After Mendeleyev published the first periodic table, chemists continued to discover new elements, and they continued to have the properties that Mendeleyev predicted. It was clear that he had found more than a useful arrangement of the elements; he had found a fundamental aspect of matter. Something caused elements to have periodic properties. But what was that something? And did it have anything to do with Dalton’s atomic theory?
Scientists were willing to say that matter behaves as if it is made of atoms, but many were still looking for proof that atoms actually existed. Then along came J. J. Thomson (1856—1940) of Cambridge University in England, who in 1897 described his discovery of tiny bits of matter that he called corpuscles and we now call electrons.
By then, scientists understood electricity quite well. They knew that two positively charged bodies or two negatively charged bodies would repel (push away) each other, while a pair of bodies with opposite electric charges would attract each other. The attractive or repulsive forces get much stronger as the charged bodies get closer together. Decreasing their separation to half its value multiplies the force by four (2 × 2). If the distance is shortened to one-third of its original value, the force increases by nine (3 × 3).
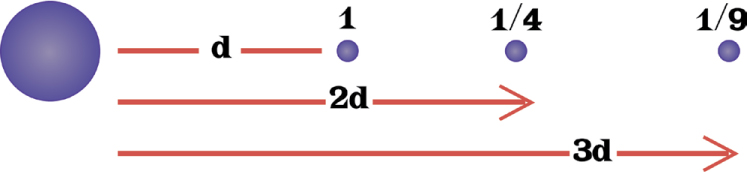
The Law of Electrical Forces. By the end of the nineteenth century, scientists recognized that chemistry and electricity are closely related. They used the law of electrical forces, illustrated here and described on this page, to determine in 1897 that the newly discovered electron had a mass that was much smaller than the smallest atom. That made it the first known subatomic particle.
They also knew that electricity was related to chemical reactions and valences, and thus it was probably important in atoms. Thomson’s corpuscles had unexpected properties. They carried negative electric charge and were less than a thousandth as heavy as the lightest atom, hydrogen. (We now know that a hydrogen atom is about 1,800 times as heavy as an electron.)
Yet despite that huge difference in mass, the corpuscles carried as much negative charge as a hydrogen atom might carry in its most positively charged state. That result suggested that atoms are not indivisible but are made of even smaller subatomic particles—tiny negatively charged electrons and much heavier positively charged particles—held together by electric forces.
Evidence of Atoms
Still many scientists felt that atomic theory was incomplete without evidence of actual atoms. The problem was that they weren’t looking in the right places. The evidence that atoms and molecules were real was already known but was not recognized. It was called Brownian motion after the English botanist Robert Brown (1773–1858), who observed in 1827 that pollen grains suspended in water under a microscope followed random jiggling paths, even if the water was absolutely still.
Over the years, some scientists proposed that these grains moved because they were bumped about by molecules, and others studied Brownian motion more carefully, measuring the paths for different-sized particles at different temperatures. Finally, in 1905, Albert Einstein (1879–1955) calculated the motion that would be expected if atoms moving at a certain temperature collided with dust or pollen particles of a certain size, and the results matched Brownian motion perfectly. People couldn’t see individual atoms, but they could see their effects.
Rutherford’s Surprise
If atoms were real and contained tiny negatively charged electrons, what about the positively charged parts? Since atoms are electrically neutral overall, they must contain as much positive as negative electric charge. Scientist wondered if that positively charged matter was in the form of the individual particles or as a spread-out mass. J. J. Thomson put forward an educated guess. Since electrons carry so little mass, he envisioned the positively charged bulk of atoms as a kind of pudding containing tiny electron plums.
Brownian Motion
This illustration shows what Robert Brown observed when he followed the movement of pollen grains suspended in water through his microscope in 1827. Instead of staying in place or slowly drifting, the grain abruptly changed direction at random intervals.
At first, Brown suspected the movement was because the grains had come from living things. So he tried it with pollen from dead plants. The movement was the same. Then he tried tiny bits of fossilized wood. Finally he tried material that had never been alive—bits of window glass and dust from a stone from The Great Sphinx. No matter what he tried, the particles followed similar random, jerky paths.
Brown tried to explain the phenomenon in various ways: There were currents in the water due to evaporation; there were tiny vibrations that he couldn’t feel; it was due to the light striking the particles so he could see them. Yet none of these were satisfactory.
In the 1860s and 1870s, physicists studying thermodynamics began to suggest that the motion might be due to water molecules colliding with the grains. French scientist Léon Gouy (1854–1926) repeated the experiments with different liquids and under different conditions. He showed that the motion was not affected by strong light or electromagnetic fields. That strengthened the idea that molecular collisions were the cause. Finally, in 1905, Albert Einstein’s calculations matched the observations so well that no one could deny that Brownian motion was indeed the result of collisions with the fluid’s molecules.
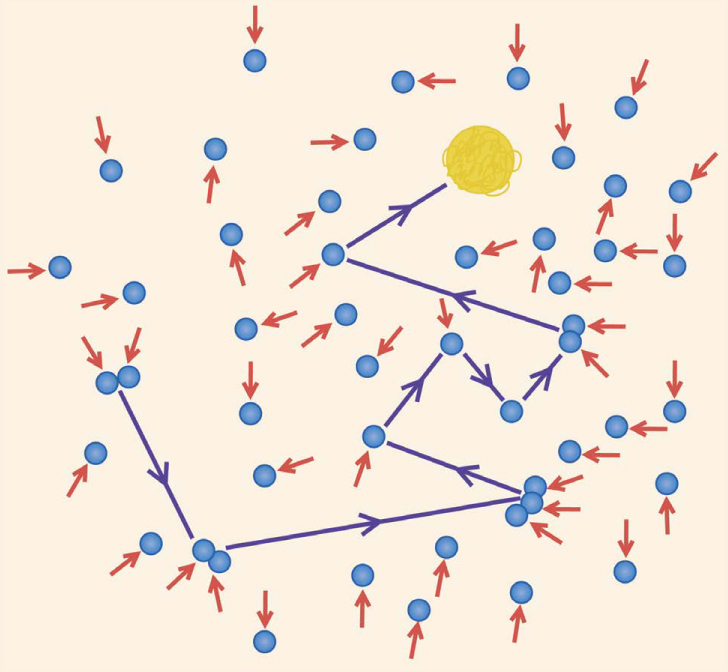
Brownian Motion. In 1827, botanist Robert Brown observed an odd phenomenon under his microscope. Pollen grains suspended in water followed jerky, irregular paths, even when the water was perfectly still. Other scientists looked at other particles, including those that were never associated with a living organism, and found similar motion. They began to suspect that it resulted from random collisions with water molecules. Calculations by Albert Einstein in 1905 matched observed results. It was the first direct evidence that atoms and molecules exist.

Ernest Rutherford (1871–1937). Rutherford, already a Nobel Prize winner for his work with radioactivity, used alpha particles to probe the internal structure of atoms and found a surprising result. Most of an atom’s mass and all of its positive electric charge was in a tiny central region called the nucleus.
Thomson’s plum pudding model was put to the test by Ernest Rutherford (1871—1937), who had come up with a way to probe matter by bombarding it with the emissions from radioactive substances. Rutherford was one of the first scientists to explore the radioactivity as Thomson’s student at Cambridge between 1895 and 1898. There he found two distinct forms of radioactivity, which he named “alpha rays” and “beta rays.”
He then went on to become a professor at McGill University in Montreal, Canada, where in 1902, he and his colleague Frederick Soddy (1877—1956), discovered a third form of radioactivity they called “gamma rays.” They also discovered that alpha and beta radiation were streams of fast-moving particles of opposite electric charge. The alphas were positively charged and much more massive than the negatively charged betas. (We now know that beta rays are electrons.)
Rutherford returned to England in 1907 as a professor at the University of Manchester and began shooting beams of alpha particles through metallic foil and measuring how the alphas deflected, or scattered, as they interacted with atoms of the metal. By studying alpha scattering carefully, he hoped to be able to determine the size, spacing, and perhaps even the shape of the atoms in the foil. His student Hans Geiger (1882—1945) devised an instrument to detect and count the alpha particles. They determined that alpha particles were helium atoms without their electrons, just as Rutherford had suspected.
In 1909, they began their scattering experiments, and the results were surprising. Nearly all the alphas passed straight through the foil or deflected only slightly. If the atoms were hard balls, Rutherford and Geiger would have expected more deflection. Also puzzling was this: a few alpha particles were unaccounted for. Geiger’s counters had been placed behind the metal foil. Had some particles been deflected so much that they had missed the detectors? If so, what was scattering those few alpha particles through such large angles?

The Proof Is in the Not-Pudding. Rutherford’s experiment produced results that, for the most part, were not very different from what would be expected from Thomson’s “plum pudding” model of the atom. Most alpha particles deflected only slightly if at all. However, an occasional alpha went missing. Searching for the missing alpha particles, Rutherford’s students discovered they had deflected in unexpected directions. They had bounced far off to the side or nearly backwards. That led him to propose the existence of the nucleus.
While Geiger continued his detailed measurements, Rutherford assigned the task of looking for large-angle scattering to Ernest Marsden (1889—1970), another of his students. Marsden found that the missing alpha particles scattered to the left or right of the original detectors and a few even scattered backward. Rutherford described this result as “almost as incredible as if you had fired a 15-inch (38-centimeter) shell at a piece of tissue paper and it came back and hit you.”
Rutherford explained his results with a new model of the atom. He envisioned an atom as a miniature solar system with electrical forces playing the role of gravity. Like the solar system, the atom is mostly empty space. Most of its mass is concentrated in a very small, positively charged nucleus (plural: nuclei) about one ten-thousandth of the size of the atom. In orbit around that minuscule but massive Sun are much tinier negatively charged planets: the electrons. Because the atoms are mostly empty space, most alpha particles passed through the foil without coming close enough to a nucleus to be scattered very much. Only on rare occasions would a fast-moving alpha particle make a nearly direct hit on a much heavier nucleus, which then scattered the alpha sideways or even backward.
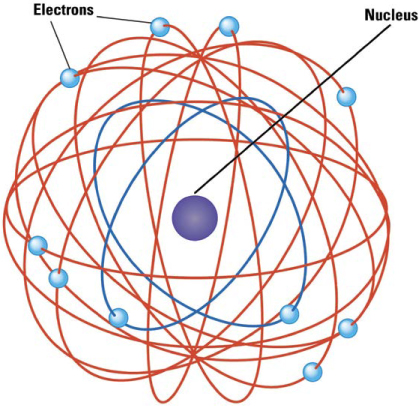
The Planetary Model of the Atom. After discovering the nucleus, Rutherford proposed that atoms were like miniature solar systems held together by electric forces rather than gravity. Most of its mass was in its compact, positively charged nucleus, while tiny negatively charged electrons orbited that nucleus like a swarm of small planets.
Numbering the Periodic Table
But what was in the nucleus? Rutherford and other scientists concluded that the nucleus was composed of positive particles, which they called protons. Each nucleus contained as many protons as that atom had electrons. They also began to realize that the elements in the periodic table could be arranged in numerical order by how many protons or electrons they had. And this they called the atomic number.
It was not difficult experimentally to remove electrons from atoms, turning them into ions. Thus scientists decided that it was better to define atomic number by the positive charge in the nucleus. The atomic number thereby became the number of protons instead of the number of electrons. Hydrogen, the simplest atom with atomic number one, has a nucleus with a single proton. Helium, with atomic number two, has two protons, and so forth. However, the issue proved to be more complicated.
The atomic mass of hydrogen is one, but the atomic mass of helium is four. Other atoms reveal similar discrepancies. Lead, for example, has an atomic number of 82 and an atomic mass of approximately 207. Protons alone could not account for even half the mass of most nuclei. What else might be there?
Perhaps this extra mass could explain another problem in Rutherford’s atomic model. If the nucleus contained only protons, they would repel each other with electric forces. Packed together in such a tiny space, that repulsive force would be enormous. Whatever else was in the nucleus to give it more mass would also have to exert an even larger attractive force to overcome the protons’ electrical repulsion. Rutherford knew that he had just barely begun to understand the nucleus and the forces that act within it.