 8
8 
 8
8 
Proteins have many effects on food and cooking, from stabilizing foams and emulsions to firming up gels. Knowing a little bit more detail about proteins will help you design and modify recipes and fix things that go wrong.
Proteins are made up of amino acids. An amino acid has a central carbon, with a carboxyl group (COOH) at one end and an amine group (NH2) at the other end. The simplest amino acid is glycine.
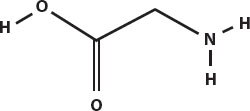
Notice that at the left end, there is a hydrogen attached to an oxygen. At the right end there is a hydrogen. Amino acids can join together by joining end to end and losing a water molecule—the OH at the left and the H at the right.
For example, two glycines can join to form diglycine. In the first drawing below, the box shows where the two glycines will join together.
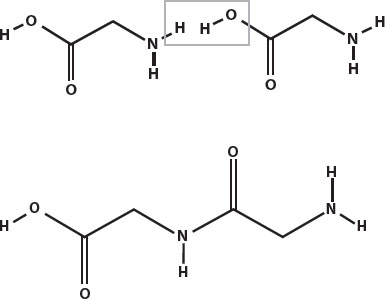
This kind of bond is called a peptide bond, and it is very strong. When only a few amino acids are joined together in this way, the molecule is called a polypeptide. Longer polypeptides are called proteins.
There are about 22 different amino acids found in the proteins that make up our bodies and the foods we eat. These amino acids differ from one another in one particular way: the things that are attached to the carbon right next to the nitrogen. In glycine, there is just a hydrogen atom there.
 Chemistry Lesson
Chemistry LessonThe sequence of amino acids in a protein gives it what we call the primary structure of the protein. You can think of the primary structure as a string of beads, in which each bead is an amino acid.
In some amino acids, long chains of atoms are attached to the carbon atom next to the nitrogen. These chains can form their own bonds with one another, to create what is called the secondary structure. Two common secondary structures are the alpha helix, in which the protein forms a coiled spring, or helix, and the beta sheet, in which the strings of beads bond parallel to one another to form sheets. These forms are held together with hydrogen bonds (see page 39).
The tertiary structure of a protein is the form it takes when it folds into a three-dimensional shape. Soluble proteins usually have a globular or almost spherical tertiary structure. As mentioned earlier, egg albumin is globular; there is also a whole class of proteins that are called globulins because of their shape. Insoluble proteins—such as collagen in connective tissue, elastin in tendons and arteries, and keratins in hair, hooves, and nails—have a fibrous tertiary structure.
Some proteins combine with other molecules to form conjugated proteins. In the nuclei of cells, for example, proteins combine with nucleic acids to form nucleoproteins. Proteins can combine with just a little carbohydrate to form a glycoprotein, or with more than about 4 percent carbohydrate to create mucoproteins. Combined with fat, they are lipoproteins. The structural properties of these combinations result in the quaternary structure of a protein—the last of the four kinds of protein structure
In their natural state, proteins like egg albumin and milk casein are soluble in water. Most of their hydrogen bond-forming parts are tucked inside the folded structure of the protein, making them unavailable for forming bonds with other molecules. They are all the same shape, so they all have the same properties and can form crystals.
There are several mechanisms that destroy these properties. Heat, acids, strong alkalis, alcohol, urea, salicylate, and ultraviolet light are among the more common ways that proteins become denatured.
A denatured protein unfolds as many of the hydrogen bonds that preserve the three-dimensional structure of the protein are broken. Instead of a uniform solution of molecules that are all the same shape, in a denatured protein, the molecules can take a staggering number of different shapes (on the order of 1,020 different shapes, depending on the size of the protein molecule). Like snowflakes, few if any of the molecules will have the same shape, and they will no longer form regular crystals.
The unfolded molecules also have more bond-forming areas exposed on the outside, so they form bonds with one another and coagulate. They become insoluble in water.
You have seen that surface effects cause proteins to denature. When you beat egg whites or whip cream, the proteins unfold as their hydrophobic parts rearrange to avoid water in favor of air or fat. The unfolded proteins can then bond to one another to create stabilizing protein films that keep the new forms in the desired shape.
In cooking, you control the denaturing of proteins in several ways. You can control the temperature, you can control the acidity, you can use copper bowls to beat the egg white and catalyze the formation of disulfide bonds in the proteins, and you can control the fat or air content when you beat the proteins.
For example, when beating egg whites, it is important to keep fats out of the egg whites. A little bit of oil or egg yolk can prevent the foam from stabilizing, as the air competes with fat for the hydrophobic parts of the molecule.
Because proteins have both acidic parts and basic parts, the acidity of the solution they are in changes their behavior. Acids release protons (hydrogen nuclei) and bases accept protons. In an acidic solution, the basic parts of the protein accept protons from the acidic solution and become positively charged. The positive charges repel one another and the protein molecules are less likely to combine with one another.
In a basic solution, the acidic parts of the protein lose a proton and become negatively charged. This also results in repulsion between the protein molecules, and combination is reduced.
Charged areas of the protein interact with water molecules because water is a polar molecule, with one end negative and one end positive. These charged ends are attracted to opposite charges on the protein.
Whether a protein forms a gel is thus affected by the acidity of the water it is dissolved in.
Nearly 80 percent of the proteins in milk are casein proteins. Caseins have a lot of the amino acid proline, which has a side chain that causes proteins to bend wherever there is a proline. This makes the proteins unlikely to stack into regular, orderly secondary structures. In addition, caseins have no disulfide bonds, and so they have little tertiary structure. This means that the hydrophobic parts of the molecule are open and exposed (not tucked inside a ball).
All of this combines to give caseins interesting properties. The hydrophobic parts end up migrating together, and the hydrophilic parts arrange themselves to the outside, facing the water. These tiny, hairy balls of protein are called micelles.
Caseins bind together with calcium and phosphorus. As a nutrient for the young mammal that needs to build bones, this is a useful property. Without the caseins, calcium phosphate would not be soluble. In the basic solution of milk, the hydrophilic parts of caseins become negatively charged and repel one another. This allows milk to stay liquid. But caseins clot in the stomach, due to acids that counteract the negative charges and enzymes that cut the proteins into smaller pieces. This clotting makes the proteins stay longer in the stomach, releasing amino acids slowly, which aids digestion and absorption of the protein.
In the stomach of young mammals, enzymes cut off part of the water-soluble casein (kappa-casein) that has the negative charges that keep the micelles apart. In making cheese, these enzymes, extracted from the stomachs of young calves, are used to make the caseins clot together into a solid.
The proteins in eggs largely determine the characteristics of foods that contain eggs. Understanding the different properties of these proteins can be helpful when cooking or creating new dishes.
When you crack an egg into a pan, you can immediately see three parts. There is the yolk, a thin watery white, and a thick gelled white.
Egg white contains several mucoproteins, in which the protein is attached to carbohydrates. In the egg, these serve as nutrients for the growing embryo, and as support and protection.
Over half the protein in egg white is of one type: ovalbumin. It denatures at 176°F (80°C), forming the solid white mass you see at breakfast.
The next most prevalent protein in egg white is ovotransferin, also called conalbumin, which makes up about 12 percent of the proteins in the white of the egg. It denatures at a lower temperature, about 145°F (63°C).
The third most prevalent protein is ovomucin, at 11 percent. It is found close to the yolk, mixed with other proteins, thickening them.
When you crack an egg into a frying pan, the thin part of the egg white has less ovomucin, and the thick part of the white has two to four times as much. Ovomucin is the main gelling agent in egg white.
The first protein to denature when heating an egg white is the ovotransferin. As it unfolds, it binds not only to other unfolded ovotransferin molecules but to other proteins that are not yet denatured. The ovomucin molecules, which do not coagulate with heat by themselves, can thus be incorporated into a strong gel with the ovotransferin and ovalbumin.
The remaining proteins make up less than a quarter of the protein in egg white, but some of them bear mentioning here. Avidin makes up a very small portion of the egg white (less than a 1/10 of a percent), but it binds very tightly to the essential nutrient biotin (vitamin B7), making the biotin unavailable as a food source. This effect is destroyed when the protein is denatured by heat or beating, but it can be a problem in a diet that contains a lot of raw egg white.
Raw meat is tough because each tiny packet of muscle fibers is surrounded by a tough sheet of connective tissue. This is the same tissue that, when boiled, makes gelatin.
When meat is cooked, the tough connective tissue denatures and becomes soft gelatin. The proteins in the muscle fibers also denature. Enzymes in the tissue no longer function when they are denatured, so cooked meat will keep longer than raw meat.
If the meat is overcooked, the water in the fiber bundles boils and the gelatin bag holding them bursts, and the meat dries out. At high temperatures, the proteins also undergo further denaturing and cross-linking, making the meat tough again. Crisp bacon is an excellent example of this. A thick, juicy steak would be inedible if cooked to the hardness of bacon.
Enzymes in foods are often a problem for food storage. As cells break open, the enzymes inside them leak out and react with other parts of the food. This causes brown soft spots in fruits and vegetables, and it makes meats smell and taste bad. The damaged parts also invite decay microorganisms.
Denaturing the enzymes can help to preserve the food. The heat of cooking is one familiar way to denature enzymes, but proteins can also be denatured by acids, strong alkalis, desiccation, or salt.
When wheat flour and water are mixed and kneaded, sheets of gluten are formed. With further kneading, these sheets stick together into larger and larger sheets.
But if oils or fats are added, the hydrophobic amino acids in the gluten attach to the fat, so that they are not available to form bonds with other gluten molecules. This changes the nature of the dough, making it more tender and less able to trap bubbles of leavening gasses.
The result is a more cakelike, less breadlike structure.
One particular amino acid has a strong effect on the taste of foods. That is glutamic acid, and salts of it are called glutamates.
Besides being an abundant neurotransmitter in the brain, glutamate activates sensors on the tongue that detect savory protein-rich foods. Meats, poultry, fish, cheese, and soy sauce are rich sources of glutamate. The commercial form of pure glutamate is monosodium glutamate, MSG, which is an additive in many foods.
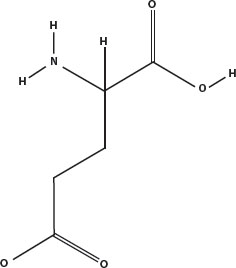
Glutamic acid
Cheese is made from milk that has been curdled by the addition of acids and an enzyme from the stomach of calves called rennet. The acid can come from almost any food source, but for the most part it is produced by bacteria that convert the milk sugar lactose into lactic acid. Yogurt is also produced this way.
Cheese can be made without rennet, but the enzyme makes the curds stronger and more rubbery. Rennet allows the milk to curdle with less acid, which in turn allows flavor-producing bacteria to colonize the curd. Cheeses made with rennet will melt easily, while cheeses made with acid alone remain solid at high temperatures.
The curds are salted and moisture pressed out so the product will not be as easily attacked by bacteria as raw milk would. Thus making cheese is a way of preserving milk.
 Recipe
RecipeOur feathered assistant, Corky, will help in the preparation, especially in the taste testing as the stuffing progresses.

Get up very early in the morning on the day before you plan to eat the turkey. It will be cooking all day and all night, and most of the rest of the next day. This bird will be cooking for an hour a pound, and this bird is 36 pounds. The turkey, not the parrot.
Because it will be cooking at a temperature below boiling, take some simple steps to ensure that nothing harmful grows in your bird.
The stuffing will be acidic and sweet, which will prevent bacterial growth. Dried fruits and nuts do not support bacterial growth. Other steps to prevent harmful bacteria will be shown later. These precautions are a good idea no matter how you cook your bird.
Ingredients:
Supplies:
Melt the butter in a 4-quart Pyrex bowl using the microwave.
Core and chop the apples into pieces about the size of a sugar cube. As you chop each apple, put the pieces into the melted butter. This will prevent them from getting brown on the cut edges.

Chop the nectarines into small pieces and add them to the apples.

Put the fruits and nuts into a huge bowl.

Add the two boxes of stuffing mix. I like to use one box of cornbread stuffing mix and one box of traditional. Mix everything well with a large spoon.

Add the two eggs, and mix very well, distributing the eggs throughout the stuffing. Add the apple cider.

At this point the stuffing is done. You and your assistant can now taste it. You might find you do a lot of tasting, as this is one of the best stuffings you will ever taste, and it needs no cooking to be enjoyed.

Now you are ready to wash and sterilize the turkey. Rinse it inside and out with hot water. Add a big handful of salt to the inside of the turkey, and use it as a scrubbing aid. Rinse out all of the salt, then use 2 quarts of hydrogen peroxide to sterilize the bird inside and out. Let the hydrogen peroxide sit inside the bird for a few minutes. Hydrogen peroxide kills any bacteria and molds that might be in or on the turkey. It breaks down into water and oxygen. You will see the oxygen bubbles scrubbing away inside the bird, and on the outside.

Don’t rinse the peroxide off the bird. Almost all of it will drain out of the turkey as you tip it in the sink, but a little will remain inside as you stuff the bird. This will provide extra oxygen, which prevents the growth of botulism bacteria.
Now stuff both cavities of the turkey. Go ahead and pack it in tightly—this stuffing can take it.

Use metal or bamboo skewers to close up the openings. Some people lace them shut with string, but I find that the skewers work just fine by themselves.
Now make a basket weave of bacon on top of the turkey. I used to do a simple one-layer weave, but each year my guests keep demanding “More bacon!” and I accommodate them. You will see why it is so popular in a minute.

Depending on how large your turkey is, and how hungry you and your assistant are, there will be enough stuffing left over to put into its own casserole dish for your vegetarian friends. My assistant is more interested in the stuffing than the bacon-wrapped turkey.

Now take a clean paper shopping bag and cut out one side so you can place it over the turkey. This will prevent splattering in the oven and keep the steam close to the bird as it cooks.
I like to use two thermometers, one for the breast meat and one for the dark meat. It doesn’t matter, however, as they both end up reading the same when the bird is done. I just like the comfort of redundancy.

A 36-pound turkey will just barely fit into a large oven.

The trick to a moist and tender turkey is to cook it at a temperature below the boiling point of water, but slightly above the temperature you want it to end up when it is done. I use a temperature of 205°F (96°C).
Cook the turkey at 350°F (176°C) for the first two hours to further sterilize the outside of the bird before you turn it down to 205°F (96°C).
In any meat, the tiny bundles of muscle cells are surrounded by a tough layer of connective tissue. To make the meat tender, raise the temperature until the connective tissue melts into gelatin. But if the temperature gets above the boiling point of water, the steam will burst open these little soft sacks of gelatin, and all of the juices will end up at the bottom of the pan instead of waiting to ooze out of the meat as it is sliced.
Because you have ensured that the bird is free of harmful bacteria, you can cook it to a lower temperature than is usually called for with poultry. This will prevent the breast meat from becoming dry. This is the way I like my turkey, cooked to about 150°F (66°C).
However, this results in the thigh meat looking red, especially around the joints, and this terrifies some of my guests. For them, I allow the turkey to cook to 160°F or even 170°F (71°C or 77°C), which makes the breast meat a little dry to my taste, but the dark meat is still moist and delicious. Being a dark meat fan, I find this compromise perfectly acceptable.
The turkey should be done a couple of hours before you start dinner. This allows you to use the oven for all of the other trimmings you want to have with the meal. More important, it allows time for the turkey to cool, and the little gelatin packets to gel, so the bird carves without falling apart.

For my guests, it also gives them time to pick off all of the bacon and eat it. This is not just an important ritual for this group, but it is also a necessary step before carving. All of that hard, brittle bacon shell has to be removed so the knife can find something to slice into.
