician and physicist who in the 1860s argued that our perceptions were not a direct projection of the world around us but rather a constructed model of the world based on a succession of learned “unconscious inferences.”14
More recently, British neuroscientist Karl Friston, building on Helmholtz’s theory, proposed that our brains are prediction machines. Friston contends that in order to keep us safe and able to operate efficiently in a noisy, stimulus-filled world, we cut down on background noise and focus in on the most important stimuli in a given instant.15 To be able to do this, our brains construct models of what we will experience in the upcoming moment. Instead of waiting to take in and process all available information, we predict our experiences—what we will see and how we will feel—sometimes before we are fully aware that there is something to see or feel. In so doing, we constrain our perceptions and reactions to what we expect will happen next. In concentrating on only a subset of incoming information, we are more efficient with our finite energy resources.
As one of the most recent and compelling models of placebo analgesia, predictive coding benefits from the power of neuroimaging to capture the timing and magnitude of brain activity in response to stimuli that vary in intensity, and thus vary in precision. Placebo neuroimaging studies designed to test the validity of using a predictive coding model in placebo analgesia not only support predictive coding as a model that explains how placebo effects work but also demonstrated that the precision or surety of our expectations can have a profound influence on the nature of our experience.
We predict our experiences—what we will see and how we will feel—sometimes before we are fully aware that there is something to see or feel.
The model holds that the expectations induced by associative learning and/or placebo treatment recruit top-down opioid-mediated signaling at key regions in the spinal cord, limiting incoming signals to the pain matrix. In so doing, a patient’s experience of pain can be reduced by their expectations. Hence the precision of a subject’s expectation can be manipulated by repeatedly exposing them to variable pain levels during the associative learning phase of an experiment.16 For instance, subjects who get consistently lowered heat stimuli with the administration of a placebo cream have a more precise expectation and consequently more robust placebo response. In contrast, subjects exposed to greater variability in heat stimuli levels during the associative learning phase of the experiment have a weaker placebo response. This study therefore supported the hypothesis that both the expectation and incoming stimulus are weighted by their relative precisions to shape the experience of pain and placebo effects. Dickinson, ahead of Helmholtz and Friston, alludes to this transactional nature of precision in the relationship between “the object absolute” and how we situate or “set it fair” with our perception: “Precise the Object’s loss— / Perception in itself a Gain / Replying to its Price.”
Other Conditions and Models
While pain is a convenient model to study placebos, placebo analgesia studies are limited by being mostly performed in healthy volunteers. Understanding the neurological mechanisms in conditions that are characterized by high placebo response rates in clinical trials, like Parkinson’s disease and major depression, is critical to being able to identify and evaluate effective treatments for these illnesses.
Parkinson’s is a neurodegenerative disease characterized by reduced motor function with tremors, slowness, and general difficulty controlling movement. A common neuropathic anomaly in patients with Parkinson’s disease is a loss of dopamine-signaling neurons specialized in instructing and implementing motor functions. The placebo effects in Parkinson’s disease are high but variable, ranging from 0 to 55 percent, and can result in tangible long-term improvements in motor function.17
In a seminal study on placebo effects in Parkinson’s disease, the placebo treatment induced the release of endogenous dopamine in the areas of the brain responsible for planning movement, learning, reward, motivation, memory, emotion, and action.18 Not surprisingly, the magnitude of the dopamine release was greater in the patients who reported a placebo benefit compared to those who did not. These findings, replicated in numerous subsequent studies, point to dopamine and dopamine signaling in the striatum (a brain region involved in decision-making and movement) as another important neurological region involved in the production of placebo effects.19 In placebo studies in patients with Parkinson’s, it appears that the expectation of receiving a reward (a benefit from the drug), not the reward itself, is linked to increased dopamine release in the ventral striatum. Recordings from individual neurons revealed that the response to a placebo was associated with changes in the basal ganglia circuit, a region implicated in Parkinson’s disease.
Major depression is another condition characterized by a high placebo response in clinical trials.20 Studying the antidepressant effects of placebos is difficult because of the typical delay in responses to pharmaceutical treatment, which tend to occur across weeks to months rather than within minutes to hours. Still, several studies have made important contributions to our understanding of placebo-induced neurological mechanisms in depression. In an early placebo antidepressant imaging study, patients with depression were scanned using PET before and after a six-week treatment with the antidepressant fluoxetine (Prozac) or a placebo.21 In this pivotal study, placebo and fluoxetine responders showed overlapping neural activity in several brain regions, suggesting common pathways shared by placebo and antidepressant treatments.
Since this early study, there has been extensive research to assess the placebo response in individuals with depression, particularly because the rates of a placebo response in patients with depression continue to be quite high, stumping the development of novel antidepressants. Recent PET studies found that antidepressant placebo effects in patients with depression increased brain activity through the transmission of both dopamine and opioid neurotransmitters.22 These results support theories that the opioid and dopaminergic pathways are involved in placebo responses, though they might have separate and compounding effects.
The Brain and Placebos
Pain, Parkinson’s disease, and depression are models that show significant changes in the brain in response to placebo treatments, allowing for the dissection of mechanisms underlying placebo effects in healthy controls and patients, but they are not the only ones that show neurobiological changes. Additional placebo effect models are being developed in other conditions, including irritable bowel syndrome (IBS), asthma, and Alzheimer’s disease. These models suggest that there are multiple disease-specific and common pathways engaged in the formation of placebo effects. Importantly, an expectation of benefit plays a critical role in the magnitude of the alleviation of the perceived symptoms and response to placebo treatment across disease models. Expectations are significant, and the mechanism that placebo recruits is powerful. The vmPFC has emerged as a key region in using our expectations to shape placebo effects. Thus we might consider the vmPFC to be a back door to how we see and experience the world—a back door that is susceptible to being hijacked by manipulative messaging. While underlying placebo effect mechanisms can be used for good, negative information and expectations can have the opposite and sometimes negative effects. This phenomenon, termed the nocebo effect, is where I begin in the next chapter.