Review the anatomy of the peripheral nerves and brachial and lumbosacral plexi.
Understand the utility of different magnetic resonance imaging (MRI) sequences and magnetic resonance neurography (MRN) for identifying peripheral nerve diseases.
Describe the imaging characteristics of different peripheral nerve diseases to help narrow the differential diagnosis.
Discuss the role of advanced imaging techniques.
20.1 Introduction
The diagnostic work-up of peripheral neuropathies relies on the patient’s clinical history, physical examination, and electrophysiological studies, which usually provide enough information about the location, severity, and the etiology of the underlying nerve injury in the majority of patients. However, electrodiagnostic studies, such as electromyography (EMG), do not display the anatomic detail needed for precise localization and treatment planning because of the deep location of nerves and the variable innervation of regional muscles. EMG sensitivity for lumbosacral radiculopathy ranges from 49 to 86%. Therefore, imaging particularly with magnetic resonance imaging (MRI)/magnetic resonance neurography (MRN) and ultrasound (US) has an increasing role in the evaluation of peripheral neuropathies [1]. Although US is more operator-dependent than MRI and less effective in cases of deep nerves of the pelvis and lumbosacral plexus, it is very effective for dynamic assessment of superficial peripheral nerves abnormalities, such as changes in nerve caliber, continuity, and echogenicity [2].
20.2 Anatomy of Peripheral Nerves
Peripheral nerves range from 1 to 20 mm in size, the largest being the sciatic nerve. Peripheral nerves are formed by multiple axons, grouped into fascicles, the number of which depends on the size and length of the nerve. Endoneurium invests the Schwann cell–axon complex, with the inner border represented by the Schwann cell basement membrane and its outer border by the perineurium. The epineurium, the outermost connective tissue sheath, envelops the nerve and provides mechanical support for the axons. Endoneurial fluid within each fascicle is isolated from the general extracellular space by tightly adherent epithelial-like cells of the perineurium and from the circulating blood by the tight junctions between the endothelial cells of the endoneurial capillaries. The endoneurium and perineurium form a functional, relatively impermeable barrier known as the blood–nerve interface that protects the peripheral nervous system against toxic and infectious agents.
20.3 Brachial Plexus (BP)
The ventral rami of the C5 through T1 nerves give rise to the BP. The BP structures travel through the supraclavicular fossa paralleling the subclavian artery. Individual ventral rami or roots are best seen on axial and coronal images. The sagittal plane is helpful for following the BP structures from the spine to the axilla as they lie perpendicular to the plane. The upper (C5-C6), middle (C7), and lower (C8-T1) trunks travel between anterior and middle scalene muscles, forming the lateral border of the scalene triangle [3]. In the supraclavicular triangle, the trunks divide into anterior and posterior divisions just before passing posterior to the clavicle and going from the lateral aspect of the anterior scalene muscle to the lateral border of the first rib [3]. The cords (lateral, medial, and posterior) course from the mid-clavicle to the inferomedial coracoid process and are named according to location relative to the axillary artery. At the pectoralis minor muscle, just medial to the coracoid, axillary vein, and axillary artery, the cords change into the five terminal branches (radial, axillary, musculocutaneous, median, and ulnar nerves).
20.4 Lumbosacral Plexus (LSP)
The ventral rami of the L1-L4 spinal nerve roots and a small contribution from the 12th thoracic nerve coalesce within or posterior to the psoas major muscle to form the lumbar plexus [2]. The lumbar plexus gives rise to the iliohypogastric (L1), ilioinguinal (L1), genitofemoral (L1-L2), femoral (L2-L4), and lateral femoral cutaneous (L2-L3) nerves, which emerge lateral to the psoas major muscle. The obturator nerve and lumbosacral trunk come forward from the medial border of the psoas muscle. A minor (peroneal) branch of L4 combines with the ventral ramus of L5 to form the lumbosacral trunk. The latter descends over the sacral ala and joins the S1 to S3 ventral rami on the anterior aspect of the piriformis muscle to form the sacral plexus [4]. The sacral plexus gives rise to the sciatic (L4-S3), pudendal (S2-S4), superior gluteal (L4-S1), and inferior gluteal (L5-S2) nerves.
20.5 Magnetic Resonance Neurography (MRN)
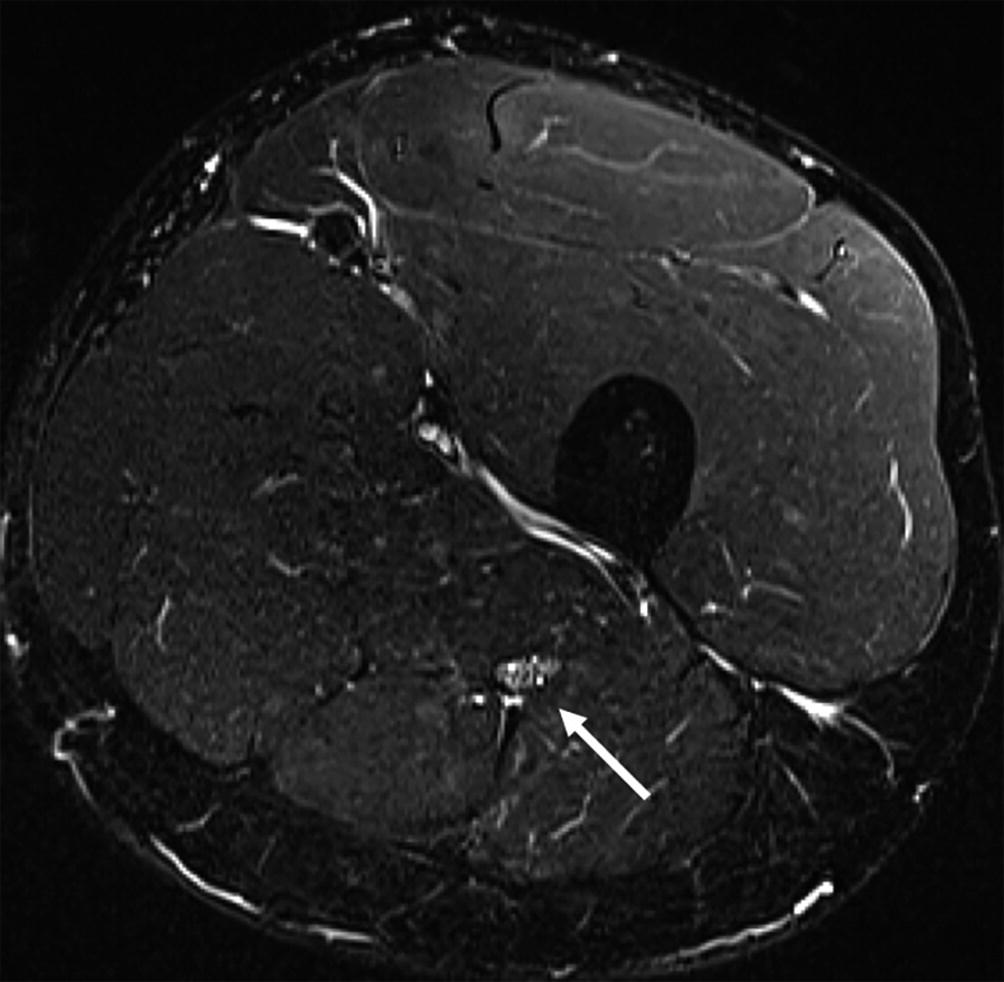
MRN (3 T), axial T2-STIR section at mid-thigh. Normal subject. The right sciatic nerve (arrow) is moderately hyperintense compared to the adjacent muscles and its transverse fascicular pattern is clearly identifiable
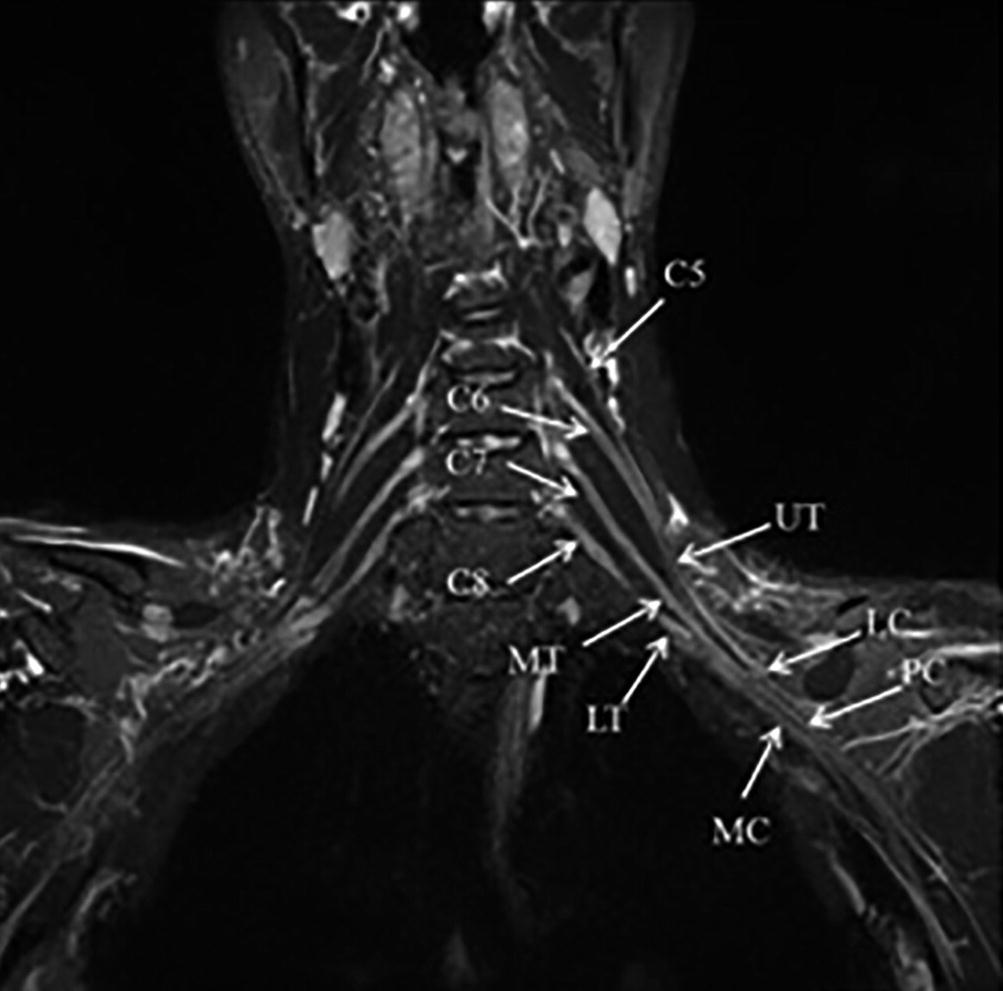
3D MRN (3 T) of the brachial plexus. Oblique coronal reformat. The supra and infraclavicular segments of the brachial plexus are simultaneously displayed in a single image. Upper trunk (UT), Middle trunk (MT), Lower trunk (LT), Lateral cord (LC), Posterior cord PC), Medial cord (MC)
A comprehensive MRI protocol for the investigation of peripheral nerves should include MRN, which provide both structural and functional information on the nerves and muscle denervation, T1 sequences which are helpful for a precise anatomical identification of nerves and for the identification of muscular atrophy, and contrast-enhanced T1sequences for the evaluation of the blood–nerve barrier integrity. MRN is effective in the diagnostic work-up of traumatic nerve injuries [8], nerve entrapment syndromes [9], and nerve tumors [10]. Lumbosacral MRN may demonstrate abnormal intraneural T2 signal in a substantial portion of patients with clinical symptoms of lower extremity radiculopathy and correlates with findings of active radiculopathy on EMG [11]. Recently MRN has been proposed for the evaluation of hereditary and immune-mediated disorders of peripheral nerves [12].
20.6 Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) is a novel technique which has been recently applied to the investigation of peripheral nerve disorders. DTI provides “microstructural” information about nerve integrity. Nerves are characterized by greater water diffusion anisotropy compared to the surrounding tissues, due to a barrier to water diffusion formed by axonal myelin sheaths. Diffusion imaging of the peripheral nerves is challenging because of their small size and course, particularly the BP because of the geometric distortion and artifacts along the course of the nerves between the neck and the shoulder. Additionally, DTI gives quantitative information about the degree and direction of water diffusion: fractional anisotropy (FA), apparent diffusion coefficient (ADC), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) [13]. Reconstructing the diffusion information to characterize the integrity of the white matter tracts (diffusion tensor tractography, DTT) yields 3D representations of anisotropic nerve fibers. Successful tracking of the major peripheral nerves can be obtained using the same approach as deterministic tractography of the brain white matter bundles. DTI has been extensively applied to the median nerve at the carpal tunnel and more recently to the BP and LSP, although its overall diagnostic value in clinical routine is still to be ascertained. Although DTI and tractography are not widely used clinically, good reproducibility of DTI quantitative analysis with only small variations in calculated FA and ADC in the BP of normal volunteers has been shown [14].
MRN can characterize nerve morphology, longitudinal variations in signal intensity and caliber, and connections and relations to other nerves or plexuses to help identify pathology in correlation with the patient’s clinical history, physical examination, and electrophysiological studies.
20.7 Traumatic Injuries of Peripheral Nerves
MR imaging in peripheral nerve injuries according to Seddon’s classification
Classification | MR |
|---|---|
Neurapraxia | – T2 hyperintensity of the nerve (within 24 h of trauma) – No muscle denervation |
Axonotmesis | – T2 hyperintensity and enlargement of the nerve with fascicular hypertrophy, with or without neuroma in continuity – Muscle denervation |
Neurotmesis | – Nerve disruption with stump neuroma – Muscle denervation |
20.8 Traumatic Injuries of the Brachial Plexus
MR imaging findings in different peripheral nerve injuries on MR Myelography
Brachial plexus injuries | MR |
|---|---|
Complete avulsion | Traumatic pseudomeningoceles Ventral and dorsal roots not identifiable Denervation edema of the posterior paraspinal muscles |
Partial avulsion | Reduced number of rootlets on MIP projections Ventral or dorsal root absent on axial sections with mild abnormalities of dural sleeves |
Post-ganglionic Injuries | Swelling and increased signal intensity of the roots, trunks and cords of the brachial plexus Tortuosity and increased intensity of the infraclavicular brachial plexus Post-traumatic neuromas along the course of the roots and/or primary trunks |
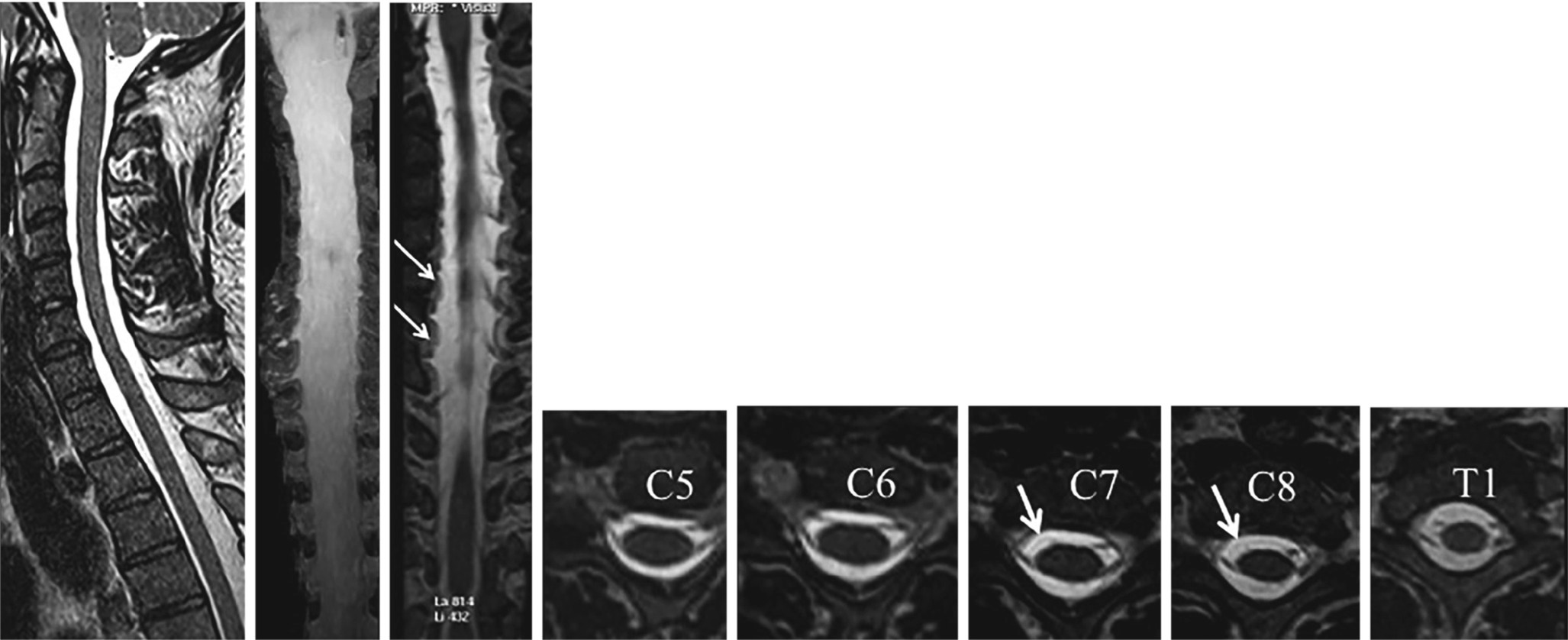
22-year-old male, motor cycle accident, with complete right brachial plexus palsy. 3D MR Myelography. Right C7 and C8 (partial) avulsions of the ventral nerve roots (arrows)
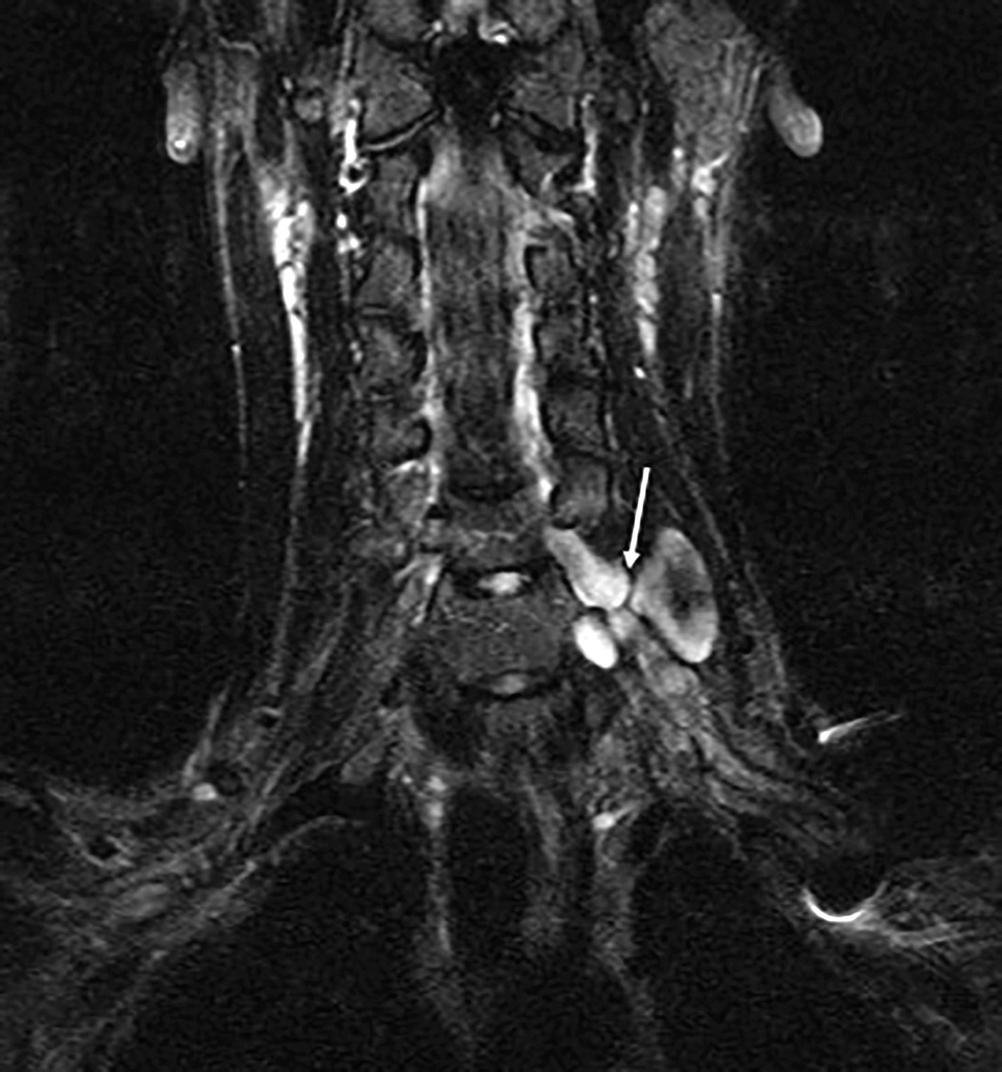
MRN, traumatic injury of the left brachial plexus. Neurotmesis of the left C6 nerve root: stump neuroma (arrow)

Traumatic injury of the right brachial plexus. (a) MRN: the right brachial plexus is barely identifiable (arrow). (b) Tractography: right C7, C8, T1 nerve root avulsions (open arrows)
For traumatic injuries of the upper limb, US is the examination of choice and can be performed immediately after trauma to demonstrate neurotmesis-type lesions to show nerve discontinuity. MRN can identify axonotmesis-type lesions, which appear as global nerve enlargement with mild fascicular hypertrophy that extends longitudinally and requires longer time for nerve regeneration.
DTI can be used to assess the nerve regeneration after surgery [19].
20.9 Traumatic Injuries of the Lumbosacral Plexus, Sciatic and Femoral Nerves

24-year-old male, road accident, with left femoral shaft fracture, sciatic nerve injury. MRN (1.5 T), axial T2-STIR at pelvic floor (a), proximal and mid-thigh (b, c), sagittal oblique reformat (d). Enlargement and hyperintensity of the left sciatic nerve, which is characterized by fascicular hypertrophy (arrows). Increased signal intensity of semimembranosus, semitendinosus, and long head of the biceps femuri, due to acute denervation
A pseudomeningocele is a preganglionic injury that is seen in 80% of traumatic avulsions. It results from traction on the cervical roots and meninges with CSF leakage into contiguous areas.
Iatrogenic injury to the LSP may result from surgical gynecologic or anesthetic procedures, compression, traction, and vascular insults [20]. Traumatic injuries of the sciatic nerve can also occur as a complication of prosthetic hip surgery, most frequently involving the fibular division. Injuries of the femoral nerve can be secondary to pelvic trauma or iatrogenic in nature with nerve compression from hematoma or pseudoaneurysm during femoral artery puncture, respectively. The intrapelvic femoral nerve may show increased T2 signal and size or deviation by hematoma, with denervation of the iliopsoas muscle. Although nerve abnormalities in the thigh can be difficult to detect, denervation of the quadriceps femoris may be a clue to femoral nerve injury distal to the groin ligament.
20.10 Entrapment Neuropathies
Common nerve entrapment syndromes
Nerve entrapment syndrome | Involved nerve | Site of entrapment |
|---|---|---|
Carpal tunnel syndrome | Median nerve | Carpal tunnel, at the level of hamate (proximal alterations at the level of pisiform) |
Ulnar neuropathy | Ulnar nerve | Cubital tunnel |
Fibular nerve entrapment | Fibular nerve | At fibular head or deep to the origin of peroneus longus muscle |
Piriformis syndrome | Sciatic nerve | Sciatic notch |
Tibial nerve entrapment | Tibial nerve | Tarsal tunnel |
20.11 Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS) is the most common peripheral nerve entrapment syndrome and has various etiologies, including repetitive trauma, metabolic and hormonal conditions, and ganglion cysts. While US has 77.6% sensitivity and 86.8% specificity for diagnosing CTS, sensitivity of MRN is above 90%. On MRN, abnormal nerve signal length and cross-sectional area at the distal radioulnar joint are the best predictors of the severity [21]. Correlation between median nerve DTI metrics in the carpal tunnel and electrophysiology has been recently reported [22]. MRN is particularly useful for the evaluation of atypical CTS, when assessing for space-occupying lesion, and in patients with persistent symptoms after carpal tunnel release.
20.12 Ulnar Neuropathy at the Elbow
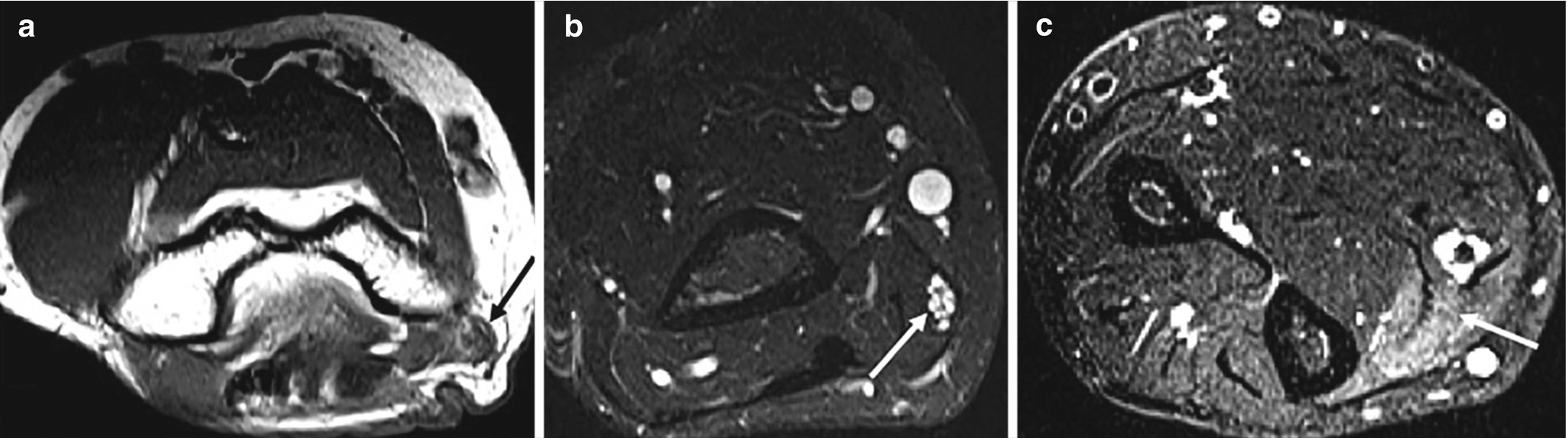
48-year old male with acute onset of right forearm pain and paresthesia. Ulnar nerve entrapment at the cubital tunnel. (a) Axial T1 image at the right elbow (a), MRN at distal arm (b), and forearm (c). Deformation of the right ulnar nerve (black arrow in a). Proximal to the entrapment site, the ulnar nerve is enlarged and hyperintense with fascicular hypertrophy (arrow in b). Denervation edema of the flexor carpi ulnaris (arrow in c)
Although both US and MRN offer anatomic delineation of entrapped nerves, US has the advantage of providing dynamic evaluation of nerve entrapment.
20.13 Non-traumatic Brachial and Lumbosacral Plexopathies
Idiopathic brachial plexitis or idiopathic neuralgic amyotrophy [INA] (aka Parsonage Turner syndrome) is a self-limiting inflammatory disorder usually affecting the unilateral BP, more commonly in males. Acute symptoms include neck, shoulder, and scapula pain. Muscle weakness ensues days or weeks after the initial pain, and the symptoms may last several months up to 3 years. MRN will show diffuse T2 hyperintensity and enlargement of affected neural structures, commonly C5 and C6 nerve roots and the upper trunk, and denervation edema in various muscles that does not follow a typical innervation pattern (e.g., isolated edema in the teres minor muscle).
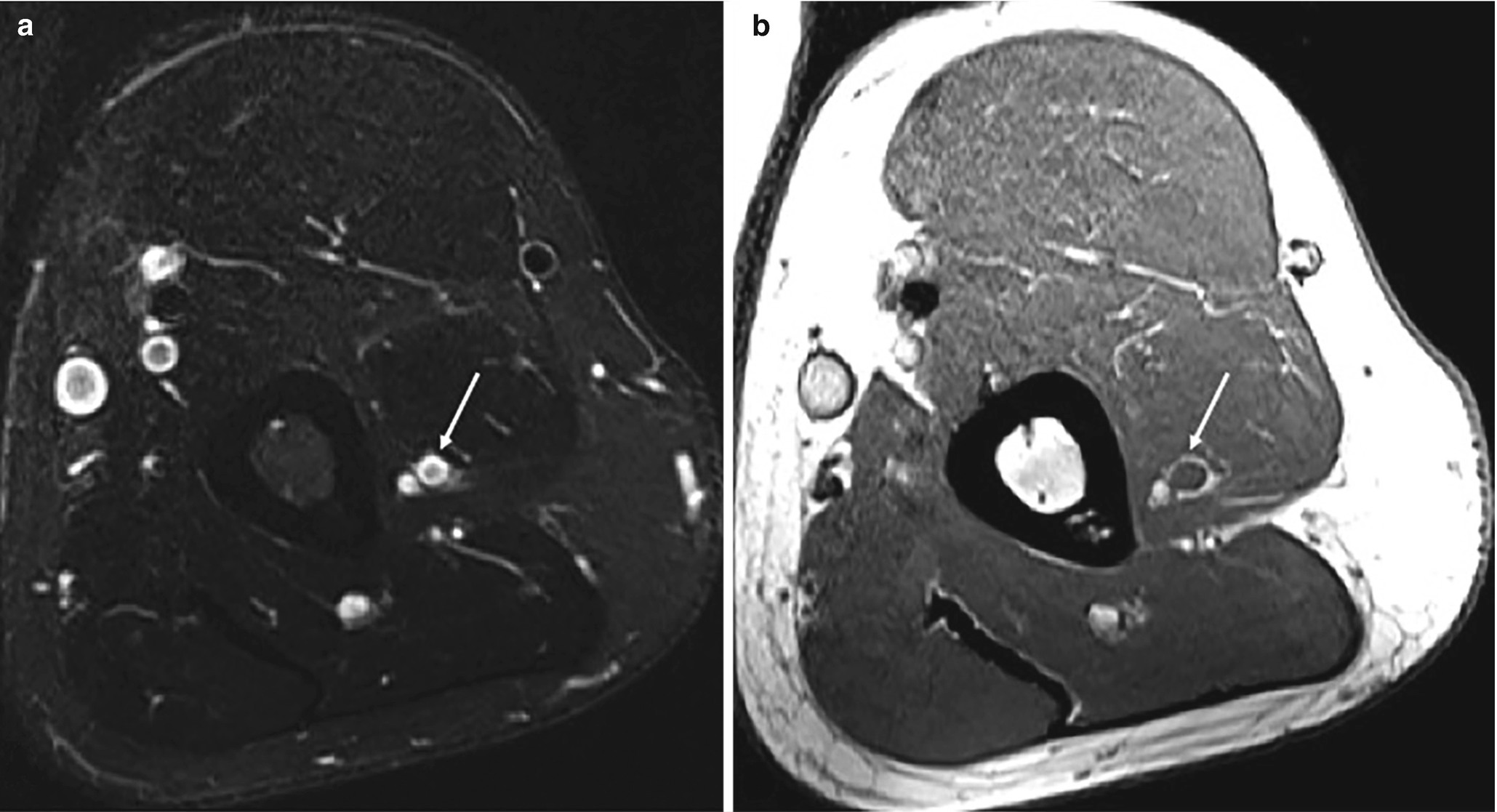
62-year-old male with acute idiopathic neuralgic amyotrophy. (a) Axial T2 STIR, (b) axial T1. Swelling and increased signal intensity of the left radial nerve at mid-arm, with the bull’s eye sign (arrow)
Idiopathic lumbosacral plexopathy (ILSP) is a self-limiting condition that presents with (sub)acute, severe, asymmetrical leg pain, followed by asymmetrical multifocal weakness and atrophy in the subsequent weeks or months. Though some studies have hypothesized an immune-mediated etiology, biopsies of distal cutaneous nerve segments have shown features of an inflammatory microvasculitis causing ischemic damage of the nerves [24]. Diabetic LSP neuropathy has similar clinical and pathological findings, suggesting that inflammation may form part of the final common pathway in both conditions. MRN shows increased signal intensity and mild contrast enhancement asymmetrically involving multiple nerve roots and terminal branches of the LSP as well muscle denervation changes.
LSP may be involved in retroperitoneal disorders, such as psoas abscess, hematoma, retroperitoneal fibrosis, and malignant disease (soft tissue or osseous), either by extrinsic compression or infiltration. Inflammatory sacroiliac joint arthritis may also involve the sacral component of the LSP.
Radiation plexopathy may manifest from a few months to years after brachytherapy or intraoperative radiation therapy, usually with doses exceeding 6000 cGy. This type of plexopathy is often painless and progresses slowly, in contradistinction to severely painful tumor-related plexopathy. Expected radiation-induced changes on MRN are uniform, symmetric thickening, T2 hyperintensity, and faint to no contrast enhancement of BP and LSP within the irradiated region. It may be difficult to separate the neural structures from each other. Recurrent tumor often shows a focal or diffuse heterogeneously enhancing mass [25]. Follow-up imaging is helpful to differentiate radiation-induced inflammation from tumor recurrence as the former will show fibrotic changes over time.
20.14 Thoracic Outlet Syndrome (TOS)
TOS includes three disorders involving the neurovascular bundle in its course through the interscalene triangle and costoclavicular space: classic TOS (or neurogenic TOS), vascular TOS, and non-specific TOS. Classic TOS is the most frequent form, affecting middle-aged adults, especially women. It is characterized by chronic neck pain radiating to the supraclavicular region and arm, exacerbated by arm elevation, sometimes associated with paresthesias and numbness in C8/T1 dermatomes. Electrophysiological and clinical provocative tests are non-specific; however, MRI can reveal static or dynamic compression of the BP by structural abnormalities such as cervical or anomalous first rib, long C7 transverse process, muscular anomalies, or narrowed costoclavicular space [26].
20.15 Immune-Mediated Neuropathies (Table 20.4)
MRI findings in inflammatory neuropathies
MRI | Distribution | |
|---|---|---|
AIDP | Enhancement of spinal nerve roots | Bilateral and symmetrical |
CIDP | Hypertrophy and T2 hyperintensity of BP and LSP nerve roots, with gradual normalization in distal nerves | Bilateral and symmetrical |
MADSAM | Hypertrophy and T2 hyperintensity of peripheral nerve trunks | Multifocal and asymmetrical |
MMN | Hypertrophy and T2 hyperintensity Contrast enhancement of BP | Asymmetrical |
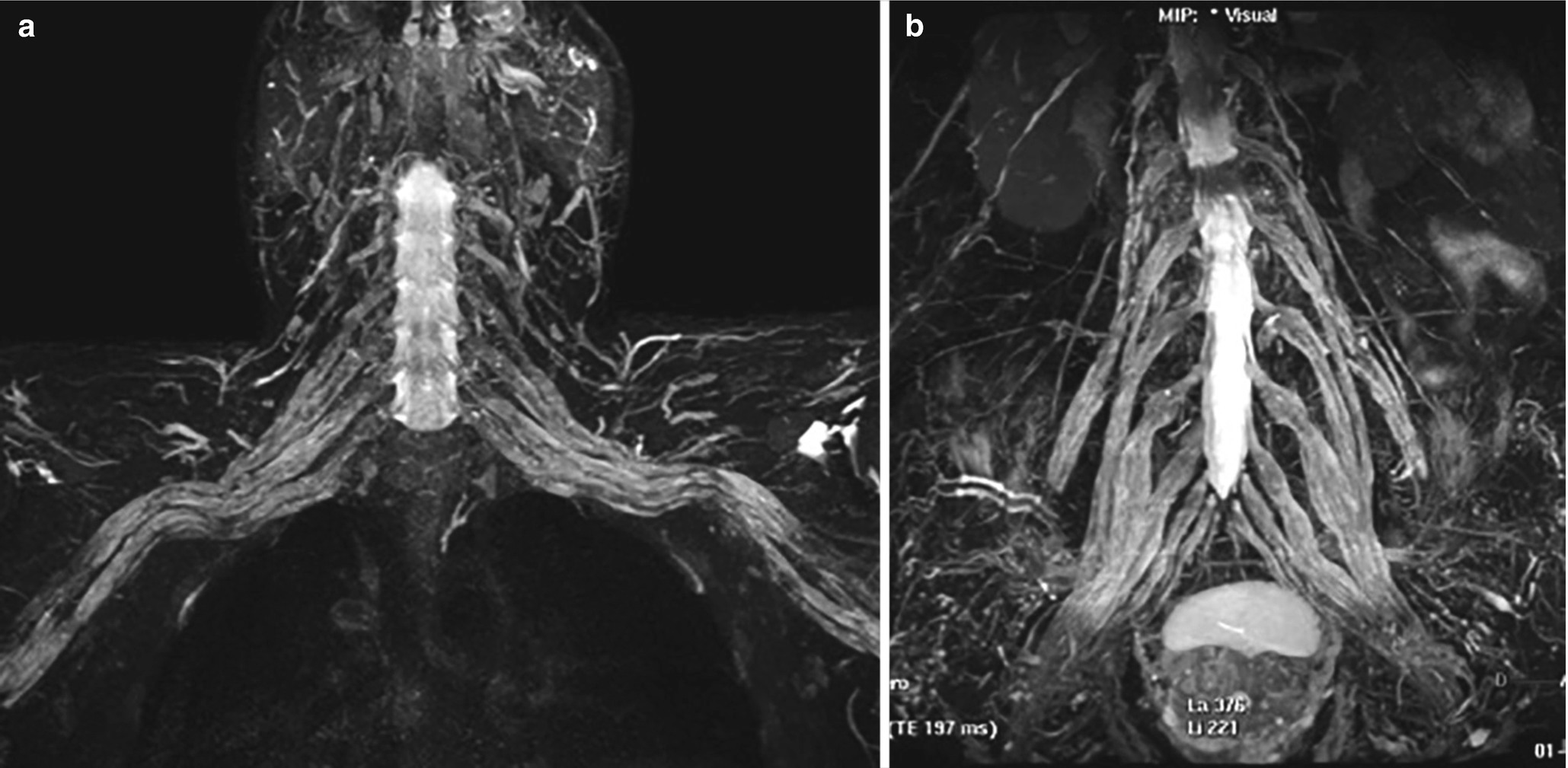
50-year-old male, CIDP: 3D MRN, MIP views bilateral hypertrophy of the brachial (a) and lumbosacral plexus (b)
Hereditary neuropathies such as Charcot–Marie–Tooth disease can mimic the appearance of CIDP on MRI with findings of focal or diffuse peripheral nerve enlargement and fatty degeneration of the involved muscles. The cross-sectional measurement of the sciatic nerve area in mid-thigh can be a helpful distinguishing feature [30].
Multifocal acquired demyelinating sensory and motor neuropathy (MADSAM) is characterized by an asymmetric multifocal pattern of motor and sensory loss, conduction block, and other features of demyelination in nerve conduction studies. Nerve hypertrophy is usually asymmetric and multifocal in the peripheral nerve trunks [31].
Multifocal motor neuropathy (MMN) is a chronic, slowly progressive immune-mediated neuropathy, characterized by progressive, predominantly distal, asymmetric limb weakness, mostly affecting upper limbs with minimal or no sensory impairment. The clinical presentation of MMN may mimic motor neuron disease, particularly in patients with predominant lower motor neuron impairment. MRI can be valuable to differentiate MMN from other neuropathies. About 40–50% of the patients with MMN show asymmetric hypertrophy and signal intensity abnormalities or contrast enhancement on MRI, and the pattern of signal alterations closely correlates with the distribution of muscle weakness [32].
20.16 Peripheral Nerve Tumors
The most common neurogenic tumors of the BP and LSP are schwannomas and neurofibromas. On MRI, peripheral nerve sheath tumors are typically fusiform or round-shaped soft tissue lesions in continuity with the nerve and show classic findings such as the target, fascicular, and tail signs on T2 images, split fat sign on T images, and the bag of worms sign for plexiform neurofibromas [33]. Conventional MRI sequences cannot reliably differentiate solitary neurofibromas from schwannomas, although certain imaging features such as the surrounding T2 hypointense epineurium may be more typical of schwannomas than neurofibromas.
When MRN shows diffuse T2 hyperintensity and enlargement of affected neural structures in a patient with acute onset including neck, shoulder, and scapula pain, look for the characteristic “bulls’ eye sign” to suggest idiopathic brachial plexitis or idiopathic neuralgic amyotrophy.
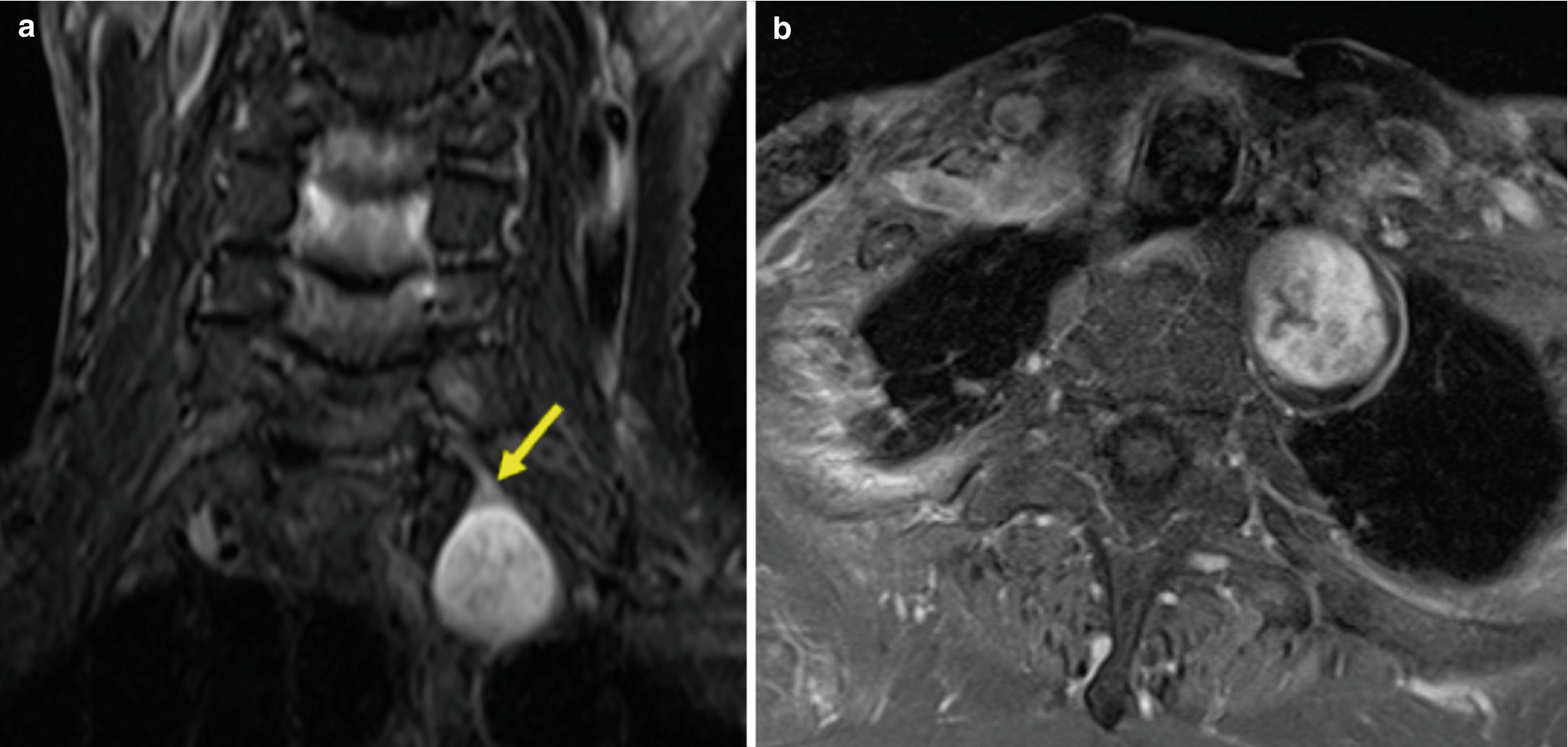
(a) Coronal STIR and (b) axial contrast-enhanced T1 images of a schwannoma demonstrate the origin from a single nerve (arrow) and STIR hyperintensity. The heterogeneous enhancement may reflect internal cystic change

Coronal MRN of a patient with NF1: neurofibroma of the left sciatic nerve with the “target” sign (empty arrow), neurofibroma with intermediate signal intensity of the pelvic floor (arrow), plexiform neurofibroma of the right sciatic nerve (double arrow)
Malignant peripheral nerve sheath tumors (MPNST) have higher prevalence in patients with NF1 and can develop as a long-term side effect of radiation therapy. Malignant degeneration of plexiform neurofibromas can occur in 5–13% of cases. The imaging distinction between benign nerve sheath tumors and MPNST can be challenging. Imaging features, such as advanced local invasion, bone destruction, poorly defined margins, absence of the target sign on T2 images, and larger size (>5 cm) suggest malignancy [34]. The combination of MRI findings (e.g., rapid growing, peripheral enhancement, peritumoral edema, intralesional cysts) has been reported to provide 61% sensitivity and 90% specificity in detecting MPNST [35]. 18Fluorodeoxyglucose positron emission tomography is a complementary diagnostic tool, particularly for identifying tumors with aggressive behavior [36].
Intraneural perineurioma is a rare benign and slowly growing nerve tumor arising from the perineurial cells surrounding the peripheral nerve fibers, most commonly occurring in teenagers and young adults without sex predilection and presenting with progressive muscle weakness.
Because of the insidious clinical presentation, the diagnosis is often delayed. Perineuriomas have typical MRI features: enlargement of the involved nerve over a considerable length, fusiform shape, mild increase in T2 signal intensity, T1 isointense signal, and moderate to marked contrast enhancement. Individual fascicles are uniformly enlarged, and their preserved fascicular architecture gives a “honeycomb” appearance on enhanced T1-fat-sat sequences.
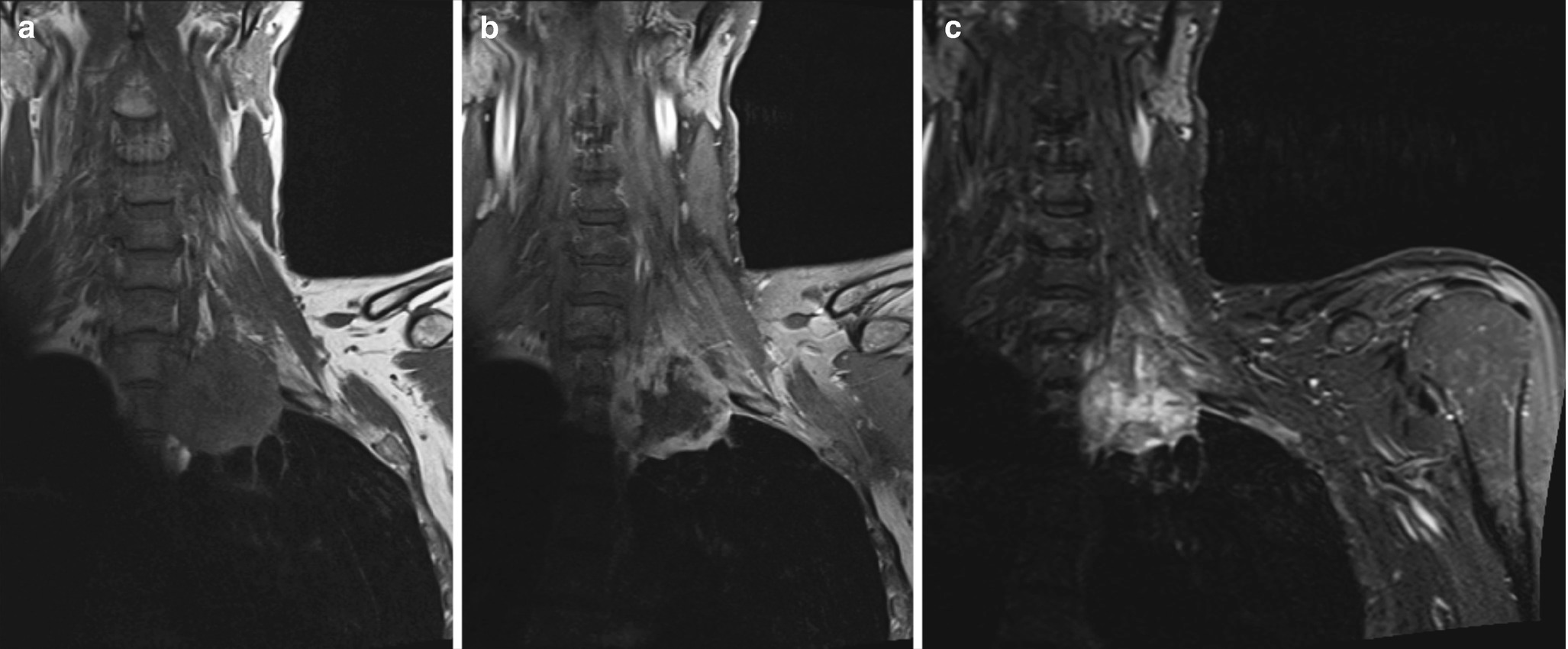
Coronal (a) T1, (b) contrast-enhanced T1, and (c) STIR images reveal a T1 hypointense, heterogeneously T2 hyperintense, heterogeneously enhancing lesion in the left lung apex in keeping with a bronchogenic carcinoma (Pancoast tumor) infiltrating into the interscalene triangle and involving the left C7, C8, and T1 (arrow). This patient presented with persistent left shoulder pain
MRN can accurately display the anatomical relationship of a schwannoma attached to the main trunk of a nerve while a neurofibroma, composed of all peripheral nerve cellular elements, expands radially entrapping native neural elements within the tumor. A plexiform neurofibroma involves multiple nerve roots and has a “bag of worms” appearance. A perineurioma shows fusiform enlargement and mild T2 hyperintensity of the involved nerve and preserved fascicular architecture giving the “honeycomb” appearance on enhanced images.
Non-tumoral masses, such as aneurysms/pseudoaneurysms can cause a compressive plexopathy. DTT can provide relevant topographical information on non-neurogenic soft tissue tumors and adjacent nerves when conventional MRI is unable to depict the course of the involved nerves or distinguish possible nerve infiltration [38]. DTT and low FA provide insight into neural integrity, with low diffusivity values indicating malignancy [10]. As increasing ADC suggests benignity of lesions, this diffusion metric can also be potentially followed as a biomarker to detect tumor response/necrosis if the lesions are being followed longitudinally or after adjuvant medical treatment.
20.17 Concluding Remarks
Understanding of the brachial plexus and lumbosacral plexus anatomy is important for the accurate interpretation. Using an anatomic landmark-based approach to interrogate each individual component of the brachial plexus and lumbosacral plexus in combination with the direct and indirect imaging findings enables the development of a relevant differential diagnosis. By incorporating the clinical history, ancillary test results, and physical examination with the imaging findings, one can arrive at the correct diagnosis.
Diagnostic work-up of peripheral neuropathies should include clinical history, physical examination, electrophysiological studies, and high resolution MRI/MRN (US when nerves are superficial).
MRN affords the advantage of simultaneous exploration of nerves, muscles, and other surrounding tissues which can assess secondary effects of nerve pathology (e.g., muscle denervation), and compressive or infiltrative pathologies.
It is important to distinguish between preganglionic and postganglionic traumatic plexopathy as it affects treatment options, with the former requiring nerve transfer and the latter often being managed conservatively.
In peripheral nerve entrapment syndromes, US has the advantage of providing dynamic evaluation of nerve entrapment. MRN is able to identify a variable degree of signal intensity change within the nerve that is commonly higher proximal to the site of entrapment.
Neurogenic tumors are identified as well-circumscribed, round-shaped soft tissue lesions in continuity with the nerve, and MRI findings of advanced local invasion, poorly defined margins, absence of the target sign on T2-weighted sequences, peritumoral edema, intralesional cysts, and bone destruction, suggest malignancy.

Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.