Rutherford was already famous when he discovered the nucleus. His studies of radioactivity had earned him the 1908 Nobel Prize in Chemistry for his careful measurements of alpha, beta, and gamma rays. He, along with his former colleague Soddy and other scientists, learned to identify different radioactive elements by distinct characteristics of their emissions. In that work, they realized that nature was succeeding where had failed: transforming one element into another. The process, which was called transmutation, hinted that atoms contained other particles besides electrons. Just as electrons were responsible for chemical reactions, those other particles were responsible for radioactivity and transmutation.
Once Rutherford, Geiger, and Marsden discovered the nucleus, it was clear that radioactivity originated within that small central region of an atom. Rutherford, and especially Soddy, began tracking the elements from their original form to their new forms. They found that when a nucleus emits an alpha particle, it loses four units of atomic mass while its atomic number decreases by two. Nuclei that emit beta particles do not change atomic mass, but their atomic numbers increase by one unit. In both of those cases, an atom of one element transmutes into an atom of a different element. The new “daughter” atom is often radioactive, more so than its “parent,” so there is a chain of radioactive decay from one atom to another.
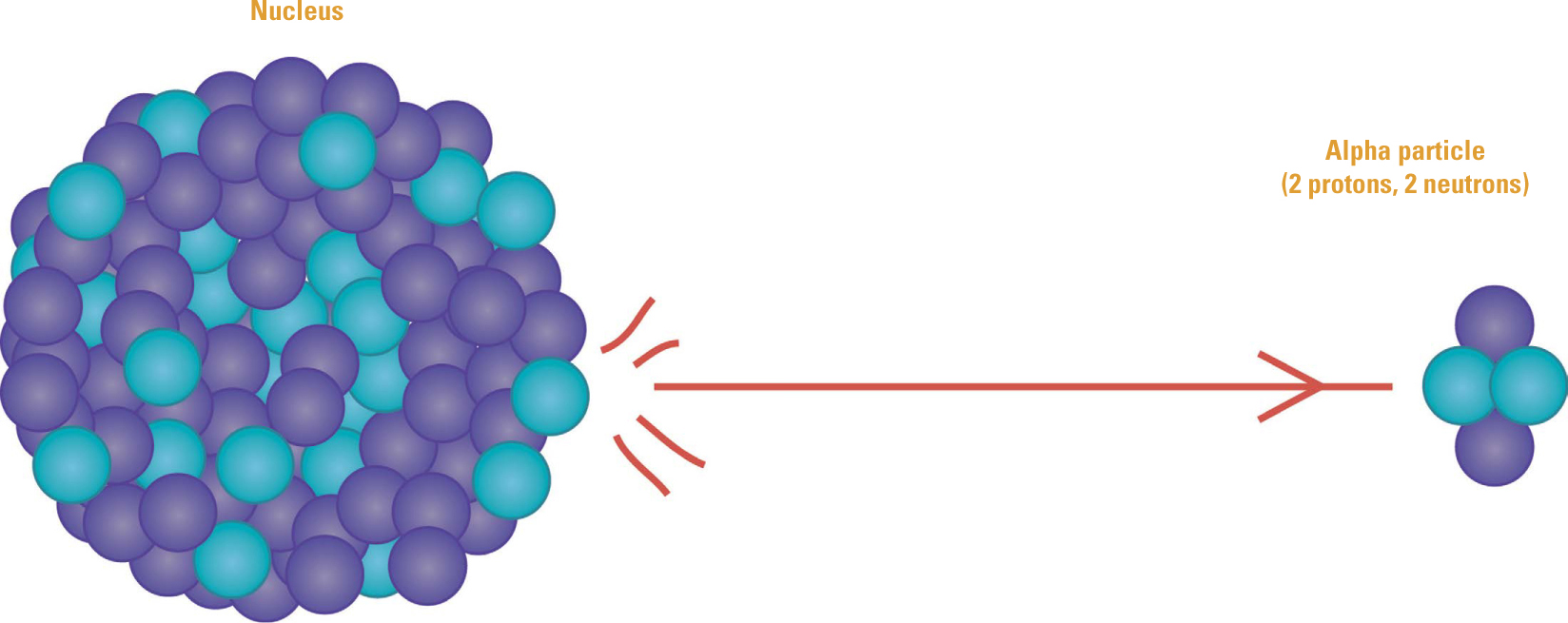
Transmutation in Alpha Decay. Rutherford and Soddy described the process of radioactive decay as transmutation that produces a “daughter” nucleus from a “parent.” In alpha decay, the daughter has an atomic number that is two less than the parent’s and an atomic mass that is reduced by four. That led them to conclude that an alpha particle is a helium nucleus.
Rutherford and Soddy also discovered that several of the daughter atoms in different radioactive decay chains behaved the same way chemically, which meant that they have the same atomic numbers, yet their atomic masses were different. Soddy suggested calling such atoms isotopes (from the Greek for “equal place”). Chemists soon discovered that many non-radioactive atoms also had more than one isotopic form as well. To distinguish one isotope from another, they began denoting atoms by their chemical symbol and their atomic mass. Chlorine, for example, has naturally occurring isotopes with atomic masses of 35 and 37. The more common of the two is Cl35, which explains why chemists measure the atomic mass of natural chlorine as 35.47.
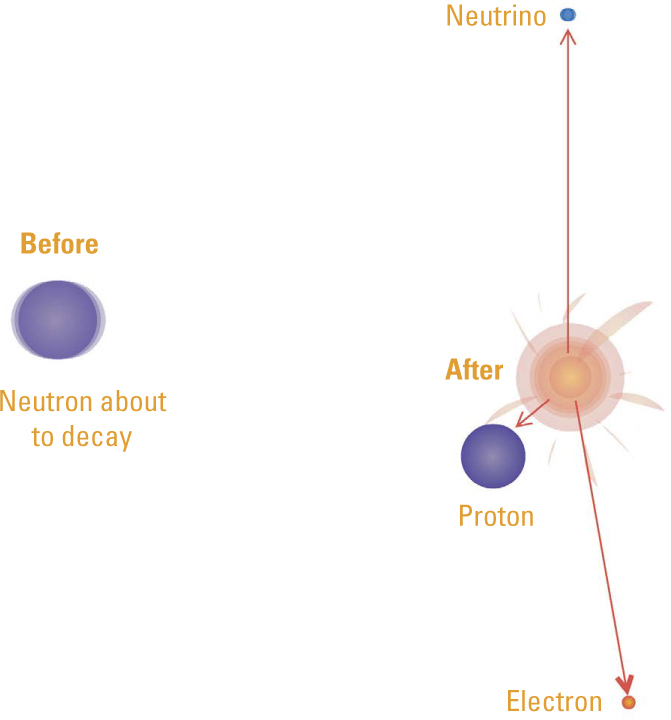
Transmutation in Beta Decay. In beta decay, the daughter nucleus has an atomic number that is one greater than its parent and an atomic mass that is virtually unchanged. After Rutherford proposed that a nucleus contained neutrons as well as protons, he envisioned that a beta particle was the electron resulting from the decay of a neutron into a proton. That was not quite correct. In order to obey the law of conservation of energy, the emission of an additional particle called the neutrino was also necessary.
More Than Protons
Once Rutherford discovered the nucleus, he naturally drew on his work with radioactivity when considering what was inside nuclei. No one questioned that the hydrogen nucleus was a single proton that carried the same amount of positive charge as the electron’s negative charge. Rutherford’s studies had shown that alpha particles were helium nuclei, which carried two units of positive charge but had an atomic mass of four units. Looking at the atoms that produced the radioactivity, some scientists suggested that the extra mass in a nucleus was due to extra protons plus an equal number of electrons. Rutherford didn’t agree, and in 1920, a year after replacing the retiring J. J. Thomson as leader of the Cavendish Laboratory at Cambridge University, he explained it in this way.
Chemistry vs. Alchemy
Before Rutherford discovered the nucleus, he did not realize that the radioactivity was coming from deep inside the atom or that transmutation was very different from alchemy and its scientific successor, chemistry. Chemical changes are related to the atoms’ electrons and the bonds they form between atoms, but chemistry never changes atomic number or atomic mass. Radioactivity, however, comes from the within the nucleus and transforms one atom into another. Unfortunately for those people who hoped to achieve the alchemists’ dream of transforming lead into gold, radioactive transformations went the other way, beginning with rare and valuable elements such as uranium and ending—often billions of years later—with much less valuable lead.
An electron inside a nucleus would experience a powerful electrical attraction to any proton it would encounter and the pair would quickly bind together in a single, electrically neutral unit, a subatomic particle he called a neutron. He theorized that the alpha particles were made of four subatomic particles, two protons each with one unit of positive electric charge plus two uncharged neutrons.
Rutherford had already established that a radioactive atom that emits an alpha particle experiences a decrease of two in atomic number and four in atomic mass. Now he was saying that those decreases were due to a smaller helium nucleus—a unit of two protons and two neutrons—bursting out of the larger unstable nucleus of a radioactive atom. He explained beta emission as the result of the splitting of a neutron into a proton and an electron. Since electrons are so light, the new nucleus would have approximately the same atomic mass but its atomic number would increase by one.
Even scientists who agreed with Rutherford’s theories wouldn’t accept them without proof. “Show me neutrons,” they would insist, and Rutherford set out to do just that. It took more than a decade, during which physicists devised many new devices and techniques to observe the paths of subatomic particles through matter, though not the particles themselves. Those techniques were very effective for revealing the passage of electrically charged particles, but the neutron, if it existed, would remain invisible.
Finally, in 1932, James Chadwick (1891–1974), one of Rutherford’s colleagues at the Cavendish Laboratory, figured out a way to detect neutrons indirectly but convincingly. The basic structure and components of atoms as we now know them—positively charged nuclei of protons and neutrons carrying most of the mass while filling only about a ten-thousandth of the atom’s diameter, surrounded by light electrons—had been established.
Other Revolutions in Physics
While Rutherford concentrated on the nucleus, two other revolutions in physics were also taking place: quantum mechanics and relativity. Together, they transformed our scientific understanding of matter and energy, and space and time. Albert Einstein had a hand in both. Amazingly, he published his groundbreaking ideas in both fields in 1905, the same year that his explanation of Brownian motion proved that atoms and molecules are real.

James Chadwick (1891–1974). Detecting an electrically neutral particle like the neutron was a challenging problem. In 1932, Chadwick found a way to detect neutrons indirectly. His experiment is described in detail in Understanding Neutrons in this series.
Where Rutherford Was Not Quite Right
Rutherford’s explanation of beta rays was not quite right. He was correct that a neutron within the nucleus transforms into a proton and an electron. But later research revealed that he had left something out. When the nucleus of a particular radioactive isotope nucleus emits an alpha particle, that alpha always carries the same amount of energy. That allows scientists to identify the isotope by the energy of its alpha particle. The same is true of gamma rays.
But beta rays are different. They can have any amount of energy up to a certain maximum. That caused a problem, because the scientific principle of conservation of energy was never violated in any other circumstance. Energy could change form, but it was never gained or lost. Einstein’s famous equation E = mc2 showed that mass was a form of energy. When a neutron transforms into a proton and an electron, the nucleus loses a small amount of mass that transforms into energy. The proton stays in place, but the electron leaves the nucleus as a beta particle and carries some of that energy with it.
Experiments showed that the energy the beta particle carried was never more than the lost mass. But it could be much less. To conserve energy, Wolfgang Pauli (1900–1958) proposed in 1930 that another particle was also emitted. That particle could not carry electric charge or have much mass, which made it very hard to detect. He called it the neutrino, for “little neutral one.” The neutrino was a central part of a theory of beta decay published by Enrico Fermi (1901–1954) in 1933, and it was finally detected in 1956.
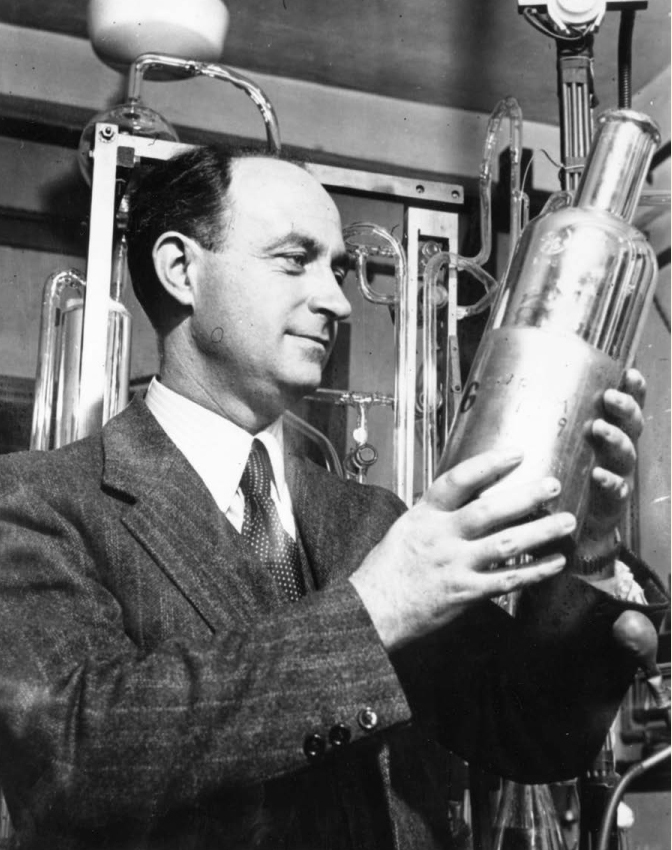
Enrico Fermi (1901–1954). Fermi developed a full theory to explain beta decay. Besides producing an electron (the beta particle), the decay process also led to the emission of a very light, electrically uncharged particle, the “little neutral one,” or neutrino in Fermi’s native Italian.
The most famous scientific equation of all time is probably Einstein’s E = mc2, which he wrote almost as an afterthought to his theory of relativity. Just as relativity uncovered surprising relationships between space and time, this equation expressed the unexpected fact that mass (m) and energy (E) are two sides of the same coin. They are measured in different units, so a conversion factor is needed to match them. Nature’s conversion factor between mass and energy is the speed of light (c) squared, or multiplied by itself. As scientists began to measure the masses of protons, neutrons, and the nuclei of different isotopes, they began to see the power of that simple equation. For example, when a radioactive nucleus emits an alpha particle, the mass of the daughter nucleus plus the mass of the alpha particle add up to less than the original mass. The missing mass turns out to be exactly the energy carried by the alpha particle.
Einstein’s third great idea of 1905, and the one that led to his winning the Nobel Prize in 1921, changed the relationship between matter and energy in another way. He looked at two puzzling recent discoveries. The first was the photoelectric effect, in which light could knock electrons free from metals but only if its frequency was high enough. The second was an odd idea developed a few years earlier by Max Planck (1858–1947) in his calculations of the spectrum (the mix of colors) produced by a hot body. Planck’s equation depended on having light energy coming not in smooth waves like water, but in a stream of packets called quanta. He didn’t believe that quanta were real, but they fixed a serious problem with his calculations. Einstein realized that the photoelectric effect was evidence that Planck’s quanta, which later became known as photons, were real.
Other scientists soon recognized that all subatomic particles are quanta, just like photons, and they behave like waves at the atomic scale. They developed a new field called quantum mechanics that described the situation. For example, electrons in atoms had certain allowed wavelike states, each corresponding to a certain energy level specified by four “quantum numbers.” Quantum mechanics worked spectacularly well for hydrogen, and when scientists imagined building larger atoms by adding more protons and electrons to them, they discovered something even better. The atoms had periodic properties. More than fifty years after Mendeleyev’s dream, the periodic table made sense.
Quantum mechanics also changed the way science understood the basic forces of nature. For example, the laws of electromagnetism were recast in a new mathematical form called quantum electrodynamics, developed in the 1940s. These laws explain electromagnetic attraction and repulsion as the result of exchanging photons between electrically charged quanta, such as electrons and protons. In fact, in the quantum world, all forces are the result of such particle exchanges—and that takes us back into the nucleus. If protons repel one another by exchanging photons, what keeps the nucleus from blowing itself apart? There must be another force at work inside the nucleus, and it must have something to do with both protons and neutrons. That force is known as the strong nuclear force, or simply the strong force. (There’s another force called the weak nuclear force that explains beta decay.)

The Power of Nuclear Forces. The universe is comprised of billions or trillions of galaxies like this one, each containing billions or trillions of stars. Stars produce their vast amounts of light by nuclear processes that rely on the strong and weak nuclear forces.
The strong force must have some unusual properties. Unlike electromagnetism, which extends its influence a long way, the strong force must become insignificant beyond nuclear distances. Otherwise, nuclei of different atoms would be drawn together and the universe would collapse into one giant atom. It must be much more powerful than the electromagnetic force within the nucleus, yet there must be a limit to its power outside the nucleus so that particles are not crushed to nothingness.
Such a force can be understood by mathematical approaches that are similar to those used in quantum electrodynamics, with a few differences. Nucleons—protons and neutrons—attract through a property that physicists call color, the strong-force equivalent of electric charge, and instead of trading massless photons, they exchange particles called pi-mesons that have a mass of about 250 times that of an electron.
Of all the known forces in nature, the strong nuclear force is the most powerful of all. It is also very important in other ways. It is not only responsible for holding nuclei together, but it is also the source of the energy of the stars.