1
Introduction
William G. Fahrenholtz1, Eric J. Wuchina2, William E. Lee3, and Yanchun Zhou4
1 Department of Materials Science and Engineering, Missouri University of Science and Technology, Rolla, MO, USA
2 Naval Surface Warfare Center, Carderock Division, West Bethesda, MD, USA
3 Centre for Advanced Structural Ceramics, Department of Materials, Imperial College London, London, UK
4 Science and Technology of Advanced Functional Composite Laboratory, Aerospace Research Institute of Materials and Processing Technology, Beijing, China
1.1 Background
The impetus for this book was the conference “Ultra-High Temperature Ceramics: Materials for Extreme Environment Applications II,” which was held on May 13–18, 2012, in Hernstein, Austria. As the title implies, this was the second conference on this topic that has been organized by Engineering Conferences International (ECI). The four editors served as the co-organizers of the conference. Both the U.S. Navy Office of Naval Research Global and the U.S. Air Force European Office of Aerospace Research and Development provided funding for the conference that helped support participation of invited speakers and students. The conference brought together about 60 researchers from around the world to discuss the latest research related to this remarkable class of materials. The first conference in the series was organized by Eric Wuchina and Alida Bellosi and was held in August 2008 at Lake Tahoe, California, United States. A third conference is planned for Australia in April 2015.
The book is our attempt to capture a snapshot of the current state of the art in ultra-high temperature ceramic (UHTC) materials. The chapters in this volume represent the key areas that were discussed in the meeting. The chapter authors are leaders in their fields from around the world, and all of the lead authors participated in the conference. Rather than a narrow focus on the latest scientific progress as would be expected in an article in a peer-reviewed journal, the chapters in this book provide a broader look at recent progress and information on the current understanding of this family of materials.
1.2 Ultra-High Temperature Ceramics
Recent interest in UHTCs has been motivated by the search for materials that can withstand extreme environments. The extremes can include, individually or in combination, the effects of temperature, chemical reactivity, mechanical stress, radiation, and wear. Some potential applications for this class of materials include microelectronics, molten metal containment, high-temperature electrodes, and wear-resistant surfaces. However, a majority of the research has been motivated by unmet material needs for hypersonic aviation. Specifically, improved materials are needed to withstand the conditions encountered by wing leading edges and propulsion system components in hypersonic aerospace vehicles as well as the extreme conditions associated with atmospheric reentry and rocket propulsion. The combination of extreme temperature, chemically aggressive environments, and rapid heating/cooling is beyond the capabilities of current engineering ceramics. Recent interest in UHTCs has been high as indicated by a number of special journal issues [1–3] and review articles [4–9] devoted to the topic. Despite this interest, no clear criteria have been established to differentiate UHTCs from other structural ceramics.
Broadly, ceramic materials can be defined as inorganic, nonmetallic solids [10]. This definition encompasses most materials that are typically considered to be ceramics such as clay-based traditional ceramics, alumina, piezoelectric materials, and silicon carbide. However, the definition still leaves some gray areas such as glass, carbon, and intermetallic compounds. In some cases, the definition is expanded to include other characteristics. For example, Barsoum defines ceramics as “solid compounds that are formed by the application of heat, and sometimes heat and pressure, comprising at least one metal and a non-metallic elemental solid, a combination of at least two nonmetallic elemental solids, or a combination of at least two nonmetallic elemental solids and a nonmetal” [11]. In other cases, the definition involves characteristics such as melting temperature, bonding type, or electrical properties [12]. Likewise, several different definitions have been espoused for UHTCs. The three main classifications are melting temperature, ultimate use temperature, and chemical composition.
The most common definition for a UHTC is a material that melts at a temperature of 3000°C or higher. As shown in Figure 1.1, very few materials meet this criterion. For example, only three elements have melting temperature above 3000°C, W, Re, and Ta, all of which are metals. Note that carbon was not included in this group because of the complexity of its behavior, which has been reviewed in detail elsewhere [13, 14]. Interestingly, ThO2 is the only oxide ceramic that has a melting temperature above 3000°C. Most of the materials that have melting temperatures above 3000°C are borides, carbides, and nitrides of early transition metals. Consequently, most studies on UHTCs focus on compounds such as ZrB2, HfB2, TaC, TaB2, ZrC, and HfC. While this definition is probably the most common, significant uncertainty exists in melting temperatures for these compounds. For example, ZrB2 is commonly reported to melt at 3250°C based on the phase diagrams reported by Rudy [15] and Portnoi et al. [16], but others report different melting temperatures including 3040°C by Glaser and Post [17] and 3517°C by Rogl and Potter [18] The discrepancies indicate that not only are temperatures difficult to measure precisely in the ultra-high temperature regime, but also that these compounds may decompose or dissociate before melting, which would also be difficult to detect in the experimental setups used for these studies. So, while melting temperature is a clearly defined metric, assessment includes uncertainty. Further, the selection of 3000°C as the criterion was arbitrary and could be set at other temperatures.
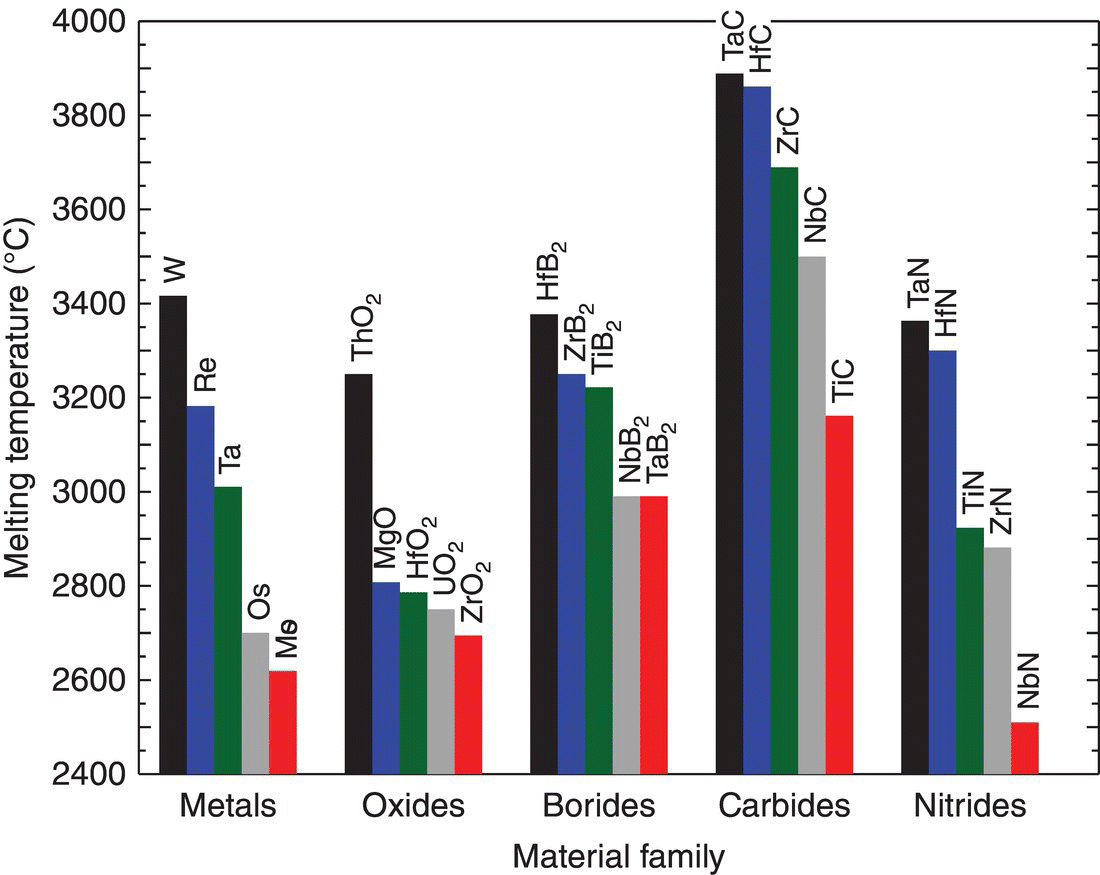
Figure 1.1. Materials with the highest reported melting temperature grouped by material family.
Reprinted with permission from Ref. [6].
A second method that can be used to define UHTCs is the highest temperature for use in air. This practical definition fits well with the nature of engineering materials, but introduces additional questions. As with melting temperature, the selection of a use temperature to define the ultra-high temperature regime is also somewhat arbitrary. At the time of publication of this chapter, a number of choices are available for use in air at temperatures up to about 1600 °C including alumina, magnesia, silicon carbide, and silicon nitride. Hence, the minimum use temperature for the ultra-high temperature regime should be above that level. Many hypersonic applications involve higher temperatures, so 2000 °C has been cited as the cutoff of the ultra-high temperature regime [5–19]. Despite a seemingly clear definition, both use temperature and duration blur the distinction. The application-driven criterion to define UHTCs has obvious attractions as a definition for engineering materials, but this metric also has some shortcomings.
The final method that can be used to define UHTCs is chemistry, which is the least quantitative, but probably most widely used. Most UHTC compounds are borides, carbides, or nitrides of early transition metals. Hence, any compound containing a transition metal such as Zr, Hf, Ta, W, or Nb along with B, C, or N has the potential to be a UHTC.
No one definition has emerged as the way to identify UHTCs. As an example of the shortcomings of all of the three definitions described earlier, consider one of the most commonly cited UHTC compositions, ZrB2–SiC. When compounds with melting temperatures above 3000 °C such as ZrB2 are combined intentionally with other phases (i.e., sintering aids, grain pinning additives, oxidation-enhancing additives, etc.) or when impurities are present, the temperature at which liquid forms or melting occurs can be below 3000°C. For ZrB2–SiC, the solidus temperature is about 2300°C due to a eutectic reaction [20]. Hence, one could argue that one of the most widely researched UHTC compositions does not meet any of the criteria defined earlier because: (i) liquid forms below 3000°C; (ii) one of the constituents (SiC) cannot be used for extended times above 1600°C; and (iii) SiC is not a boride, carbide, or nitride of an early transition metal.
Despite the uncertainty in the definition of UHTCs, a close-knit global community of researchers who focus on these materials has emerged over the past decade or so. Groups in the United States, Italy, China, Australia, Russia, and the United Kingdom, have worked competitively and collaboratively to advance our understanding of the fundamental behavior of materials that can be used in extreme environments. This book, in keeping with the spirit of the ECI conference series, takes a pragmatic approach in defining UHTCs and includes the community of researchers who focus on materials with the potential for use in extreme environments such as those associated with hypersonic flight, atmospheric reentry, and rocket propulsion. Since the first conference in the series in 2008, the community of researchers has grown to include those working on materials for nuclear applications and researchers who are investigating new methods for characterizing and testing materials under conditions that are representative of the extreme environments encountered in use.
1.3 Description of Contents
The sequence of the chapters in this book was selected to represent the progression of a typical experimental study. After this introductory chapter, the next chapter provides background information on previous research. The chapter focuses on historic studies on what we now consider UHTCs that were conducted in the United States in the 1950s, 1960s, and 1970s. These studies accompanied decisions that were being made about the design of the next generation of launch and reentry vehicles. The next group of chapters describes synthesis and processing. The third chapter describes synthesis of boride compounds, while the fourth focuses on calculations of fundamental bonding properties. The fifth chapter describes aqueous processing and surface chemistry. The sixth is the final chapter in the synthesis and processing section, and it describes densification and microstructure of UHTCs. The middle section of the book contains chapters describing the properties of UHTCs. This section includes contributions focused on thermomechanical properties, elevated temperature mechanical properties and deformation, and oxidation. The final section is focused on the performance of UHTC materials. The section includes a review of UHTC-based composites. Other chapters focus on TaC, UHTC nitrides, TiB2, and UHTCs for nuclear applications. The final chapter describes the testing of UHTCs in relevant environments. That chapter is based on the conference keynote presentation.
References
- 1. Joan Fuller and Michael Sacks, issue editors. Special Issue of the Journal of Materials Science, 39(19), October 2004, 5885–6066.
- 2. Joan Fuller, Yigal Blum, and Jochen Marschall, guest editors. Special Issue of the Journal of the American Ceramic Society, 91(5), May 2008, 1397–1502.
- 3. Joan Fuller, Greg Hilmas, Erica Corral, Laura Riegel, and William Fahrenholtz, guest editors. Special Issue of the Journal of the European Ceramic Society, 30(11), August 2010, 2145–2418.
- 4. Telle R, Sigl LS, Takagi K. Boride-based hard materials. In: Riedel R, editor. Handbook of Ceramic Hard Materials. Weinheim: Wiley-VCH; 2000. p 802–949.
- 5. Gasch MJ, Ellerby DT, Johnson SM. Ultra-High temperature ceramics. In: Bansal N, editor. Handbook of Composites. Boston (MA): Kluwer Academic Publishers; 2004. p 197–224.
- 6. Fahrenholtz WG, Hilmas GE, Talmy IG, Zaykoski JA. Refractory' diborides of zirconium and hafnium. J Am Ceram Soc 2007;90 (5):1347–1364.
- 7. Guo S-Q. Densification of ZrB2-based composites and their mechanical and physical properties: a review. J Eur Ceram Soc 2009;29 (6):995–1011.
- 8. Fahrenholtz WG, Hilmas GE. Oxidation of Ultra-High temperature transition metal diboride ceramics. Int Mater Rev 2012;57 (1):61–72.
- 9. Eakins E, Jayaseelan DD, Lee WE. Toward Oxidation-Resistant ZrB2–SiC Ultra-High Temperature Ceramics. Met and Mat Trans A 2011;42A: 878–887.
- 10. Kingery WD, Bowen HK, Uhlmann DR. Introduction to Ceramics. New York: John Wiley & Sons, Inc.; 1976.
- 11. Barsoum MW. Fundamentals of Ceramics. New York: McGraw-Hill; 1997.
- 12. Richerson DW. Modern Ceramic Engineering. 2nd ed. New York: Marcel-Dekker; 1992.
- 13. Bundy FP. Pressure-temperature phase diagram of elemental carbon. Phys A 1989;156 (1):169–178.
- 14. Bundy FP, Bassett WA, Weathers MS, Hemley RJ, Mao HK, Goncharov AF. The pressure-temperature phase and transformation diagram for carbon; updated through 1994. Carbon 1996;34 (2):141–153.
- 15. Rudy E. Ternary phase equilibria in transition metal-boron-carbon systems: part V, compendium of phase diagram data. Technical Report AFML-TR-65-2. Wright Patterson Air Force Base (OH): Air Force Materials Laboratory; 1969.
- 16. Portnoi KI, Romashov VM, Vyroshina LI. Phase diagram of the zirconium-boron system. Poroshkoviaia Metallugia 1970;10 (7):68–71.
- 17. Glaser FW, Post B. System zirconium-boron. Trans Metallurgical Soc AIME 1953;197:1117–1118.
- 18. Rogl P, Potter PE. A critical review and thermodynamic calculation of the binary system: zirconium-boron. Calphad 1988;12 (2):191–204.
- 19. Opeka MM, Talmy IG, Zaykoski JA. Oxidation-based materials selection for 2000°C+ hypersonic aerosurfaces: theoretical considerations and historical experience. J Mater Sci 2004;39 (13):5887–5904.
- 20. McHale AE, editor. Phase Diagrams for Ceramists Volume X: Borides, Carbides, and Nitrides. Westerville (OH): The American Ceramic Society; 1994. Figure 8672.