CHAPTER 28
Formulary
Karen Watson1 and Aisling Ryan2
1 Orpington, Kent, UK
2 King's College Hospital, London, UK
Introduction
Modern dermatology treatment continues to be dominated by the use of traditional topical therapy. However, there is an increased emphasis on injectable biological therapies targeting specific inflammatory pathways.
Topical therapy
The skin has the advantage of being readily amenable to treatment with topical therapy. Relatively high concentrations of medication can be applied to the skin safely, with good efficacy and comparatively few side effects. Several factors govern the choice of topical treatment, such as formulation, frequency of application, site and severity of skin disease and patient preference and ability to apply local therapy. Complications tend to be local irritant or allergic reactions. The choice of topical treatment depends on the disease process, pharmaceutical properties of the drug, site of application and cosmesis.
Emollients
Emollients are important in the treatment of dry, scaly, and inflammatory skin conditions as they help reduce transepidermal water loss from a damaged epidermal barrier. They soften dry skin by filling in the spaces left by desquamating keratinocytes. There is even some evidence pointing towards emollients applied daily to a baby's skin from birth possibly preventing atopic eczema.
The constituents of an emollient or topical base have significant properties. Lipids, for example, cover the stratum corneum to prevent evaporation of water. White and yellow soft paraffin and liquid paraffin are extracted from crude oil. They are stable, inert hydrocarbons, which form the basis of most commercially available ointments and emollients. Emulsifying agents are used to stabilise emulsions, which are immiscible mixtures of aqueous and oily constituents, and penetration enhancers, such as urea and propylene glycol, may be used to increase penetration of an active component through the skin. Humectants are compounds with a high affinity for water, which can draw water into the stratum corneum and have useful emollient properties.
The properties of various formulations of topical therapy are outlined in Table 28.1. Emollients can be applied liberally and regularly to all areas of dry skin.
Table 28.1 Comparison of formulations for topical therapy.
| Formulation | Characteristics | Advantages | Disadvantages |
| Ointments | Oil‐based. Provide occlusive film over skin and help retain water. Aid skin hydration and penetration of topical treatment | Tend not to require preservatives as lack of water in preparation prevents microbial growth | Greasy and cosmetically less appealing to use |
| Creams | Emulsions containing water and oil. May be composed of oil in water or water in oil (oily creams). Aid skin hydration, but generally less effectively than ointments | Cosmetically acceptable | Contain preservatives, which may cause sensitisation |
| Lotions | Watery suspensions, often containing alcohol | Easily spread over a large area. Evaporation of water or alcohol has a drying, cooling effect Cosmetically acceptable Useful for hair‐bearing areas, such as the scalp |
Contain preservatives and therefore have sensitising potential. Alcohol may cause stinging |
| Gels | Semisolid emulsion in alcohol base. Useful for suspending insoluble drugs Good absorbent properties |
Tend to dry on skin Useful for hair‐bearing areas. Cosmetically acceptable especially for use on the face |
Relatively high irritant and sensitising potential |
Moisturising soap substitutes applied to the skin and washed off are an important part of managing inflammatory skin disease, as regular soaps are irritant detergents that remove intercellular lipids and disrupt the barrier function of the stratum corneum.
Topical immunomodulatory treatments
Topical corticosteroids
Mode of action
Topical corticosteroids have been used to treat a wide range of inflammatory dermatoses since the 1950s. Steroid diffuses through the stratum corneum, cell membrane and into the cytoplasm of keratinocytes where it binds to the glucocorticoid receptor causing activation. The ligand‐bound receptor enters the nuclear compartment and interacts with glucocorticoid response elements (GREs), resulting in the modulation of gene transcription. In addition, the ligand‐bound receptor may also inhibit other transcription factors. The overall effect is to suppress inflammatory cytokines, inhibit T‐cell activation, and reduce cell proliferation.
Classification of topical steroids
Topical corticosteroids are classified according to their potency, which is thought to be related to their glucocorticoid receptor affinity.
Topical steroids are divided into four classes:
- Class 1 Super‐potent (600 times as potent as hydrocortisone): for example, clobetasone propionate (Dermovate®) and mometasone (Elocon®).
- Class 2 Potent (150 times as potent as hydrocortisone): for example, betamethasone valerate (Betnovate®).
- Class 3 Moderate (25 times as potent as hydrocortisone): for example, clobetasone butyrate (Eumovate®).
- Class 4 Mild – hydrocortisone.
This classification allows determination of the relative strength and therefore the efficacy and potential side effects of therapy. Generally, the weakest steroid to effectively treat the skin condition should be chosen. Milder steroids should be used on the face and flexural sites (Table 28.2 provides a detailed outline of topical steroids and their relative potencies).
Table 28.2 Relative potency of topical corticosteroids.
| Generic name | Proprietary name | Potency |
| 1% hydrocortisone | Efcortelan® | Mild |
| 1% hydrocortisone acetate and 1% fusidic acid | Fucidin H® | Mild |
| 1% hydrocortisone, 1% nystatin 100 000 units/g and 3% oxytetracycline | Timodine® | Mild |
| Clobetasone butyrate 0.05% | Eumovate | Moderate |
| Alclometasone dipropionate 0.05% | Modrasone® | Moderate |
| Betamethasone valerate 0.1% | Betnovate | Potent |
| Mometasone furoate 0.1% | Elocon | Potent |
| Diflucortolone valerate 0.1% | Nerisone® | Potent |
| Betamethasone dipropionate 0.05% and 3% salicylic acid | Diprosalic® | Potent |
| Betamethasone valerate 0.1% and fusidic acid 3% | Fucibet® | Potent |
| Clobetasol propionate 0.05% | Dermovate | Super‐potent |
| Clobetasol propionate 0.05%, neomycin sulfate 0.5% and nystatin 100 000 units/g | Dermovate NN® | Super‐potent |
Topical corticosteroids should be applied ‘sparingly’. However, this is difficult to define and therefore the finger‐tip unit (FTU) system was devised. One FTU (a line of ointment from the tip of the finger to the first skin crease) is enough steroid to treat a hand‐sized (palmar and dorsal surface) area of affected skin. This assumes a 5 mm nozzle and equates to 0.5 g of ointment/cream. In medical practice, it is common for patients to use insufficient amounts of topical steroids due to the fear of potential complications.
Side effects of topical steroids
- Skin atrophy (Figure 28.3)
- Telangiectasia
- Striae (Figure 28.1)
- Ecchymosis
- Hirsutism
- Folliculitis
- Perioral and periorbital dermatitis (Figure 28.2)
- Steroid‐induced acne/rosacea (Figure 28.3)
- Absorption and suppression of the hypothalamic pituitary axis (HPA)

Figure 28.1 Liberal application of a potent topical steroid resulting in striae formation.
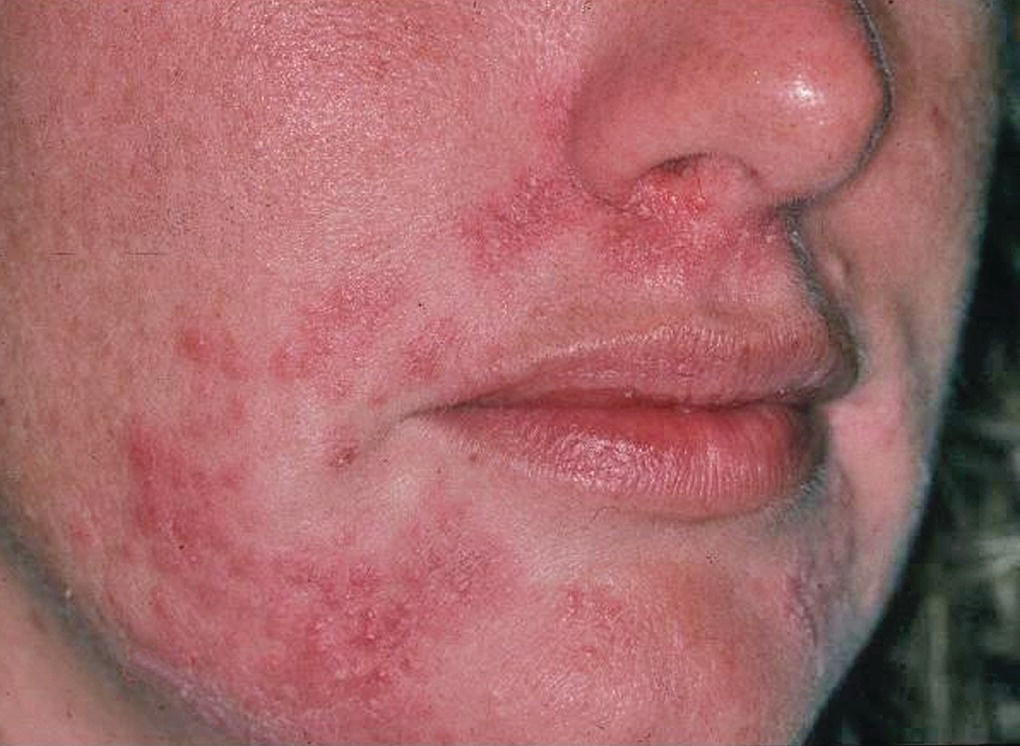
Figure 28.2 Perioral dermatitis caused by local application of topical steroids.
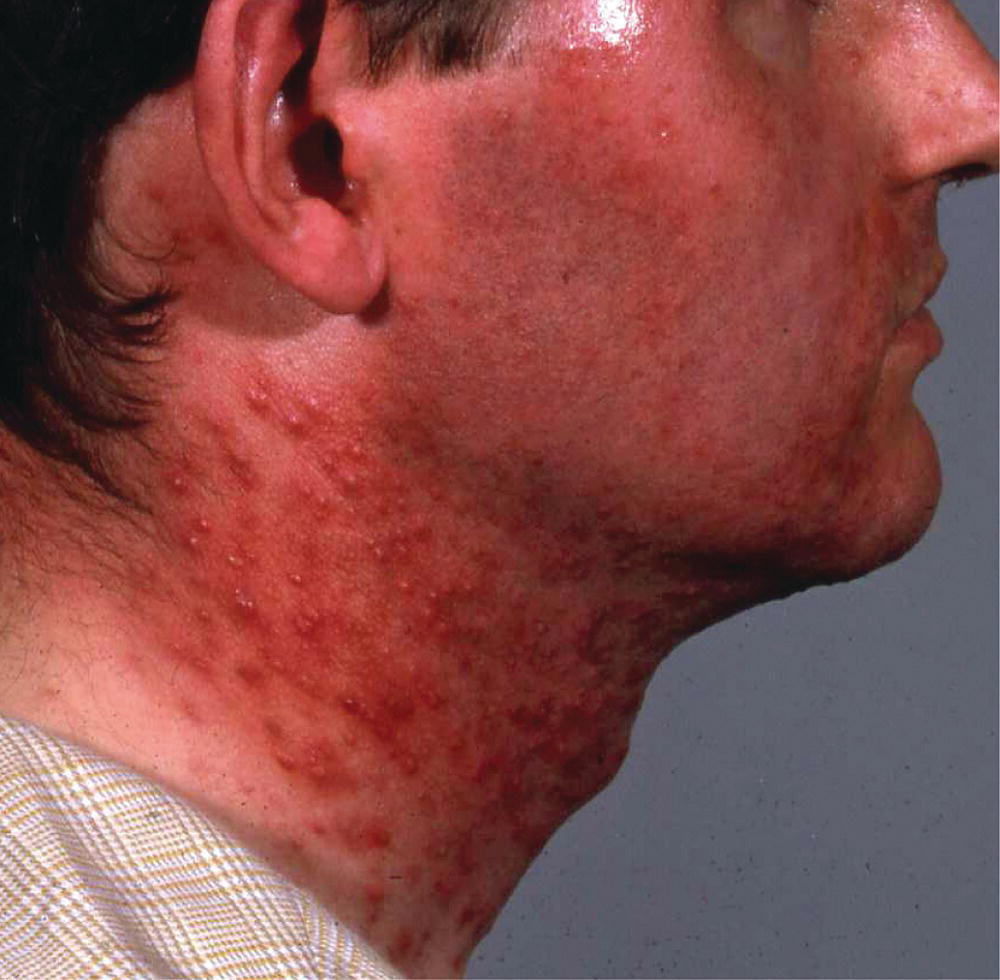
Figure 28.3 Potent topical steroid‐induced atrophy and acne.
Calcineurin inhibitors
Topical tacrolimus (ointment) and pimecrolimus (cream) were originally developed for the treatment of eczema (in patients over the age of two years). These agents inhibit calcineurin (a calcium and calmodulin‐dependent serine/threonine phosphatase) and suppress T‐cell activation. Topical tacrolimus has also been used to treat alopecia areata, oral and genital lichen planus, and vitiligo, with varying degrees of success. Pimecrolimus is less potent than topical tacrolimus and is used predominantly in the treatment of eczema in children as a steroid‐sparing agent.
Topical antimicrobials
Several topical antimicrobial preparations are available, some of which are summarised in Table 28.3.
Table 28.3 Topical antimicrobials used in the treatment of superficial infections.
| Preparation | Indications | Weaknesses | |
| Topical antibiotics | Fusidic acid (Fucidin ointment®) Mupirocin (Bactroban ointment®) Silver sulfadiazine (Flamazine®) |
Staphylococcal infections Gram‐positive and some gram‐negative organisms Treatment of nasal staphylococcal carriage Pseudomonal infection and some prophylaxis against staphylococcal infection |
Resistance Resistance Minimal absorption and renal impairment when applied to extensive burns |
| Topical antibiotics used in the treatment of acne | Tetracyclines Erythromycin Clindamycin |
Acne May be used in combination with keratolytics such as benzoyl peroxide |
Resistance May stain clothing yellow |
| Topical antifungals | Allylamines Terbinafine cream (Lamasil cream®) Imidazoles Clotrimazole (Canesten®) Econazole Ketoconazole Miconazole Tioconazole Amorolfine (Loceryl lacquer®) |
Fungicidal against dermatophyte infections | Ineffective against dermatophyte infections of the nails and scalp |
| Fungistatic Active against Candida and Pityrosporum May be used in combination with topical steroids Used in the treatment of intertrigo, pityriasis versicolor, and some dermatophyte infections |
Concurrent use of topical steroid may mask infection | ||
| Fungistatic Used in the treatment of onychomycosis Some activity against Scytalidium Synergistic activity with systemic antifungals |
Poor cure rates in dermatophyte infections affecting the nail matrix when used as sole therapy | ||
| Topical antivirals | Aciclovir cream (Zovirax®) Penciclovir cream (Denavir®) |
Used to treat labial and genital herpes simplex | Needs to be applied as early as possible in the episode for maximum benefit |
| Anti‐parasitic agents | Permethrin Malathion Ivermectin |
5% cream used in the treatment of scabies and pubic lice. 1% rinse used to treat head lice Used in the treatment of scabies, head lice, and pubic lice 1% cream used to treat rosacea, cutaneous larva migrans |
Require two treatments one week apart Alcoholic lotions can irritate skin and can exacerbate eczema |
Miscellaneous topical therapy used in the treatment of psoriasis
These are outlined in Table 28.4.
Table 28.4 Miscellaneous preparations used in the treatment of psoriasis.
| Preparation | Mode of action | Indications | Complications |
| Crude coal tar and coal tar solution | Unclear | Psoriasis | Messy to use |
| Derived from the distillation of organic matter | Tar has anti‐proliferative effects on the epidermis | Used in combination with ultraviolet radiation with additive effects | Potent odour Scrotal squamous cell carcinoma |
| Dithranol | Unclear | Psoriasis | Local reactions and irritation of normal surrounding skin |
| Available in cream formulation or in Lassar's paste in concentrations from 0.1% to 3% | Dithranol has potent anti‐proliferative effects | Short contact regimens used in outpatient settings | Skin staining |
Vitamin D3 analogues:
|
Regulate cell growth, differentiation, and immune function | Psoriasis | Hypercalcaemia Irritation Prolonged use of betamethasone and calcipotriol may precipitate the formation of pustules on withdrawal |
Topical anti‐proliferative agents
Topical 5 fluorouracil
5 fluorouracil (5% cream, 0.5% solution) is an antimetabolite, which blocks DNA synthesis by inhibiting thymidylate synthetase. It is used topically to treat actinic keratoses, Bowen's disease, and superficial basal cell carcinomas (BCCs). Treatment should be applied daily for four to six weeks. Main adverse effects include local erythema and irritation and, with continuous use, marked inflammation and erosions. These adverse effects may be ameliorated by treatment breaks and use of topical steroids.
Topical diclofenac
Diclofenac is a non‐steroidal anti‐inflammatory drug available in a 3% gel formulation for the treatment of mild actinic keratoses. The mechanism of action is unclear. It is generally well tolerated, although there may be some localised inflammation.
Topical imiquimod
Topical imiquimod (5% and 3.75% cream) is an immunomodulatory preparation, used to treat genital warts, vulval intra‐epithelial neoplasia (VIN), extra‐mammary Paget's disease, actinic keratoses, superficial BCCs, and lentigo maligna. It stimulates the innate immune system and promotes the development of antigen‐specific cell‐mediated responses via Toll‐like receptor 7. It causes considerable inflammation with oedema, erosions, and occasional ulceration. It is usually applied three times weekly for up to four months, depending on the indication.
Topical ingenol mebutate
Ingenol mebutate is derived from the plant Euphorbia peplus, which is grown in Queensland specifically to produce this gel, used for the treatment of actinic keratoses. The mechanism of action is unclear, but it appears to cause rapid lesion necrosis and neutrophil‐mediated antibody‐dependent cellular cytotoxicity. It is applied for two to three days depending on the site and may cause discomfort and irritation.
Miscellaneous agents
Keratolytics
Keratolytic agents are topical preparations used in the treatment of hyperkeratosis and acne. They help soften the skin and aid the removal of scale. They may also have anti‐comedogenic activity, although they can cause local irritation with erythema and dryness. Examples include salicylic acid and vitamin A derivatives such as tretinoin and adapalene.
Sunscreens
The aim of sunscreens is to block both ultraviolet A (UVA) and ultraviolet B (UVB) penetration of the skin and thereby inhibit the ageing and carcinogenic effects of ultraviolet (UV) radiation. Compounds used to achieve sun protection may either reflect and scatter UV light or absorb it. Examples of physical agents blocking UV include zinc oxide, titanium dioxide, and ferrous oxide. They tend to be used in combination with light absorbers such as para‐aminobenzoic acid (PABA) and benzophenones. The sun protection factor (SPF) of a sunscreen is an indication of the level of protection from UVB. An SPF over 15 is considered to confer good UVB protection when the sunscreen is applied adequately; however, evidence suggests most people apply insufficient amounts. UVA protection is measured on a 1–5‐star basis although there is little standardisation. Therefore, high factor sunscreen with UVA and UVB protection should be applied to exposed skin before going out in the sun, and this should be reapplied regularly to maintain protection.
Cosmetic camouflage
Cosmetic camouflage plays an important role in the treatment of patients with disfiguring conditions such as scarring, dyspigmentation, and port wine stains. Proprietary preparations are readily available (e.g. VitiColor®, Dermablend®) and the charity Changing Faces provides a volunteer‐led skin camouflage service for patients in the United Kingdom (www.changingfaces.org.uk).
Phototherapy
Phototherapy (see Chapter 3) is the treatment of skin disease with UV radiation alone and photochemotherapy is UV irradiation in combination with psoralen ultraviolet A (PUVA). Both are used extensively in dermatological practice to treat a wide range of skin disorders. Phototherapy involves the use of artificial UVB irradiation delivered by fluorescent lamps. UVB consists of electromagnetic energy of wavelength 290–320 nm and represents that part of the spectrum that is largely responsible for sunburn. UVA consists of energy of wavelength 315–400 nm. Both PUVA and narrow‐band UVB phototherapy are now widely used for the treatment of psoriasis, atopic eczema, polymorphic light eruption, mycosis fungoides, and vitiligo, among others.
Systemic therapy
Drugs used for infectious disorders
Antibacterial drugs
Antibiotics are used widely in dermatology for a range of conditions from acne to impetigo and cellulitis. They may be required for prolonged courses over a period of weeks to months. Host factors, drug properties, and causative pathogens should all be considered when choosing a suitable antibiotic. Host factors include underlying disease, age, previous adverse reactions, and pregnancy. Drug parameters include interaction with concomitant therapy, side effect profile, dosage, route of administration, and cost. Causative pathogens and their sensitivity/resistance patterns should ideally be identified through swabs taken for microbiology. Table 28.5 illustrates some of the antibiotics most commonly used in dermatology (mode of action, indications, and complications).
Table 28.5 Antibiotics used in dermatology, their method of action, indications, and complications.
| Antibiotic group | Method of action | Antibiotic | Indications | Considerations |
| Penicillin's | Inhibition of bacterial cell wall synthesis Activation of autolytic bacterial enzymes Bactericidal β lactamase resistant penicillin |
Penicillin | Gram‐positive infections, e.g. Streptococcus Cellulitis Erysipelas |
Hypersensitivity reactions which may be severe Dose reduction in renal impairment |
| Flucloxacillin | β lactamase producing organisms, e.g. Staphylococcus aureus Cellulitis Impetigo | Hypersensitivity reactions | ||
| Macrolides | Penetration of bacterial cell wall and inhibition of RNA‐dependent protein synthesis by reversible binding to ribosomes | Erythromycin | Gram‐positive infections Penicillin allergy Cellulitis Erysipelas Impetigo Acne Erythrasm |
Nausea, diarrhoea |
| Clarithromycin | Gram‐positive and gram‐negative cover Erysipelas | Fewer gastrointestinal side effects | ||
| Azithromycin | Short courses, long acting | |||
| Tetracyclines | Inhibition of protein synthesis by ribosomal binding | Oxytetracycline Minocycline Doxycycline Lymecycline |
Gram‐positive and gram‐negative organisms Mycobacteria Acne Rosacea Perioral dermatitis Bullous pemphigoid Lyme disease Fish tank granuloma |
Nausea, vomiting. Brown discolouration of teeth and delayed bone growth in children. Contraindicated in children under 12 years Hypersensitivity reactions Blue‐black pigmentation of nails and skin Photosensitivity |
Antifungal drugs
Most cutaneous fungal infections can be effectively treated with topical therapy. However, systemic treatment is required for fungal infections of the nails and hair. Terbinafine is a fungicidal allylamine, which binds to plasma proteins and is found in high concentrations in the hair, nails, and stratum corneum. In the treatment of tinea capitis oral terbinafine is more effective against endothrix organisms (Trichophyton tonsurans) than ectothrix infections (Microsporum canis). Terbinafine is licensed for use in children in several countries. Multiple studies have shown terbinafine to be safe and effective (severe liver injury is recognised but unusual). Treatment dosage is calculated according to the patient's weight (62.5 mg up to 20 kg; 125 mg up to 40 kg; 250 mg over 40 kg) and given daily for one month. Prolonged courses of three months or more are required in the treatment of onychomycosis involving the nail matrix.
Griseofulvin has fungistatic activity and has been used for many years to treat tinea capitis in children (weight < 50 kg; 10–20 mg/kg) given daily for six to eight weeks. However, terbinafine and itraconazole are often used in preference to griseofulvin as they are better tolerated and have a broader spectrum of activity. Griseofulvin is ineffective against pityriasis versicolor or yeast infections such as Candida albicans.
Itraconazole is a triazole used in pulsed therapy (one week per month) or continuously for the treatment of onychomycosis, tinea capitis, particularly in young infants (available as an oral solution) and pityriasis versicolor resistant to topical therapy.
Antiviral drugs
Systemic antivirals are available for the treatment of human herpes virus (HHV) infections such as herpes simplex virus (HSV) type 1 and type 2 (causing herpes labialis and genital lesions respectively) and varicella zoster virus (VZV) causing chickenpox and herpes zoster (shingles).
Aciclovir is a well‐established antiviral drug used in the treatment of HHV. It inhibits viral DNA polymerase and irreversibly inhibits viral DNA synthesis. The underlying diagnosis determines the dose and treatment duration. Primary genital herpes simplex requires 200 mg three times daily for five days while herpes zoster and chickenpox in adults require 800 mg three times a day for seven days.
Aciclovir tends to be most effective if therapy is started within 72 hours of disease onset. Secondary prophylaxis for recurrent and frequent attacks of HSV may be given at a dose of 200–400 mg twice daily. Intravenous administration is preferable in severely ill patients at risk of disseminated HSV (immunocompromised, eczema herpeticum). A topical preparation of acyclovir/penciclovir is also available for the treatment of mild herpes labialis.
Alternative oral antivirals include valaciclovir and famciclovir, which are licensed for the treatment of herpes zoster and primary and recurrent genital herpes. Their advantage is that they are better absorbed through the gut and are useful in the treatment of acyclovir‐resistant HHV infections (most common in patients with HIV or post bone marrow transplantation), though they are more expensive.
Antiparasite drugs
Scabies and pediculosis are usually adequately treated with topical therapy. Most studies show that 5% permethrin cream applied to the skin, left on overnight and repeated after seven days, is highly effective in treating scabies. However, Norwegian/resistant scabies and pediculoses refractory to conventional topical preparations may be amenable to treatment with oral ivermectin (200 μg/kg). Ivermectin causes paralysis and death of parasites and a single dose is usually sufficient. Ivermectin is available on a named‐patient basis only in the UK. Topical 1% ivermectin cream has also been shown to be effective in the treatment of rosacea, scabies, and cutaneous larva migrans.
Larva migrans (hookworm) and larva currens (strongyloides) are effectively treated with oral albendazole (400 mg once daily for three days) or ivermectin 200 mcg/kg two doses (see Chapter 17).
Systemic immunomodulatory drugs
Corticosteroids
Systemic corticosteroids are used in the treatment of a wide range of inflammatory dermatoses. They are effective and often life‐saving immunosuppressant and anti‐inflammatory agents but need to be used with caution as they may have adverse effects. These include hyperglycaemia, hyperlipidaemia, hypertension, sodium and fluid retention, atherosclerosis, suppression of the HPA, growth retardation, osteoporosis, avascular necrosis of bone, alteration of fat distribution, myopathy, increased incidence of infection, reactivation of tuberculosis, peptic ulceration, glaucoma, cataracts, striae and psychiatric disorders. The indications, risks, benefits, potential adjuvant steroid‐sparing therapy, and gastro and bone protection should therefore be carefully considered. However, systemic corticosteroids are particularly useful in controlling vasculitis, connective tissue disorders, sarcoidosis, erythroderma, lichen planus, and neutrophilic dermatoses among others. They are relatively contraindicated in psoriasis as withdrawal of the steroid may precipitate an exacerbation or generalised pustular psoriasis.
Patients treated with corticosteroids should be monitored closely for adverse effects, and they should be weaned off therapy slowly. A reducing course over six weeks is often used to treat severe exacerbations of atopic dermatitis. Patient education is important for those on long‐term treatment. They should be provided with a steroid treatment card, which outlines important information for patients and carers.
Methotrexate
Methotrexate is an antimetabolite and is a potent inhibitor of the enzyme dihydrofolate reductase. It competitively and irreversibly binds to dihydrofolate reductase with a much greater affinity than its natural substrate folic acid, thereby preventing the conversion of dihydrofolate to tetrahydrofolate. This is an important step in the synthesis of thymidylate and purine nucleotides needed for DNA and RNA synthesis, and results in inhibition of cell division.
Methotrexate is very useful in the treatment of psoriasis and atopic dermatitis. It is thought to act as an immunomodulator by inhibiting DNA synthesis in lymphocytes rather than having an antiproliferative effect. It is also used in sarcoidosis, connective tissue disease, bullous pemphigoid, vasculitis, and morphoea.
Methotrexate is taken once a week, the dose being carefully titrated by 2.5 mg increments. It has several side effects including bone marrow suppression, hepatotoxicity, nausea and vomiting, pulmonary fibrosis and teratogenicity, and patients need careful monitoring. Liver biopsy and procollagen III measurements have been surpassed by the use of ultrasound FibroScan technology as an indirect measure of liver fibrosis. Folic acid 5 mg once/week or several times per week is taken in addition to prevent folate deficiency, reduce nausea and hepatotoxicity. Acute methotrexate overdose or toxicity may be treated with folinic acid, which bypasses the metabolic effects of methotrexate. Methotrexate also has several potentially serious drug interactions including non‐steroidal anti‐inflammatory drugs, antibiotics, corticosteroids, and omeprazole.
Azathioprine
Azathioprine is an antimetabolite, which inhibits DNA and RNA synthesis, and the differentiation and proliferation of lymphocytes. It is an immunosuppressant and is often used in conjunction with corticosteroids as it has steroid‐sparing effects. Azathioprine is an effective treatment in a wide range of dermatological conditions, such as severe atopic eczema, chronic actinic dermatitis, immunobullous disorders, systemic lupus erythematosus, and dermatomyositis. Although it is usually well tolerated, it has several side effects including bone marrow suppression, nausea and vomiting, hypersensitivity reactions, hepatotoxicity, macrocytosis, pancreatitis, and diffuse hair loss. Bone marrow suppression may be predicted in susceptible patients who have low levels of the enzyme thiopurine methyl transferase (TPMT), and who are therefore unable to metabolise the drug efficiently. Unfortunately, the other side effects of azathioprine cannot be predicted by the TPMT activity.
Ciclosporin
Ciclosporin is an immunosuppressant drug derived from the fungus Tolypocladium inflatum. It suppresses the induction and proliferation of T‐lymphocytes and inhibits the production of inflammatory cytokines. It is effective in the treatment of severe psoriasis (including erythrodermic psoriasis and palmo‐plantar pustulosis), atopic eczema, and possibly in severe drug eruptions such as toxic epidermolytic necrolysis (TEN). Its advantages include rapid onset of action (one to two weeks) and lack of bone marrow suppression. However, it has several side effects, such as renal toxicity, hypertension, hypertrichosis, tremor, and gingival hyperplasia. There is also an increased risk of malignancy. Ciclosporin therefore tends to be used for short periods to treat severe flares of disease, or as part of a rotational regimen. It is metabolised by cytochrome P450 and interacts with several other drugs. Ciclosporin is usually given between 3 and 5 mg/kg/day in two divided doses.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an immunosuppressant agent, which acts by selectively and irreversibly inhibiting inosine monophosphate dehydrogenase, resulting in the depletion of intracellular guanine nucleotides. It seems to have a selective effect on activated T‐lymphocytes. In dermatology, it is mainly used for the treatment of immunobullous disorders and pyoderma gangrenosum. Side effects are predominantly gastrointestinal, with nausea, vomiting, and diarrhoea. The elderly generally more susceptible to the adverse effects of MMF, which also include bone marrow suppression, infection, fatigue, headaches, and weakness. Treatment doses in dermatology usually range from 250 mg to 1 g twice daily.
Dimethyl fumarate
Self‐experimentation by the German Chemist Schweckendiek led to the discovery that fumaric acid esters were useful in the treatment of psoriasis. Dimethyl fumarate (DMF) is approved for use in patients with severe psoriasis unresponsive to or ineligible for standard systemic therapies. The mode of action of DMF and its metabolite monomethyl fumarate is not fully understood but is thought to be intracellular, resulting in a shift in T‐helper cells from the Th1 and Th17 profile to a Th2 phenotype. DMF is less effective than biologic therapy and associated with significant symptomatic adverse events. Gastrointestinal upset occurs in two‐thirds of patients and typically includes diarrhoea, abdominal pain, nausea, and flatulence. Flushing occurs in up to 20% of patients. Lymphopenia occurs in 10% of patients and necessitates regular monitoring of full blood count. A lymphocyte count below 0.7 × 109/l is an indication for dose reduction.
Apremilast
Apremilast is approved for severe chronic plaque psoriasis in adults unresponsive to or ineligible for standard systemic therapies. Apremilast is an oral small molecule which inhibits the enzyme phosphodiesterase‐4 (PDE4) which plays a key role in intracellular T‐cell signalling. Inhibition of PDE4 down‐regulates the expression of key cytokines in psoriasis including tumour necrosis factor alpha (TNF‐α) and IL‐23.
Apremilast is significantly less effective than biologic therapy. It is associated with several adverse events early in treatment. Gastrointestinal upset is the most commonly reported adverse event (about 15%) and typically involves mild to moderate nausea and diarrhoea. It is usually self‐limiting and resolves within four weeks. Less commonly reported adverse events include upper respiratory tract infections and headache. Clinical trials and post‐marketing surveys suggested an increased risk of serious psychiatric symptoms, including depression, suicidal thoughts, and suicidal behaviours in patients taking apremilast. As such these symptoms are known to be more common in patients with psoriasis, the true significance of the reports therefore is unclear. Nonetheless, caution is indicated.
Systemic retinoids
Retinoids are derived from vitamin A and include acitretin, isotretinoin, alitretinoin, and bexarotene. They activate nuclear receptors and regulate gene transcription. They have anti‐inflammatory, anti‐keratinising, anti‐sebum, anti‐tumour, and anti‐proliferative effects. Acitretin is used in the treatment of psoriasis, Darier's disease, pityriasis rubra pilaris, ichthyosis, keratodermas, and in transplant recipients who are at high risk of developing cutaneous malignancies. Isotretinoin is the drug of choice for treating severe nodulocystic acne and timely initiation of treatment is aimed at preventing significant scarring. It may also be used in hidradenitis suppurativa, dissecting cellulitis of the scalp and severe recalcitrant papulopustular rosacea. Alitretinoin is used for the treatment of severe chronic hand eczema, which is refractory to treatment with topical corticosteroids. Bexarotene is reserved for the treatment of cutaneous T‐cell lymphoma.
Systemic retinoids have several side effects, the most important of which is teratogenicity. Women of child‐bearing age must use a robust form of contraception for at least a month prior to and during treatment. Isotretinoin, alitretinoin, and bexarotene have a relatively short elimination half‐life and contraception needs to be continued for at least a month after discontinuation of therapy. Acitretin has a much longer half‐life and pregnancy needs to be avoided for at least three years after treatment has stopped. The side effect profile of systemic retinoids is summarised in Table 28.6. Acitretin is usually prescribed at doses ranging from 10 to 50 mg daily. Isotretinoin dosage is based on weight between 0.5 and 1 mg/kg/day with a treatment course for severe acne usually being given as a total target dose of 120–150 mg/kg. The dose of alitretinoin is 30 mg daily, reducing to 10 mg daily in patients with side effects on the higher dose.
Table 28.6 Side effects of systemic retinoids.
| Teratogenicity |
| Depression |
| Cheilitis |
| Hypercholesterolaemia |
| Hypertriglyceridaemia |
| Elevation of transaminases |
| Hepatitis |
| Pancreatitis |
| Myopathy |
| Reduced night vision |
| Dry eyes |
| Epistaxis |
| Facial erythema |
| Photosensitivity |
| Hair loss |
| DISH |
| Premature epiphyseal closure |
| Leucopeniaa |
| Agranulocytosisa |
| Hypothyroidisma,b |
DISH, diffuse interstitial skeletal hyperostosis.
a Predominantly a risk with bexarotene.
b Predominantly a risk with aliltretinoin.
Antihistamines
Histamine has numerous effects on the skin, causing itching, vasodilatation, and increased vascular permeability predominantly through its action on H1 receptors. Antihistamines reversibly block H1 receptors. First‐generation antihistamines tend to be sedating and include chlorpheniramine, hydroxyzine, and promethazine. Second‐generation antihistamines tend to be non‐sedating, have a slower onset and longer duration of action. They include cetirizine, loratidine, fexofenadine, levocetirizine, and desloratidine. They play a central role in the treatment of urticaria, angioedema, type 1 hypersensitivity reactions, anaphylaxis, pruritus, cutaneous mastocytosis, and acute insect bite reactions. They tend to be well tolerated although side effects include drowsiness, anticholinergic activity, and arrhythmias. Topical antihistamines should be avoided because of the risk of developing allergic contact dermatitis.
Miscellaneous drugs
Dapsone
Dapsone is a sulfonamide, traditionally used in the treatment of leprosy. Its mode of action is unclear, but it is particularly useful in the treatment of disorders where neutrophils or IgA immune complexes play a role, for example, dermatitis herpetiformis, bullous pemphigoid, mucous membrane pemphigoid, linear IgA disease, and pyoderma gangrenosum. Side effects include dose‐related haemolysis and haemolytic anaemia, which are more common in those individuals with glucose‐6‐phosphate dehydrogenase (G6PD) deficiency. G6PD should therefore be measured prior to starting treatment. Other adverse effects include agranulocytosis, methaemoglobinaemia, hypersensitivity syndrome, and peripheral neuropathy.
Antimalarials
Hydroxychloroquine, mepacrine, and chloroquine are used to treat systemic lupus erythematosus, discoid lupus erythematosus, subacute cutaneous lupus erythematosus, sarcoidosis, polymorphic light eruption, and porphyria cutanea tarda. Their mode of action is thought to involve interruption of antigen processing and inhibition of inflammatory cytokines. Chloroquine can cause irreversible retinopathy, and mepacrine is unlicensed in the United Kingdom. Hydroxychloroquine therefore tends to be the antimalarial of choice in the treatment of dermatological disorders. It is well tolerated at doses of 200 mg once or twice daily (lower doses should be used in low body weight patients) although retinal toxicity can rarely occur. Visual acuity should be monitored by an optician for those patients on long‐term therapy.
Biological therapies
Biologics are drugs whose active substance is made by a living organism. Small‐molecule medicines (SMOLs) are conventional drugs made by chemical synthesis. Biologics are large, complex molecules; most are proteins or polypeptides. In contrast, SMOLs have a low molecular weight. Being large protein molecules, biologics are intrinsically unstable; they are poorly absorbed from the gastrointestinal tract and easily degraded by gastric acids and enzymes. They are usually administered parenterally.
Biologics used in psoriasis
Psoriasis is a complex immunologic disease which occurs in genetically susceptible individuals exposed to appropriate environmental stimuli. Our understanding of psoriasis has evolved significantly in the last decade. It is now recognised that the key cytokine in psoriasis is interleukin‐17 (IL‐17), which is produced by T‐helper 17 (Th17) cells in response to differentiation, which is induced by interleukin‐23 (IL‐23). TNF‐α is also over‐expressed in psoriasis. Its effects are mediated via the IL‐23/Th17 pathway, with which it interacts at two points. Upstream, TNF‐α stimulates myeloid dendritic cells to produce IL‐23. Downstream, TNF‐α and IL‐17 interact synergistically in keratinocytes to increase transcription of many psoriasis‐related genes. As the importance of TNF‐α in psoriasis was recognised long before that of IL‐17, biologics targeting TNF‐α were among the first developed for psoriasis.
There are currently three tumour necrosis factor inhibitors (TNFi) approved for use in psoriasis in the UK: infliximab, adalimumab, and etanercept. A fourth, certolizumab, is currently being assessed by NICE (the National Institute for Health and Care Excellence) and approval is expected in April 2019. There are a few key structural differences between these TNFi. Etanercept is a soluble receptor; infliximab and adalimumab are monoclonal antibodies. Infliximab is chimeric (it has a murine variable region and a human constant region). Adalimumab is fully human. Certolizumab is an antibody fragment, which is pegylated (conjugated with polyethylene glycol). Because certolizumab lacks an Fc portion, it is not transported across the placenta in pregnancy and will be useful in women of child‐bearing age.
TNFi are effective and are well tolerated. Like other biologic agents, the TNFi lack traditional end‐organ toxicity (hepatotoxicity, nephrotoxicity) and require less frequent monitoring of laboratory parameters. Important but uncommon adverse events include tuberculosis (reactivation and de novo disease), reactivation of hepatitis B, demyelination, and exacerbation of heart failure.
IL‐12/23 inhibitors were developed after TNFi. Ustekinumab is a monoclonal antibody to the p40 subunit of IL‐12 and IL‐23. It prevents these cytokines binding to their T‐cell receptors and stimulating differentiation into T‐helper 1 (Th1) and Th17 cells, respectively. The efficacy of ustekinumab is similar to adalimumab. It is dosed less frequently and has shown better drug survival than the TNFi. Its side effect profile is similar.
IL‐17 inhibitors followed IL‐12/23 inhibitors. These target IL‐17 specifically, downstream of IL‐23. There are currently three IL‐17 inhibitors approved for psoriasis in the UK: secukinumab and ixekizumab are monoclonal antibodies to IL‐17A, brodalumab blocks its receptor. Where head‐to‐head trials have been undertaken, IL‐17 inhibitors have shown superior efficacy to TNFi and ustekinumab. They have thrown up some unique adverse events, however: such as candidiasis and induction or exacerbation of inflammatory bowel disease (IBD). This is not entirely surprising as IL‐17 is known to play a key role in defence against extracellular pathogens and maintaining gut mucosal integrity.
The latest class of biologics target IL‐23, upstream of IL‐17. Unlike ustekinumab, which targets the p40 subunit common to IL‐12 and IL‐23, IL‐23 inhibitors target the p19 subunit only, which is unique to IL‐23. Although early indications are that their efficacy is broadly similar to the IL‐17 inhibitors, they may be better tolerated. Candidiasis and induction or exacerbation of IBD were not seen in clinical trials. This may be due to the preservation of IL17 produced by macrophages, monocytes, and neutrophils, which are not dependent on IL‐23. Just one IL‐23 inhibitor is currently approved for use in psoriasis in the UK (guselkumab). Approval for others is expected soon.
Biologics used in atopic dermatitis
Atopic dermatitis is a T‐helper 2 (Th2) cell mediated disease in which the cytokines IL‐13 and IL‐4 play a key role. Dupilumab is a monoclonal antibody that binds competitively to the α‐subunit common to the type I and type II IL‐4 receptors, blocking IL‐4 and IL‐13 binding and signalling. Dupilumab was recently approved for use in moderate to severe atopic eczema in the UK. It is effective and generally well tolerated. Common adverse events reported in phase III trials were non‐serious, and included nasopharyngitis, upper respiratory tract infections, injection site reactions, skin infections (particularly HSV), and conjunctivitis. Following a long period with little drug development in atopic eczema, there are now many drugs in the pipeline, including topical, oral, and biological therapies.
Biologics used in chronic spontaneous urticaria
Chronic spontaneous urticaria (CSU) is characterised by recurrent wheals for at least six weeks. The immunopathogenesis of urticaria is not fully understood but immunoglobulin E (IgE)‐mediated histamine release from mast cells and basophils plays a key role. Omalizumab is a monoclonal antibody which binds to free IgE and prevents it from binding to its receptor on mast cells and basophils. Omalizumab is recommended as an add‐on therapy for patients with severe CSU which has not responded to standard treatment with H1‐antihistamines and leukotriene receptor antagonists (montelukast). Omalizumab is generally well tolerated: commonly reported adverse reactions include headache, upper respiratory tract infections, sinusitis, arthralgia, and injection site reactions.
Miscellaneous
Rituximab is an anti‐CD20 humanised monoclonal antibody originally developed for the treatment of non‐Hodgkin's lymphoma (NHL), which leads to transitory B‐cell depletion. It has been used in the treatment of cutaneous graft‐versus‐host disease, primary cutaneous large B‐cell NHL, paraneoplastic pemphigus, pemphigus vulgaris, pemphigus folliaceus, bullous pemphigoid, and epidermolysis bullosa acquisita.
Further reading
- Smith, C.H., Jabbar‐Lopez, Z.K., Yiu, Z.Z. et al. (2017). British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. British Journal of Dermatology 177: 628.
- Wakelin, S.H. (2014). Handbook of Systemic Drug Treatment in Dermatology. London: Manson.
- Wolverton, S.E. (2001). Comprehensive Dermatologic Drug Therapy. Toronto: WB Saunders.