Chapter 13
The Anxious and Fearful Brain
Fear is the oldest and strongest emotion of mankind.
—H. P. Lovecraft
All animals have been shaped by evolution to approach what is life sustaining and avoid what is dangerous. The success of rapid and accurate approach-avoidance decisions determines if an organism lives to reproduce and carry its genes forward to the next generation. Because vigilance for danger is a central mechanism of the process of natural selection, evolution may well favor an anxious gene (Beck et al., 1979). Some anxieties appear to be hard wired, specific to primates, and linked to both our present and past survival needs. Fear of spiders, snakes, heights, and open and closed spaces all harken back to the survival of our forest-dwelling ancestors. From an evolutionary perspective, our complex neural systems have all been sculpted to better serve the prime directive of survival.
The neural circuitry involved in fear and anxiety, although biased toward the right hemisphere, involves both hemispheres and all levels of the triune brain. The most primitive subcortical fight-or-flight circuitry, shared with our reptilian ancestors, interacts with the most highly evolved regions of the cortex. This results in the capacity to experience anxiety about everything from an unexpected tap on the shoulder to an existential crisis. The connection between every kind of anxiety and the core biological mechanisms of physical survival supports the philosophical notion that all anxiety, at its core, may be the fear of death (Tillich, 1974).
Anxiety and fear are the conscious emotional aspects of the body’s ongoing appraisal of threat, telling us to be prepared to take action. Anxiety can be triggered by countless conscious or unconscious cues and has the power to shape our behaviors, thoughts, and feelings. At its most adaptive, anxiety encourages us to step back from the edge of a cliff, or to check to see if we signed our tax forms before sealing the envelope. At its least adaptive, anxiety steers us away from taking important and appropriate risks, pushing ourselves to reach personal goals, or engaging in new and potentially beneficial behaviors.
The response to stress, or general adaptation syndrome (Selye, 1979), results in a range of physiological changes designed to prepare the body for fight or flight. Energy is mobilized through increased cardiovascular and muscular tone, whereas digestion, growth, and immune responses are inhibited. As part of the stress response, a cascade of biochemical changes occur in the hypothalamus, pituitary, and adrenal glands (the HPA axis), as well as in the sympathetic nervous system. These biochemicals mediate the physical and psychological changes experienced during stress. Increased levels of glucocorticoids, epinephrine, and endogenous opioids are particularly relevant to a discussion of the psychological impact of stress and trauma, in that they alter attention, cognition, and memory. We experience the effects of the general adaptation syndrome in situations such as automobile accidents, at crucial moments during sporting events, or when engaging in public speaking. The dangers can be real, imagined, or experienced vicariously as we watch others in stressful or dangerous situations.
With the expansion of the cerebral cortex and the emergence of imagination, we have become capable of being anxious about situations we will never experience. We can now worry about monsters living under our beds and the incineration of the earth resulting from the sun’s expansion. Because our imaginal capabilities have allowed for the construction of the self, we can also become anxious about potential threats to our psychological survival. Psychotherapists deal with a wide variety of anxiety disorders based in the fear of a social death. The expectation of rejection by another can result in social withdrawal; the fear of forgetting one’s lines in a play can result in stage fright. Systems of physical survival have been conserved in the evolution of consciousness and the ego, to be triggered when threats to these abstract constructions are activated.
Consciously experienced anxiety provides the opportunity to face and work through one’s fears. The common wisdom of getting back on the horse that threw you is advice clearly aimed at preventing the use of avoidance to control anxiety. In fact, the reduction of anxiety through avoidance reinforces the behavior and makes the feared stimulus seem all the worse. Unfortunately, anxiety can be paired with all kinds of automatic and internal sensations, emotions, and thoughts, which shape behavior outside of conscious awareness. Compounding the problem, the left hemisphere interpreter provides a rationale supporting and reinforcing avoidance: “It’s inhuman to ride horses!” “Who needs planes?”
“Why go out when it’s so comfortable at home?” The avoidance of thoughts and feelings associated with feared stimuli both reflects and perpetuates a lack of integration among neural networks. Facing one’s fears is a core component of all forms of psychotherapy.
We see this, for example, with adult women who were sexually or emotionally abused as children who sometimes come to therapy with chronic and severe weight problems. They do well on diets until they begin to be noticed by men, associated in implicit memory with the pain and shame of their childhood experiences. These negative emotional reactions lead them to return to behavioral patterns associated with an avoidance of such feelings, such as eating. The act of eating is doubly reinforcing because it provides nurturance, while gaining back the weight serves to protect against sexual advances.
Thus, what started out as a straightforward survival-based alarm system has also become a nuisance. This is another downside of the design compromises between speed and accuracy mentioned earlier (Mesulam, 1998). Evolution designed a brain that reacts quickly to a variety of subtle environmental cues. These same capabilities have negative consequences when applied to a complex and largely nonconscious psychological environment. An understanding of the neuroscience of anxiety and fear is helpful in both the conceptualization and treatment of most clinical disorders. In the following pages, we will look at the two loops of fear circuitry outlined by Joseph LeDoux, the role of the amygdala in the regulation of fear and anxiety proposed by Michael Davis, and Robert Sapolsky’s work on the negative impact of long-term stress.
Fast and Slow Fear Networks
Fear is an emotion indispensible for survival.
—Hannah Arendt
Through his research with animals, LeDoux (1994) demonstrated the existence of two separate yet interrelated neural circuits which regulate fear. The conservation of these systems during evolution allows us to apply these findings to human experience (Phelps, Delgado, Nearing, & LeDoux, 2004). The two systems (which we will call fast and slow) each play a somewhat different role in our reaction to danger. This model can be clinically useful for anxious and fearful clients by helping them understand the neurobiological mechanisms underlying their unsettling experiences.
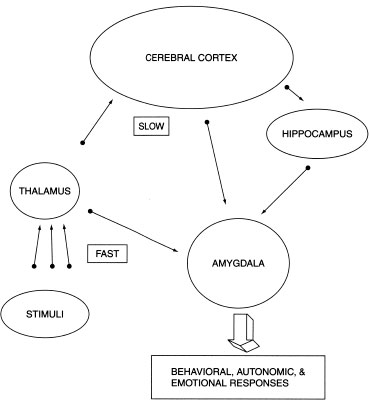
The reflexive fast system acts immediately, sending information directly from the sense organs (eyes, ears, skin, nose, tongue) through the thalamus to the amygdala. And when I say fast, I mean fast: All of this processing can occur in one twelfth of a second. The amygdala evaluates the sensory input and translates it into bodily responses via its many connections with the autonomic nervous systems. The thalamus may aid in this rapid evaluation by maintaining crude representations of potentially dangerous things often encountered in the environment such as spiders, snakes, and dangerous predators (Brosch, Sander, & Scherer, 2007). These subcortical structures play an executive role in rapid appraisal because the increased time it would take to include the cortex might have too large a survival cost.
Simultaneously, the slow system sends sensory information on to the hippocampus and cortex for further evaluation. This system is slower because it contains more synaptic connections and involves conscious processing. Cortical circuits of memory and executive processing examine the information more carefully, compare it to memories of similar situations, and decide how to proceed. The slow circuit aids in fear processing by contextualizing the information in time and space. This slow system in humans—with its apex in the prefrontal cortex—has the additional task of making sense of the behavioral and visceral reaction already set into motion by the fast systems. In this way, our conscious executive functions discover the decisions that have already been made by our unconscious executives. We find ourselves already scared when we initially perceive what is frightening us; or ecstatic as our loved one comes into view. Figure 13.1 depicts the neural circuits of the fast and slow fear networks.
This dual circuitry helps us to understand why we often react to things before thinking and then have to apologize later on. In therapy, we often attempt to utilize the conscious linguistic structures of the slow circuit to modify or inhibit dysfunctional reflexes and emotional appraisals of the fast circuit. Coupled with relaxation techniques and enhanced awareness, exposure to a feared stimulus can serve to enhance the regulatory input of the slow cortical circuits by building new neural connections. Cognitive and behavioral interventions therefore increase the ability of the cortex to inhibit the amygdala.
FIGURE 13.1
Fast and Slow Fear Circuits
A depiction of the two pathways of information to the amygdala—one directly from the thalamus and the other through the cortex and hippocampus (adapted from LeDoux, J. Emotion, memory, and the brain. Copyright ©1994 by Scientific American, Inc. All rights reserved.)
There are many examples of these two systems in action. I walked into my garage one day to look for a tool when, out of the corner of my eye, I saw a small brown object near my foot. There are plenty of little critters in my neighborhood and they often crawl, burrow, or fly into my house. I immediately jumped back, my heart rate increased, my eyes widened, and I became tense, ready to act. Moving backward, I oriented toward the shape, saw that it looked more like a piece of wood than a rodent, and began to relax. After a few seconds, my heart rate and level of arousal were back to normal; the potential danger had passed.
Analyzing this experience in terms of the two systems, my peripheral vision saw the object and my amygdala appraised it in an overgeneralized fashion to be a threat. My amygdala activated a variety of sympathetic responses including startle, increased respiration, and avoidance. In the split second while my body was reacting, I reflexively oriented my head toward the shape, which brought it to the fovea of my retina, providing my hippocampus and cortex with more detailed visual information, allowing them to appraise it more accurately than my skittish amygdala. I suppose that a species-specific fear accounts for such a strong reaction to an animal weighing just a few ounces. This example, trivial as it may be, leads to a more serious application of LeDoux’s theory to interpersonal relationships.
As the core of our social brain, the amygdala organizes the appraisals of what we have learned from our relationship history. In interpersonal situations, our amygdala reflexively and unconsciously appraises others in the context of our past experiences. From moment to moment, the reflexive activations of our fast systems (organized by past learning) shape the nature of our present experience (Bar et al., 2006). This is a powerful mechanism by which our early social learning influences our experience of the present. So, by the time we become conscious of others, our brain has already made decisions about them. In the case of prejudice, skin color triggers a set of assumptions upon which we evaluate other people (Olsson, Ebert, Banaji, & Phelps, 2005). At the opposite extreme, love at first sight is a sort of positive prejudice triggered by emotional memories projected onto another person.
The Amygdala’s Role in Anxiety and Fear
No passion so effectually robs the mind of all its powers of acting and reasoning as fear.
—Edmund Burke
The amygdala plays a central role in the activation of fear. It has been conserved and expanded during evolution in order to process increasingly complex cognitive, sensory, and emotional input. Its central role in appraisal and the triggering of the biochemical cascade of the fight-or-flight response makes it vital for processing memory, emotional regulation, bonding, and attachment. Electrical stimulation of the amygdala’s central nucleus results in the experience of fear, whereas destruction of the amygdala will eliminate fear reactions altogether (Carvey, 1998). In fact, the destruction of the amygdala in animals results in an inability to acquire a conditioned fear response.
Although we are genetically programmed to become anxious about things like snakes or abandonment, fear can be learned by pairing any thought, feeling, or sensation with a noxious stimulus, such as a loud noise or an electric shock (Corcoran & Quirk, 2007). Learning to be anxious can occur at conscious and unconscious levels related to both internal and external stimuli. Like the hippocampus, the lateral areas of the amygdala are capable of long-term potentiation (LTP) involved in reinforcing connections among neurons. Remember that LTP is the process through which the association among neurons becomes strengthened and learning is established. The amygdala can learn, throughout life, to pair any stimulus (even physical affection or praise) with fear.
As we saw earlier in our discussion of memory, the hippocampus and amygdala organize interacting but dissociable systems of memory. Bechara and colleagues (1995) reported that a patient with bilateral (left and right) amygdala damage was unable to acquire a conditioned autonomic response to sensory stimuli. The patient was, however, able to consciously remember the conditioning situation because his hippocampi were still intact. Another patient with bilateral damage to the hippocampus showed no conscious memory for the conditioning situation but did acquire autonomic and behavioral conditioning. The authors concluded that the amygdala is “indispensable” for coupling emotional conditioning with sensory information while the hippocampus is required for conscious recollection (Bechara et al., 1995).
The neural projections from the amygdala to numerous anatomical targets cause the multiple physical expressions of anxiety, fear, and panic. Projections from the amygdala to the lateral hypothalamus result in sympathetic activation responsible for increased heart rate and blood pressure. The amygdala’s stimulation of the trigeminal facial motor nerve even causes the facial expressions of fear (Davis, 1992). The amygdala is also essential in reading the fearful facial expressions of others (Baird et al., 1999). As you can see from Figure 13.2, the amygdala is well connected, making the fear response a powerful whole-body experience.
FIGURE 13.2
Some Targets of the Amygdala in the Fear Response
Some of the many anatomical targets of the amygdala in the fear response, and their biological and behavioral contribution.

The triggering of the autonomic nervous system by the amygdala causes a racing heart, sweating, and other physiological symptoms as the body prepares for fight or flight. In the absence of real external danger, this is experience is called a panic attack. Sufferers often go to emergency rooms thinking they are having a heart attack. Individuals with panic disorder have increased neural activity in the amygdala (Reiman et al., 1989). Psychologically, victims report a sense of impending doom, feelings of unreality, and the thought that they are going crazy. Panic attacks are often triggered by stress or other conflicts in the sufferer’s life, but he or she seldom makes the connection between these events and the panic attacks. Because the neural connections are contained within hidden neural layers, they are experienced as “coming out of the blue,” leaving victims struggling to comprehend what is happening to them.
The amygdala’s tendency toward generalization results in panic being triggered by an increasing number of internal and external cues (Douglas & Pribram, 1966). Because panic attacks are experienced as unpredictable and life threatening, they result in a limitation of activities. Agoraphobia, or fear of open spaces, develops as victims of panic attacks associate fear with a broader variety of situations. Hoping to avoid these attacks, sufferers restrict their activities to the point where they eventually become housebound. Simultaneously, the amygdala becomes conditioned to respond faster in people who become phobic, creating a vicious cycle of anxiety and fear (Larson et al., 2006). The behavior of these individuals becomes so shaped by fear that they come to avoid most of life. On the other hand, those of us with a slower and less active amygdala experience greater psychological well-being (van Reekum et al., 2007). One gift of aging is that the amygdala also appears to become less sensitive to fear as we grow older.
The development and connectivity of the amygdala have many implications for both early child development and psychotherapy. Without the inhibitory impact of the later-developing hippocampal-cortical networks, early fear experiences are unregulated, overwhelming full-body experiences. Because the amygdala is operational at birth, the experience of fear may be the strongest early emotion. Part of the power of early emotional learning may be the intensity of these unregulated negative affects in shaping early neural infrastructure. The infant is very dependent on caretakers to modulate these powerful experiences. Amygdalaand hippocampus-mediated memory systems are dissociable from one another, which means that early and traumatic memories can be stored without conscious awareness or cortical control. They will not be consciously remembered, but instead will emerge as sensory, motor, and emotional memories like traumatic flashbacks.
Another limbic structure closely connected to the amygdala is the bed nucleus of the stria terminalis (BNST). Like the amygdala, it is connected upward to the prefrontal cortex, as well as down into the autonomic nervous system. Unlike the amygdala, the BNST is sensitive to abstract cues and is capable of long-term activation, suggestive of both its later evolution and its role in anticipatory anxiety (Davis, 1998; Kalin et al., 2001). It appears that perhaps the amygdala specializes in fear while the BNST evolved to deal with the more complex triggers for anxiety that emerged as our brains became capable of imagining multiple potential outcomes. Interestingly, the BNST in rats is a structure that grows in response to maternal responsibilities. We have to wonder if, as the brain became specialized for caretaking, the scaffolding we need to create around our children pushed the evolution of a constant focus on potential dangers.
The Locus Coeruleus and Norepinephrine
Worry gives a small thing a big shadow.
—Swedish proverb
One important descending projection from the amygdala and BNST connects them with the locus coeruleus (LC). The LC is a small structure with extensive projections throughout the brainstem, midbrain, and cerebral cortex. It is, in fact, connected with more parts of the brain than any other structure so far discovered (Aston-Jones, Valentino, VanBockstaele, & Meyerson, 1994). The LC is the brain’s primary generator of norepinephrine (NE), which drives the activity of the sympathetic branch of the autonomic nervous system responsible for the fight-or-flight response. One effect of NE is to enhance the firing of neurons that are highly relevant to a present experience based on past learning (past fear responses), while inhibiting those involved in baseline activities.
This means that stimulation of the LC prepares us for danger by activating circuits dedicated to attention and preparation for action. NE activation makes us become vigilant, scan for danger, and maintain a posture of tense readiness. It also heightens our memory for danger, creating a sort of “print now” command for amygdala memory circuits (Livingston, 1967). The pathways containing these traumatic memories become hyperpotentiated, meaning that they are more easily triggered by less severe subsequent stressors. This allows us to be reminded in the future of similar dangers. During times of lowered hippocampal-cortical involvement (e.g., intoxication or near-sleep states), these stressful traumatic memories may become disinhibited as intrusive images and flashbacks. Translated into human and clinical terms, this means that surges of NE during periods of safety may result in past traumatic associations (anxiety, startle, visual images, etc.) being brought to awareness, which overshadow current experiences.
Stimulating the LC in animals results in a disruption of ongoing behavior and triggering of an orienting reflex, like the one I had to the small piece of wood. This is seen in patients with PTSD who respond to trauma-related cues decades after their traumatic experience. LC activity in primates results in a high degree of vigilance while interrupting sleep, grooming, and eating. Through a series of connections, the central nucleus of the amygdala stimulates the LC, which, in turn, is thought to be a major control area of the sympathetic nervous system (Aston-Jones et al., 1994). An understanding of the biochemistry and functioning of the LC is an important component of any theory of causes of anxiety disorders (Svensson, 1987).
Stress and the Hippocampus
Anxiety is a thin stream of fear trickling through the mind. If encouraged, it cuts a channel into which all other thoughts are drained.
—Arthur Somers Roche
The human brain is well equipped to survive brief periods of stress without long-term damage. In an optimal state, stressful experiences can be quickly resolved with good coping skills and the help of caring others. However, people often come to psychotherapy with long histories of anxiety, which can have profound effects on the brain. Working with rats and vervet monkeys, Sapolsky and his colleagues demonstrated that sustained stress results in hippocampal atrophy and a variety of functional impairments (Sapolsky, 1990; Sapolsky, Uno, Rebert, & Finch, 1990). His research is particularly important because it may help explain some of the negative long-term effects of childhood trauma.
The biological link between prolonged stress and hippocampal atrophy appears to be mediated via the catabolic influence of stress hormones. Glucocorticoids (GC) such as cortisol are secreted by the adrenal gland to promote the breakdown of complex compounds so that they can be used for immediate energy. The first of these hormones was found to break down complex sugars, hence the name gluco corticoids. It was later found that they also block protein synthesis, inhibiting both new neural growth and the construction of proteins involved in immunological functioning. Overall, long-term learning and biological well-being are sacrificed for the sake of immediate survival. This makes great sense when stressors are short-lived. But when stress is chronic, high levels of cortisol put us at risk of physical illness, learning dysfunctions, and memory deficits. A number of roles of cortisol are seen in Table 13.1, along with its impact on the brain and its relationship to a variety of illnesses.
The focus on immediate survival supersedes all long-term maintenance, akin to burning the furniture to survive freezing in winter. Thus, these biological processes need to be reversed as soon as possible after the crisis has passed to allow the body to recover and return to functions of restoration and repair. It is apparent that this system was designed to cope with brief periods of stress in emergency situations; it was not designed to be maintained for weeks or years at a time. The complexities of cortical processing and anticipatory anxiety are poorly matched with these primitive stress systems.
Prolonged stress most affects two processes. First, it inhibits protein production in order to maintain higher levels of metabolism. Proteins are, of course, the building blocks of the immunological system (leukocytes, B-cells, T-cells, natural killer cells, etc.) and the suppression of protein synthesis also suppresses our body’s ability to fight off infection and illness. This is one of the primary reasons for the high correlations found between prolonged stress and disease. Second, sustained higher levels of metabolism continue to pump sodium into neurons, eventually overwhelming the cell’s ability to transport it out again. This results in destruction of the cell membrane and consequent cell death. This process has been found to be particularly damaging to the hippocampus, resulting in a variety of memory deficits and depression. Loss of volume in the hippocampus is related to cumulative GC exposure (Sapolsky et al., 1990). Sustained high levels of stress partly explain why early negative experiences in parenting and attachment have a lifelong impact on physical health, mental health, and learning.
TABLE 13.1
Stress and the Hippocampus
The Role of Cortisol
Breaks down fats and proteins for immediate energy
Inhibits inflammatory processes
Inhibits protein syntheses within the immune system (leukocytes, B and T-cells, natural killer cells, etc.)
Suppresses gonadal hormones that support neural health, growth, and learning
Chronic High Levels of Cortisol/Glucocorticoids Result In
Decreased plasticity1
Dendritic degeneration2
Deficits of remyelination3
Cell death4
Inhibition of neurogenesis and neural growth5
High Levels of Cortisol Correlate With:
Impaired declarative memory and spatial reasoning6
Compromised Hippocampi Result In
Deficits of new learning7
Short-and long-term memory8
Individuals With Smaller Hippocampi Include
Adult victims of early trauma9
Post-traumatic stress disorder10
Temporal lobe epilepsy11
Schizophrenia12
Cushing’s disease (hypercortisolism) 13
The hippocampus, rich in GC receptors, plays a negative feedback role with the adrenal gland to inhibit GC production. If the hippocampus detects too many GCs together, it sends a message (via the hypothalamus or the pituitary) to the adrenal gland to slow down GC production (Sapolsky, Krey, & McEwen, 1984). The more receptors we have, the greater our feedback abilities to decrease cortisol production. Prolonged high levels of GCs increase the vulnerability of the hippocampus to a number of potential metabolic insults (Sapolsky, 1985; Woolley, Gould, & McEwen, 1990). At this point it is unclear if decreased volume reflects permanent damage to the hippocampus or a reversible inhibition of the growth of new neurons. In either case, less hippocampal mass means fewer GC receptors, which, in turn, means less negative feedback to the adrenal gland. Loss of volume in the hippocampus is related to cumulative GC exposure (Sapolsky et al., 1990).
Early trauma results in hippocampal impairment, which decreases our ability to inhibit the emotions triggered by amygdaloid memory systems. Further, deficits in reality testing and short-term memory will make the process of integrating traumatic experiences into conscious awareness more difficult. Longer periods of relationship building and pragmatic interventions focused on stress reduction and the development of coping skills may be necessary prerequisites for successful long-term therapy with victims of early stress and trauma. The hippocampus is also exquisitely sensitive to oxygen deprivation, so patients who have suffered metabolic disruptions, head trauma, or seizures may have hippocampal compromise, as well as mountain climbers, divers, or individuals with heart disease (Lombroso & Sapolsky, 1998; Regard, Oelz, Brugger, & Landis, 1989). High-dose cortisol administration for autoimmune diseases may also result in hippocampal damage (Sapolsky, 1996). All of these factors should be kept in mind when taking histories of patients with cognitive and neurological symptoms.
Impairment of the hippocampus from early chronic stress may make the therapeutic process more difficult for many clients. For example, Stein and his colleagues (Stein, Koverla, Hanna, Torchia, & McClarty, 1997) found that adult women who had experienced childhood abuse had significantly reduced left hippocampal volume. They also found that the amount of reduction was significantly correlated with increased dissociative symptoms. This relationship suggests that the left hippocampus may play a role in integrating memories into a cohesive narrative. The hippocampus is also thought to be involved in the flexible incorporation of new information into existing structures of memory (Eichenbaum, 1992). If this is the case, early abuse may result in damage to neural structures required to create new and more functional narratives.
Rats and humans differ in a number of ways besides whisker length. The increased size of the human brain and its additional processing capacity make it possible for us to worry about many more potential dangers, both real and imagined. In addition, our brains allow us to create complex situations such as traffic jams and overburdened schedules, generating ever-increasing levels of stress. Stressors such as these, which are experienced as inescapable, tend to have a greater sustained cortisol activation and negative impact on the brain. Although we like to think of childhood as a time of innocence and play, many children grow up in a state of constant distress. We saw this clearly in the attachment research where adults with anxious attachment patterns demonstrated a lack of recall for long periods of their childhoods. Parental physical or mental illness, community violence, poverty, and many other factors can contribute to this. Prolonged childhood stress can have lifelong effects on functioning related to hippocampal damage, immunological suppression, and other stress-related impairments.
Learning Not to Fear
Courage is acting in spite of fear.
—Howard W. Hunter
It is an unfortunate twist of evolutionary fate that the amygdala is mature before birth while the systems that inhibit it take years to develop. This leaves us vulnerable to overwhelming fear with little to no ability to protect ourselves. On the other hand, evolution has also provided us with caretakers who allow us to link into their developed cortex until our own is ready. The way they protect us from fear and modulate our anxiety becomes a model upon which our own brain develops. Thus, we use proximity to our parents as our key method of fear regulation, just as cold-blooded animals use locomotion and change of location to regulate their internal temperature. Our attachment schemas come to reflect the success or failure of how we and our parents navigate this process. We have seen from the research with rats that maternal attention results in a brain that is better equipped to learn as well as to downregulate the immediate and long-term effects of stress. It turns out that in dealing with fear, the ability to learn as well as having a more resilient stress system are both important for facing life’s challenges.
The hippocampus is constantly remodeled to keep abreast of current environmental changes. On the other hand, the amygdala’s role is to remember a threat, generalize it to other possible threats, and carry it into the future. Because the amygdala exhibits persistent dendritic modeling, we are unable to completely forget painful and traumatic experiences (Rainnie et al., 2004; Vyas, Bernal, & Chattorji, 2003; Vyas, & Chattorji, 2004 ). The power of the amygdala and its stubbornness in the face of the hippocampus leads us to be biased toward anxiety and fear.
The phenomenon of spontaneous recovery of a phobia demonstrates how the fear we hoped was long gone was stored in our amygdala all along (Vansteenwegen et al., 2005). Getting past our fears and phobias does not entail forgetting to be afraid; rather, the extinction of fears represents new learning organized by our slow systems of the cortex and hippocampus. In other words, extinction learning represents the formation of new neural associations that somehow keep the memory stored in the amygdala from triggering the sympathetic nervous system (Milad & Quirk, 2002; Rau & Fanselow, 2007).
The ability of the prefrontal cortex in modulating amygdala activity is believed to occur through the development of descending inhibitory circuitry (Akirav & Maroun, 2007; Ochsner et al., 2004). Evidence for this includes the fact that this cortical-amygdala network exhibits a reciprocal activation pattern where more cortical activation results in less amygdala activation and vice versa. This may not only be why our problem-solving abilities can be shorted out by fear, but also why thinking about and being prepared for a situation lessens our fears. When we successfully use cognitive techniques to decrease anxiety, we are likely building these descending cortical networks to inhibit amygdala and autonomic activation (Schaefer et al., 2002).
Learning not to fear, just like secure attachment, appears to be a major contribution of the ompfc (Morgan, Romanski, & LeDoux, 1993; Phelps et al., 2004). Electrical stimulation of the homologous region in the cortex of rats results in both amygdala inhibition and a reduction of conditioned fear (Milad, Vidal-Gonzalez, & Quirk, 2004; Perez-Jaranay & Vives, 1991; Quirk, Likhtik, Pelletier, & Paré, 2003). Even the size of the ompfc in humans is positively correlated with our ability to inhibit a fearful response (Milad, Quinn, et al., 2005). Thus, it appears that top-down control of the amygdala allows us to learn to discontinue a fearful response to something that makes us afraid. In a study by Kim, those who were taught to interpret a surprised face as negative had greater amygdala activation while those who were guided to see it as positive had more ompfc activation (Kim et al., 2003). These studies support the notion that the ompfc modulates the activation of the amygdala based on contextual and motivational factors (Kim et al., 2005; Myers & Davis, 2007; Ochsner et al., 2002; Phan et al., 2005). In other words, the slow system regulates the fast system.
These top-down circuits organize, modulate, and direct attention in ways that shape experience and reinforce the existing emotional state (Bishop, 2007; Christakou, Robbins, & Everitt, 2004). Anxiety is associated with a reduced top-down control of threat cues just as there is a reduction of control over negative stimuli in depression (Bishop et al., 2004; Brewin & Smart, 2005). In other words, anxious people tend to find danger while depressed people discover the negative aspects of their environments. And while those of us with more attentional control will still have a bias to orient toward the threat, we will exert more top-down control as we become conscious of the stimulus (Derryberry & Reed, 2002). Once again, the slow system modulates the fast system.
Thus, the balance of activation among the prefrontal cortex and amygdala also guides visual attention based on relevance, emotion, and motivation (Gazzaley et al., 2007; Geday, Kupers, & Gjedde, 2007). This is one of the many networks that may become dysregulated in PTSD, resulting in disturbances of sensory processing and memory, and even causing visual hallucinations (Gilboa et al., 2004; Rauch, Shin, & Phelps, 2006). Dissociated PTSD patients have greater activation in neural networks involved in the representation of bodily states, suggesting a lack of adequate top-down modulation of these networks by frontal executive systems (Lanius et al., 2005). As one would expect, the severity of PTSD symptoms is positively associated with amygdala activation and negatively correlated with ompfc size and responsivity (Shin, Rauch, & Pitman, 2006; Williams et al., 2006).
As we saw in Figure 13.2, the central nucleus of the amygdala is an output region that projects to sites in the midbrain and hypothalamus responsible for generating different aspects of the fear response. The connections of the ompfc to the central nucleus of the amygdala are particularly strong, especially to GABAergic (inhibitory) neurons called intercalated cells (Freedman, Insel, & Smith, 2000; McDonald et al., 1999; Royer, Martina, & Paré, 1999). It is now believed that it is within the descending networks from the ompfc to the amygdala’s central nucleus that extinction learning is remembered and carries out its inhibitory influences (Gottfried & Dolan, 2004; Quirk & Mueller, 2008). Because learning in this neural circuit conforms to what is known about the neurobiology of learning in general, the role of NMDA receptors, protein synthesis, cortisol, and other factors that modulate learning are likely involved in extinction learning (Elvander-Tottie et al., 2006; Santini et al., 2004).
Research shows that subjects involved in the cognitive appraisal of fearful faces show both a decrease in amygdala activation and an increase in prefrontal activation (Hariri et al., 2000, 2003). This same amygdala-prefrontal activity shift occurs during activation of a placebo effect (Wager et al., 2004) and recovery following the presentation of negative emotional material (Jackson et al., 2003). Individuals who manage to control their fear tend to have more activation in right frontal regions than those who do not (Johanson et al., 1998).
A deficit of extinction learning could be an alternative description of PTSD. Sufferers with PTSD show amygdala dysinhibition, making them vulnerable to the hallmark symptoms of intrusion and arousal (Akirav & Maroun, 2007). Patients with PTSD have also been shown to have smaller subregions within their ompfc in regions where intercalated cells are assumed to reside (Rauch et al., 2003). Also the thickness and activity levels of these prefrontal regions in patients with PTSD during extinction training correlates with their symptoms, supporting the association between their symptoms and deficits of cortically based extinction learning (Milad, Orr, et al., 2005; Phelps et al., 2004).
The Recovery of Fears and Phobias Under Stress
Dangers bring fears, and fears more dangers bring.
—Richard Baxter
Jacobs and Nadel (1985) proposed the existence of two systems of learning and memory involved in fears and phobias. These two systems predicted and parallel LeDoux’s model of fast and slow fear circuitry. The taxon system (fast system or amygdaloid system) is responsible for the acquisition of skills and rules, and the conditioning of stimulus-response connections. This system is context free, meaning that it contains no information about the time or location in which the learning took place. Taxon learning generalizes broadly and is primarily nonconscious. This is the system in which early learning of fear, safety, and attachment is organized and stored. The taxon system is represented in what cognitive psychologists call implicit and procedural memory.
The locale system—with the hippocampus and the cortex at its core—is responsible for cognitive maps necessary for external context, mental representations, and the pairing of memories with the situations in which they were learned. The development of the locale memory system parallels that of hippocampal-cortical circuits. Thus, although there is a great deal of learning during infancy (especially in the networks of the fearful and social brains), there is no source attribution or autobiographical narrative.
For example, a mother’s fearful look when strangers approach may cause her child to develop a general wariness of the world, but not recognize the source of this apprehension in similar situations later in life. We enter middle childhood with neural networks programmed by early learning, experienced as basic emotional givens. In the absence of trauma, learning in adults involves a balanced integration of taxon and locale systems that connect sensory, motor, and emotional aspects of memory to its semantic and autobiographical components. For children and traumatized adults, the taxon system may function independently, resulting in an adaptive dissociation among various systems of memory and conscious awareness.
Jacobs and Nadel contended that stress both changes the inner biological environment activating the taxon system and suppresses the inhibitory effects of the locale system. These changes result in the emergence of earlier fears or frightening experiences that had been successfully inhibited. This theory certainly parallels the voluminous research demonstrating the contribution of stress to the emergence or worsening of psychiatric and physical disorders. They suggested that stress impairs or downgrades the functioning of the locale system, causing us to fall back on the more primitive organization of taxon (amygdaloid) systems. From a psychoanalytic perspective, this process may be understood as regression to more primitive self states and defense mechanisms. This process also parallels the return of neonatal reflexes (the cortical release signs previously discussed in patients with Alzheimer’s disease or other forms of brain damage).
Despite the apparent extinction of a phobia or fear, the original memory is maintained and can become reactivated under stress. This neural explanation addresses the Freudian notion of symptom substitution, in which one fear or source of anxiety may be replaced by another after successful treatment of the first. In other words, a new trigger reactivates the still intact underlying neural circuitry, another way of saying what we covered earlier—that neurons within the amygdala exhibit persistent dendritic modeling (Vyas et al., 2003). Because of this, Jacobs and Nadel suggested that the therapist treating fears and phobias may need to generate stress as a part of treatment to activate and have access to these amygdala circuits. In addition, treatment may need to be continued well after behavioral manifestations are eliminated, as well as include stress management training. If the overall level of stress can be decreased, the likelihood of reactivating of primitive fear circuitry decreases.
Successful psychotherapy for anxiety, fears, and phobias has been shaped by the necessity of integrating fast and slow circuits, taxon and locale systems, and affect and cognition. Educating patients about panic leads to increased participation of the cortex during anxiety states. Cognitive therapy is all about utilizing the slow locale systems to inhibit and modulate fast taxon systems that have been shaped in maladaptive ways. Stress inoculation, or cognitive preparation for future stress, leads to an increasing opportunity for descending inhibition of the amygdaloid circuits by the hippocampal-cortical networks. Exposure, response prevention, and relaxation training result in the counterconditioning of unconscious associations stored in amygdaloid memory systems. This model of memory applies to all clinical situations, regardless of the presence of panic or anxiety disorders.
Drowning in a Sea of Doom
There is no greater hell than to be a prisoner of fear.
—Ben Johnson
Tina’s cardiologist suggested that she see a psychotherapist after a third visit to the emergency room. Each time, seemingly out of nowhere, Tina would become breathless and lightheaded; her heart would race until she felt as if it were going to burst from her chest. Convinced she was having a heart attack, Tina would call the paramedics. As she waited for the ambulance, Tina reported feeling like she was drowning in a “sea of doom.” She would imagine her teenaged children growing up without her, and vividly recollect her own mother’s death when she was a child. These feelings and images, together with the fear of death, would make her even more frightened. She told me that waiting for the ambulance felt like “an eternity.”
Tina, who was actually in excellent health, was repeatedly told she was having panic attacks. It took three of these embarrassing episodes to convince her to seek therapy. She came to my office feeling defeated and very frightened, as if she was losing a lifelong battle to stay in control. During our first session, I learned that Tina had a difficult childhood, including abandonment by her father, prolonged financial difficulties, and the death of her mother when she was 15. Tina finished high school while living with an aunt, put herself through college, and became a successful real estate agent. A 4-year marriage had left her with two children, now in their teens, to raise on her own. Tina’s identity was that of a survivor and hard worker who did not allow herself to depend on others. The panic attacks had shaken her self-confidence and created a fear of returning to the chaos, pain, and dependency of her childhood. She had hoped for a medical explanation to avoid revisiting her past.
I began treatment by educating Tina about her body’s fear response and why it felt to her like she was having a heart attack. Her racing heart, lightheadedness, rapid breathing, and sense of danger were the result of the amygdala’s multiple signals to prepare to fight or run. Gaining conscious regulation of her amygdala’s alarm circuitry was the first order of business. We discussed strategies to ward off these attacks by slowing her breathing and employing relaxation techniques. During sessions, I would have Tina make herself anxious, and then assist her in calming down. This provided her with a sense of mastery in regulating amygdala activation. Understanding what was happening in her body and knowing that her life was not in danger relieved some of her fear.
The second phase of treatment focused on addressing the long-standing lifestyle issues that kept her in a chronic state of stress. We examined the heavy burden of responsibilities she carried and her lack of relaxation and recreation. Tina’s financial fears led her to overbook her work schedule to the point of exhaustion. I learned that Tina constantly criss-crossed the Los Angeles freeway system, traveling between 30,000 and 40,000 miles each year. Between showing homes and shuttling her children from school to their various activities, we calculated that she was fighting traffic up to 6 hours a day, usually behind schedule. She began to understand the panic attacks as her body’s way of telling her to make some changes to reduce her level of stress. Regular exercise, decreasing her sales territory, and making alternative arrangements for some of her children’s transportation proved to be the most helpful solutions in these areas.
As these interventions became more routine, we explored the impact of her childhood experiences on both her self-image and lifestyle. Tina harbored the fear that she would die like her mother, leaving her children alone in the world. She tried to do everything she could for them, and save all the money they would need to go to college, all the time thinking that she would not be around much longer. Her financial planning was detailed and over the years she had followed through with it almost to the letter. The problem was that it had originally been created for two incomes; now she was doing it on her own. She came to see that her fear of death might become a self-fulfilling prophecy. Tina also came to realize that her heart was still broken over her mother’s death, and that she had never allowed herself to grieve her loss, a luxury she had felt she could not afford. Opening herself to these feelings of loss was the beginning of her therapy.
Summary
The fearful brain has two interconnected systems responsible for different aspects of fear processing. The fast or taxon system—with the amygdala at its core—makes rapid, reflexive, and unconscious decisions to provide for immediate survival. This system develops first and organizes learning related to attachment and affect regulation. It involves sensory, motor, and affective memories typical of early life and later traumatic memories. The slow or locale system, based in hippocampal-cortical networks, contextualizes and makes conscious what is being processed. The slow system’s job is to regulate the activity of the amygdala by modulating its output based on a more complex appraisal of potentially dangerous situations. This system contextualizes experience in time and space, and supports conscious awareness via cortical connectivity.
These two systems, reflecting both top-down and left-right circuits, can become dissociated during prolonged periods of stress or trauma. In psychotherapy, we attempt to activate both fast and slow circuits, taxon and locale systems, and implicit and explicit forms of memory to inform and educate each about the other. When emotional taxon networks are inhibited, we use techniques to trigger them so that they can be activated and integrated with slow locale circuits. When these same networks are out of control, we recruit locale circuits to contextualize them in time and space and allow them to be tamed by the descending, inhibitory capabilities of cortical processes. The overall goal is the activation and integration of both systems.