7
Who Responds?
We found that there were no differences in sex ratios or in intelligence between reactors and nonreactors. There are however significant differences in attitudes, habits, educational background, and personality structure between consistent reactors and nonreactors.
—Henry Beecher, “The Powerful Placebo,” 1955
The Placebo Reactor
The question of who responds to a placebo treatment is a complex one. While the answer could revolutionize clinical care and drug development, this question is not top of mind among clinicians, drug developers ignore it until it’s too late, and academic placebo researchers tend to tackle the problem from their respective vantage points. Placebo psychologists look for answers in behavioral and personality differences, placebo neuroscientists look for brain signatures, and placebo geneticists seek genetic variants. More recently, interdisciplinary studies aided by machine learning (a type of artificial intelligence that learns from data and can identify patterns) are combining psychological, neurobiological, and genetic perspectives to predict who will respond to placebos in clinical trials.
As clinical trials increasingly fail to beat placebos, the race to identify predictive features becomes more urgent. Such prediction tools are becoming more attractive to trialists, and drug developers are seeking to beat placebos and counteract the threat to drug development that they pose. Several of these methods are patented and form the basis for companies. But how well do these prediction methods work, and how can physicians and drug developers use them effectively? In this chapter, I focus on what we know about placebo responders, and consider the options and subsequent challenges for what we will do when we can accurately predict who responds.
Is There a Placebo Responder Personality Type?
The short answer is not really. Findings across the scores of studies looking at personality and placebo responders have not been consistent. This is in large part due to the small study sizes with large heterogeneity across participants as well as the experimental and clinical conditions. Study participants vary by age, sex, geographic location, health status (i.e., healthy or a patient), and disease severity. Study protocols can vary by the duration of treatment, dosage, delivery of the intervention (i.e., pill or surgery), study details conveyed in the informed consent, phase of the trial, attitude and bedside manner of the clinician, and so on. Added to this is the tension in academics between the pressure to produce innovative, exploratory work, and the necessity to replicate and validate previous findings before they can be translated to clinical practices. Thus grants and journals put a premium on novelty. Replicating a finding once or twice to validate a result is important. Beyond that, repeating the same study over and over again is considered a crisis of creativity. So we have a lot of small studies that examine numerous experimental models across healthy controls and clinical conditions, making the translation of these findings to clinical practices tedious and currently beyond reach.
Psychological Markers of the Placebo Responder
You might argue that if there was a common placebo personality type, it would emerge despite the heterogeneity, but this hasn’t happened. Still, in the field of personality of placebo responders, there are some important and intriguing trends. The “big five” is a way of categorizing broad personality traits. The five traits are: openness to experience, which describes people who tend to be inventive and curious as opposed to consistent and cautious; extraversion, which captures people who are outgoing and energetic versus solitary and reserved; agreeableness, which portrays people who are friendly and compassionate versus critical and rational; conscientiousness, which is associated with people who are efficient and organized versus extravagant and careless; and neuroticism, which relates to people who are sensitive and nervous versus resilient and confident. Of the big five personality factors, openness to experience and extraversion are the two most frequently observed in placebo studies.1
Optimism and pessimism represent the two ends of another useful personality scale: the life orientation test. Dispositional optimism, which measures a generalized positive outcome expectancy for the future, is often associated with a placebo response.2 Conversely, pessimism, which measures a tendency to have negative expectations about the future, is associated with a nocebo response.3 Several other psychological traits have been examined in placebo studies, but few have been replicated. Among these studies, suggestibility, expectation, and a desire for relief were also found to be more prevalent in participants who respond to a placebo.
A dynamic transactional model was proposed as one way to better use personality traits to predict and understand placebo responses. In this model, personality traits are grouped into two constructs: “inward” and “outward.” Inward traits, like suggestibility, absorption, and acquiescence, are associated with greater sensitivity to interior states (how one feels inside). Outward traits, like extraversion, optimism, altruism, and ego resiliency, are associated with a greater permeability to external input. In this dynamic transactional model, individuals would be more or less sensitive to a given placebo treatment depending on the context and environmental cues associated with the treatment. The applicability of the dynamic transactional model is exemplified in a clinical trial in IBS that looked at three “doses” of a placebo and found that the outward trait of extraversion was associated with only one type of placebo treatment.4
What Does Neuroimaging Tell Us about Placebo Responders?
The observation that the positive effect of a practitioner can have a greater effect on a patient’s symptoms as compared to a placebo alone is not new, but it suggests that at least in part, a placebo response is a state—a temporary way of being (e.g., hungry), rather than a trait, or a more stable and enduring feature (e.g., eye color). Still, there is good evidence that brain structure and connectivity—traits that are amenable to change over time—can predict a placebo response.
Modern neuroimaging techniques allow for the identification of some of these structural and functional differences that can underlie the responsiveness to treatment. One such technique, functional connectivity, is based on fMRI, and utilizes the magnetic properties of hemoglobin to image changes in blood flow that co-occur with activity to evaluate the extent to which regions are functionally connected. In these studies, the implication is that regions that are active simultaneously, or are “coactivated,” are connected to each other, serving as a proxy for “connectivity.” In a series of two studies of patients with chronic knee osteoarthritis, neural connectivity during pain exposure was found to predict a placebo response.5 The greatest difference between responders and nonresponders was the magnitude of connectivity between the right middle frontal gyrus, a prefrontal cortical region involved in decision-making, memory, and planning, and the rest of the brain.
Using Computational Tools to Predict Placebo Responders
With the recognition that a placebo response is multifactorial, the quest for tools to predict placebo responders turned to using composite scores and machine learning to identify combinations of demographic, psychological, and neuroimaging measures that prospectively predict who responds to a placebo.
One study of patients with chronic lower back pain used machine learning to find combinations of neural or psychological traits that predicted responses to taking placebo pills.6 The participants were randomized to blinded placebo pills or no treatment, and asked to report their back pain on a phone app over the fifteen months of the study. The placebo pill patients had greater improvements in pain intensity compared to the no-pill control participants. None of the traditional psychological measures typically observed in experimental pain studies predicted the treatment response. Instead, the placebo response was predicted by the combined effect of subscales related to openness, emotional awareness, the ability to describe inner experience, sensitivity to nonpainful situations, and reduced distraction about pain and discomfort.
In the same study, neuroimaging data obtained at the baseline, before the participants were randomly sorted into groups, showed that there were differences in anatomy and connectivity in the brain that could predict placebo responses. Some of these features included cortical thickness in sensorimotor-related regions, volume asymmetry in subcortical areas, and connectivity differences between pain-associated areas, including parts of the PFC, ACC, and PAG. While the psychological and neurological models were independently predictive of placebo responses, they were not correlated, and the addition of imaging to the psychological data did not improve the predictability of the model. Thus, while it would be simpler and certainly less expensive to use a psychological test as a proxy for the neuroanatomical and neurophysiological biomarkers, this last observation suggests that these measures are predictive of distinct elements but not placebo responses as a whole.
In another study using machine learning to model placebo reactors, education was found to be a strong predictor of placebo responses in patients with late-life depression.7 In this study, among those patients with more than twelve years of education, the placebo almost outperformed the active medication. In another study of depression in adolescent patients, the placebo response was predicted by a composite score of sex, age, being nonwhite, having a history of depression, and the baseline depression score.8 Machine learning requires large data sets, and because of the preponderance of small studies, replication of these findings in the short term is unlikely. In many studies, the baseline disease severity score is a strong predictor of a response to placebo treatment.9 This is quite likely because patients seek help and are willing to enroll in clinical trials when their symptoms are at their worst. While this might seem like it is just regression to the mean, in many cases controlling for the baseline symptom severity does not eliminate the whole effect of a placebo treatment.
Genetics and the Placebo Response: The Garbage Gene
Enzymes are a type of protein that carry out chemical reactions in the body. Catechol-O-methyltransferase (COMT) is the major enzyme that eliminates dopamine in the PFC, one of the regions activated with a placebo treatment. As discussed in the chapter on the neurobiology of placebo responses (chapter 3), dopamine signaling is induced in some participants who respond to a placebo treatment. Most of this signaling takes place in the PFC. One hypothesis is that if variation in the COMT gene influences the dopamine function in the PFC, it could influence a response to a placebo treatment too. The COMT protein also metabolizes neurotransmitters and hormones that are important in the cardiovascular, autonomic nervous, and endocrine systems. COMT effects are so frequently observed in psychological, neurological, cardiovascular, and even a few cancer studies that some researchers dubbed it the “garbage gene.” But a garbage gene that, like placebos, is associated with many outcomes across many conditions, is precisely what one might look for in a placebo gene.
Genes are made up of four different molecules called nucleotides, abbreviated as A, C, T, and G. The combinations of these nucleotides across our twenty-three pairs of chromosomes make up the genetic code. Small changes in the nucleotide sequence called polymorphisms can change the way a gene, or the protein it makes, functions. These changes happen in nature all the time. A majority of them are not harmful and simply add to human diversity. Sometimes these small changes can have more subtle effects. Though the findings are inconsistent, there is some evidence that genetics might influence some behavior, like the responsiveness to a placebo.
A common single nucleotide polymorphism in the COMT gene called rs4680 changes a G nucleotide to an A around the middle of the gene. This change results in two different versions of the gene and the proteins they encode. The valine (val) version is more efficient at metabolizing dopamine than the methionine (met) version. Hence individuals who are homozygous for the val version of the enzyme (val/val) break down dopamine three to four times more efficiently than individuals who are homozygous for the met (met/met) version. As a result, met/met individuals tend to have more dopamine available in their PFC than val/val ones.10 Heterozygous individuals, val/met, have an intermediate level of PFC dopamine.
In an IBS study examining the relationship between a placebo response and the COMT genotype among three placebo treatment arms (no treatment, sham acupuncture alone, or sham acupuncture plus a supportive clinician-patient interaction), met/met participants were found to have a greater placebo response than val/met and val/val participants, and this difference was greatest in the supportive treatment arm.11 This finding was replicated in a recent IBS clinical trial in which met/met participants had a greater response to double-blind placebo pills than val/met and val/val participants (figure 10).12
Gene-Drug Interactions
We can take an example from our own work where placebos have relieved pain arising from physiological cause (surgical incision) and show how useful the screening out of reactors can be. I, with Keats, Mosteller, and Lasagna, in 1953, administered analgesics by mouth to patients having steady, severe, postoperative wound pain, and we found that when we took all patients and all data we could not differentiate between certain combined acetylsalicylic acid data and narcotic (morphine and codeine) data; however, when we screened out the placebo reactors, a sharp differential emerged in favor of the acetylsalicylic acid administered orally as opposed to the narcotics administered orally.
—Henry Beecher, “The Powerful Placebo,” 1955

Figure 10 The genetic variation in COMT rs4680 (val158met) is associated with a differential response to a placebo treatment in IBS. Source: J. Vollert, R. Wang, S. Regis, H. E. Yetman, A. Lembo, T. Kaptchuk, V. Cheng, et al., “Genotypes of Pain and Analgesia in a Randomized Trial of Irritable Bowel Syndrome,” Frontiers in Psychiatry 23 (March 2022), https://www.frontiersin.org/articles/10.3389/fpsyt.2022.842030/full.
COMT rs4680 effects are not limited to placebo treatment in IBS. The association with a response to a placebo treatment has been observed across several conditions. Interestingly, the directions of these effects not only differ across conditions but also appear to be further modified by some drugs. Examples of this include trials of tolcapone for memory, propranolol for pain, clonidine for chronic fatigue syndrome (figure 11), aspirin for cardiovascular disease prevention, and vitamin E for cancer prevention.13 In these studies, the direction of the effect differs by the treatment arm such that in some studies, the met/met participants respond to a placebo but do worse on a drug. In other studies, the val/val participants do better on a placebo but worse on a drug. Because clinical trials typically compare the average effects in the active and placebo treatment arms, these differential results are masked when we only consider the net effects in the drug and placebo arms.
One of the fundamental assumptions of the randomized placebo-controlled clinical trial is that the drug and placebo responses are additive. That is why we can simply subtract the clinical outcome in the placebo arm from that observed in the drug treatment arm to determine the efficacy of the treatment. But what if in addition to naloxone, there are other drugs that perturb the placebo response? And what if, as we saw here with the COMT gene and tolcapone, propranolol, clonidine, aspirin, and vitamin E, this pharmacogenomic effect varies depending on the genotype, disease or condition, and drug being studied? Could differential pharmacogenomic effects in the drug and placebo arms mask the true effect of the drugs in genetically defined subpopulations? These pharmacogenomic interactions and their influence on outcomes in clinical trials are precisely what precision medicine is designed to address. To date, however, precision medicine has failed to fully account for the contribution of genetic effects in the placebo arms of clinical trials. Perhaps it’s time for us to critically examine the potential for these placebo-related gene-drug interactions to influence how we determine the efficacy of drugs.
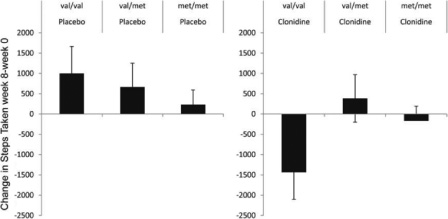
Figure 11 In patients with chronic fatigue syndrome, the change between the baseline and outcome after an eight-week treatment is plotted by COMT genotype and treatment type. Source: K. T. Hall, J. Kossowsky, T. F. Oberlander, T. J. Kaptchuk, J. P. Saul, V. B. Wyller, E. Fagermoen, et al., “Genetic Variation in Catechol-O-methyltransferase Modifies Effects of Clonidine Treatment in Chronic Fatigue Syndrome,” Pharmacogenomics Journal 16, no. 5 (October 2016): 454–460, https://doi.org/10.1038/tpj.2016.53.
A placebo response, as we are learning, is a complex phenotype, and in addition to the many demographic and psychological variables that might influence a placebo response, there are likely many other genes that contribute to this outcome. Although placebo genomics is in its infancy, a review of the literature shows that there are already many other genes that influence outcomes in the placebo arms of clinical trials and potentially interact with the drug being tested. In network medicine, proteins with similar functions form modules with distinct properties that can shed light on their mutual function. Network analyses of the proteins encoded by genes that influence a placebo response, termed the placebome (genes or the proteins that they encode that influence a placebo response), can be identified in the network of all known proteins called the interactome. The placebome module, or genetic subnetwork, appears to be proximal and sometimes overlaps with diseases and conditions known to have high placebo response rates. The placebome also maps proximal to modules of several drug classes, providing one explanation for why so many drugs appear to perturb the placebo response (figure 12).14
Perhaps it’s time for us to critically examine the potential for these placebo-related gene-drug interactions to influence how we determine the efficacy of drugs.

Figure 12 This table shows drug target categories that are significantly proximate to the placebome module in the human interactome. Note: P values were adjusted using the Bonferroni procedure. Source: R. S. Wang, K. T. Hall, F. Giulianini, D. Passow, T. J. Kaptchuk, and J. Loscalzo, “Network Analysis of the Genomic Basis of the Placebo Effect,” JCI Insight 2, no. 11 (June 2, 2017), https://doi.org/10.1172/jci.insight.93911.
If We Could Predict Placebo Responders, What Would We Do Next?
While identifying predictors of the placebo response is vital to understanding who may benefit from a placebo treatment and improving the design of clinical trials, the use of such a tool invites several questions and potential ethical concerns. If we identified a subset of the population that responded to placebos, would it be excluded from clinical trials? If this subset was excluded, would the drugs that get developed based on these trials require a black box label? And if this was the case, what drugs would we prescribe to placebo responders? Would it be ethical for them to receive a placebo? Would they have to be told that they are placebo responders? If we told them they were placebo responders, would that change the nature of their response? If one is a placebo responder in a depression trial, would they be one for a hypertension trial? Once a placebo responder, are you always a placebo responder? What group of genes influence a placebo response, and are there gene-gene as well as gene-drug interactions? Which drugs perturb a placebo response? Can we use drugs to boost a placebo response?
Clearly there are many questions we can ask. With evidence in some conditions that the placebo response is increasing in clinical trials, it likely means that there is more to the placebo response than the stable traits of genetics and brain structure. But who will invest time and resources into addressing these questions?