22
Testis Cancer: Diagnosis and Management in the Outpatient Clinic
Benjamin Patel
Testicular cancer (TC) is the most common solid cancer in men aged 20–45 with around 2400 new cases in 2016 in the UK. It constitutes 1% of male cancers and 5% of urological tumours. Since the early 1990s, the incidence has increased by 28% in males in the UK. The incidence is projected to further rise by 12% in the UK between 2015 and 2035 to 10/10 000 males. There is a peak incidence between 30 and 34 and it is rarely found in those below 15 years and above 60 years. (See Figure 22.1.) Encouragingly, mortality has fallen since the introduction of platinum‐based chemotherapy, with a 98% 10‐year survival in the UK. Indeed, in 2016 there were less than 60 deaths.
Aetiology
Aetiological factors are largely non‐modifiable. TC is more common in white western Caucasians. The most commonly affected age group is 20–45 years and there is a variable histological pattern of disease according to age. Non‐seminomatous germ cell tumours (NSGCTs) affect a slightly younger cohort (20–35 years) compared to seminomas (35–45 years). Infants and children below 10 years most commonly develop yolk sac tumours and 50% of TCs in those >60 years are lymphoma.
A previous diagnosis of TC is associated with a 12‐fold increased risk of metachronous TC, with bilateral TCs occurring in 1–2% of cases. 5–10% of TC patients have a history of cryptorchidism. In unilateral cryptorchidism, TC risk is 6 times greater in the undescended testicle and 1.7 times increased in the descended testicle. One large study indicated that those who undergo early orchidopexy (<13 years) have a twofold increased risk of TC, compared to a fivefold increased risk in those undergoing late orchidopexy (>13 years).
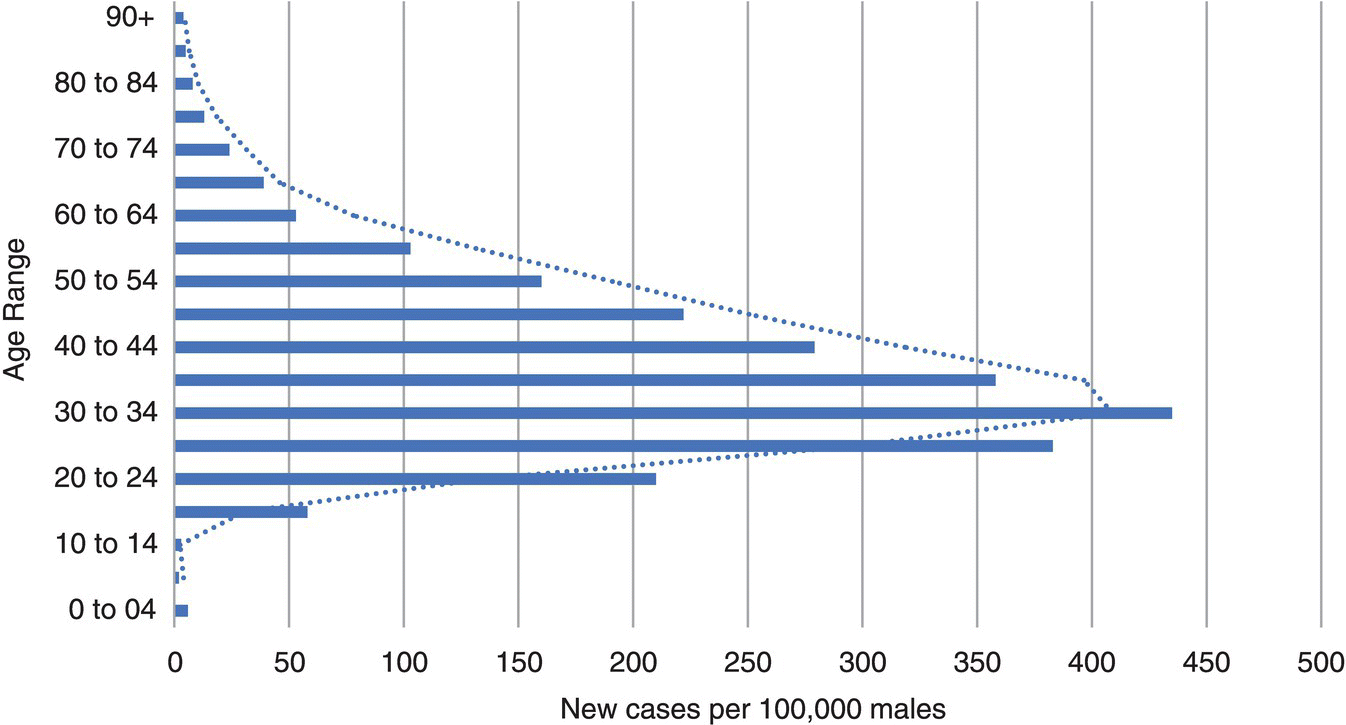
Figure 22.1 Average number of new cases per year per 100,000 males, UK.
Source: Based on graphic created by Cancer Research UK.
Genetic factors have also been identified. TC is 5 times higher in men with an affected father and 8–9 times higher in men with an affected brother. Additionally, Kleinfelter's syndrome and Kallman's syndrome are associated with increased TC risk.
In general, TC is not clearly linked to preventable factors. Human immunodeficiency virus HIV appears to increase risk of TC by 30–40%. There is weak evidence for chemical carcinogens and rural residence increasing risk. However, there is no strong evidence for smoking, alcohol, vasectomy, or trauma increasing risk.
Finally, Testicular carcinoma in situ, also known as intratubular germ cell neoplasia (ITGCN) or testicular intraepithelial neoplasia (TIN), is a precursor for TC; around 50% of men with cancer in situ (CIS) will develop TC within five years without treatment.
Symptoms and Signs
Testicular cancer most commonly presents as a hard, painless lump. It is slightly more common on the right side and bilateral in 1–2% of cases. Five percent of TCs present with acute scrotal pain, secondary to intra‐tumoral haemorrhage. Ten percent unfortunately present with symptoms of advanced disease, including weight loss, lumps in the neck, bone pain, chest symptoms and neurological symptoms. Lumbar back pain may occur if the psoas muscles and nerve roots are affected.
A proportion of TCs present during routine clinical examination, casual ultrasound (US) findings, or are revealed by scrotal trauma. On examination by bimanual palpation, testicular asymmetry may be identified. A hard, non‐tender, irregular and non‐trans illuminable mass may be felt in the testis. An associated hydrocele may be present if the tunica albuginea is breached. The epididymis, spermatic cord, and scrotal wall may be normal or involved in a small proportion of cases. Endocrine manifestations of certain TCs may results in gynaecomastia. Metastatic disease may result in supraclavicular lymphadenopathy, abdominal masses, hepatomegaly, lower limb oedema, chest signs, and cachexia.
Pathology and Subtypes
The majority of TCs are germ cell tumours (GCTs), subcategorised into seminomatous germ cell tumour (SGCT) and non‐seminomatous germ cell tumour (NSGCT) (see Table 22.1). Classic seminomas are well circumscribed, homogenous firm pale tumours. Anaplastic seminomas are similar to classic seminomas but have increased numbers of mitoses. Spermatocytic seminomas are found in an older cohort of men and are generally benign. Teratomas are heterogenous tumours composed of elements of fully differentiated tissue: mesoderm (bone, cartilage, muscle), ectoderm (neural tissue and stratified squamous including skin and derivatives such as hair follicles) and endoderm (including mucus glands).
Investigation
Ultrasound (US) is the first line investigation of scrotal lumps, with a sensitivity of almost 100% and will confirm whether a lump is intra‐ or extra testicular. It is inexpensive and should be performed to explore the abnormal and contralateral testes. Magnetic resonance imaging (MRI) of the scrotum has a greater sensitivity and specificity than US in diagnosing TC, but its high cost obviates its routine use.
Serum tumour markers play a role in diagnosis and differentiation, and they also have a prognosticating role. Alpha‐fetaprotein (AFP) (produced by yolk sac cells), human chorionic gonadotropin (hCG) (produced by trophoblasts) and lactate dehydrogenase (LDH) should all be measured before and seven days after orchidectomy. Beta‐hCG is elevated in 100% of choriocarcinomas, 40% of teratomas, and 10% of pure seminomas. Alpha‐fetaprotein can be elevated by embryonal carcinoma, teratoma, and yolk sac tumours. Pure seminomas and choriocarcinomas are not associated with raised AFP. Lactate dehydrogenase is elevated in half of TCs and is used to assess tumour burden. It is the only elevated marker in 10% of non‐seminomas. PLAP is elevated in 40% of patients with advanced germ cell tumours (GCTs), but is non‐specific and falsely elevated in smokers.
Table 22.1 Testicular cancer classification and distribution
| Germ cell tumours (90–95%) | Other tumours (5–10%) |
|---|---|
Seminoma (60%)
Non‐seminomatous (40%)
|
Stromal:
Lymphoma Metastatic from other site (<1%) Rhabdomyosarcoma Adenomatoid tumour Epidermoid cyst (benign) |
Imaging plays an important role. Computerized tomography (CT) is generally undertaken of the abdomen and pelvis to analyse extra‐testicular metastasis and lymph node involvement. Chest X‐ray (CXR) is utilised to exclude pulmonary disease, with further imaging of chest, brain, spine, and bones where clinically indicated. Importantly, biopsy is not generally advised for the evaluation of testicular masses. Diagnosis is instead established by histological analysis of the testis after removal.
All patients with suspected testicular mass should undergo inguinal exploration, alongside exteriorisation of the testis within its tunics. If a malignant tumour is identified, orchidectomy and division of the spermatic cord at the internal inguinal ring should be carried out. This is routinely performed in the ambulatory setting.
Staging of TC includes the anatomical extent of the primary tumour (pT), regional nodes (N) and distant metastases (M), alongside the assessment of serum tumour markers after orchidectomy (S).
Management of Seminomatous Germ Cell Tumours (SGCTs)
Stage I non‐locally invasive disease can be managed with surveillance, with a relapse rate of 15–20% at five years. In low‐risk groups (tumour size <4 cm and no rete testis invasion), the recurrence rate may be much lower. Chemotherapy may be utilised in the case of relapse under surveillance, although the majority of patients are suitable for radiotherapy alone because of the small volume of disease at the time of recurrence. Alternatively, stage I non‐locally invasive seminomas can be managed with single agent carboplatin chemotherapy. Whilst seminoma cells are extremely radiosensitive, the increased risk of radiation‐induced secondary non‐germ cell malignancies means that adjuvant radiotherapy is rarely used in stage I disease and has no role in young patients <40 years. Retroperitoneal lymph node dissection (RPLND) is not recommended in stage I seminoma.
Stage IIA/B seminomas may be managed with radiotherapy, with reported relapse rates of 9–24%, although long‐term radiotherapy‐associated morbidity such as secondary malignancies and cardiovascular events are a concern. Chemotherapy is an alternative, with similar reported relapse rates. Three cycles of bleomycin, etoposide, and cisplatin (‘BEP’) chemotherapy are generally employed, with four cycles of EP in cases where bleomycin toxicity is a concern.
Management of Non‐seminomatous Germ Cell Tumours (NSGCTs)
Options for stage I patients include active surveillance, adjuvant chemotherapy, and RPLND. Patients should be informed about all options, including recurrence rates and potential side effects, and the ultimate decision should take into account, risk based on vascular invasion. The largest studies of surveillance strategies suggest a cumulative relapse rate of 30%. Alternatively, patients may receive adjuvant chemotherapy with BEP, which appears to reduce relapse to under 5% with minimal long‐term toxicity. Salvage treatment of patients with recurrence during surveillance generally consists of three to four courses of BEP chemotherapy, followed by RPLND if necessary. The role of primary RPLND has now diminished in stage I disease, in view of the high cancer‐specific survival rates of surveillance with salvage treatment and the low relapse rates if adjuvant chemotherapy is employed.
In stage IIA/B NSGCTs, chemotherapy is generally employed, except for stage II disease without elevated tumour markers, in which RPLND or surveillance can be undertaken to clarify stage of disease.
Management of Metastatic Testicular Cancer
Metastatic SGCT and NSGCT are generally managed with three cycles of chemotherapy, alongside RPLND for residual or recurrent masses and salvage chemotherapy for relapsing disease.
Further Reading
- Kier, M.G., Lauritsen, J., Mortensen, M.S. et al. (2017). Prognostic factors and treatment results after bleomycin, etoposide, and cisplatin in germ cell cancer: a population‐based study. Eur. Urol. 71: 290.
- Laguna, M.P., Albers, P., Algaba, F. et al. (2019). Testicular Cancer. European Association of Urology. http://uroweb.org/guideline/testicular‐cancer.
- Tandstad, T., Ståhl, O., Håkansson, U. et al. (2014). One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann. Oncol. 25: 2167.