Looking around us, we can find many different elements. Thus we rarely think about how different our world is from most of the universe. Except for hydrogen, most of the atoms on Earth are uncommon. To understand why that is so, we need to consider how the universe began.
The best current scientific understanding is that the universe burst into being 13.7 billion years ago in an event commonly called the big bang. It began as seething, expanding matter and high-energy photons. In a short time, most of that matter became the subatomic particles we now know so well, mostly protons, electrons, and neutrinos. Initially, it was too hot for atoms to form. As the universe then expanded and cooled, the subatomic particles joined to make atomic matter.

Element Factories. Everywhere we look in the universe, we see galaxies. This image shows a relatively close group of galaxies known as Hickson 44. If we study their light we can detect small amounts of most of the familiar elements on Earth. Most of those elements were not present immediately after the big bang. It took several generations of stars, powered by nuclear fusion, to produce all the elements heavier than lithium (atomic number 3).
Most of the atoms were hydrogen. Almost all the rest were helium, except for a tiny bit of lithium (atomic number 3). None of the atoms with heavier nuclei familiar to us on Earth—carbon, nitrogen, oxygen, aluminum, iron, and so forth—were anywhere to be found. Even today, most of the matter in the universe is hydrogen, and thus most of its mass is from protons. That is even true about our bodies, although they also contain plenty of neutrons.
Most of the atoms in our bodies are hydrogen, but those atoms are combined with heavier atoms in compounds, for example with oxygen in water. Those heavier atoms contribute most of our bodies’ mass. Our bodies also need energy, extracted from plant and animal matter. That energy originally came to Earth from sunlight. So what do these facts have to do with protons? Everything!
Naming the Big Bang
Cosmology is an unusual science, since its subject is the whole universe. Although there are some competing ideas about the history of the cosmos, the most generally accepted one is called the big bang theory. The theory has its roots in the work of astronomer Edwin Hubble (1889–1953), who studied distant galaxies and discovered that, except for the very nearest ones, they were moving away from our own galaxy, the Milky Way. The farther they were from us, the faster they were receding.
Hubble published his interpretation of the results in 1929. His explanation, which came to be called Hubble’s Law, was that the universe was expanding at a constant rate. Tracing that expansion back in time led to the conclusion at one point in the distant past, all the matter and energy in the universe was concentrated in a very tiny region. It began expanding and cooling to produce the cosmos we observe today.
Every theory has its critics, and one of the most important doubters was Fred Hoyle (1915–2001). Hoyle’s competing theory described a universe in a steady state that grew by the slow but steady emergence of new matter throughout all space. During a 1949 interview on a BBC radio program, Hoyle used the phrase “big bang” to mock the idea that the universe began in an explosion. The name stuck.
Ironically, one of the best pieces of evidence supporting the big bang theory comes from a 1957 publication by Hoyle and four other scientists. In it, the scientists predicted the ratio of hydrogen, helium, and other elements that would emerge from the big bang and how much of the other elements would be forged later in the stars. Those predictions matched actual measurements so well that it would be hard to consider any other explanation—though Hoyle never stopped trying to come up with an alternative.

Giants of the Cosmos. In his study of distant galaxies in the 1920s, Edwin Hubble (right) discovered that the universe is expanding. His results have led to a theory that the cosmos as we see it now is the result of a single explosive event nearly 14 billion years ago. Noted astronomer Fred Hoyle never accepted that explanation. He developed a competing “steady state” theory and derided the idea of a massive explosion as “the big bang.” The name, intended as an insult, stuck in the public’s imagination.
The Sun is a star, and, besides making light, stars are the factories where nature has cooked its heavier nuclei. The cooking process is called nuclear fusion, and it begins with the most common nuclear raw material available in the universe: protons.
How Stars Create Heavier Elements
During the big bang, much of what happened was the opposite of what goes on in radioactive decay. For example, in beta decay, a nucleus emits a beta particle (an electron) and a neutrino when one of its neutrons gives up some of its mass to become a proton. To reverse the process and make a neutron from a proton, an electron, and a neutrino, those three particles would need to come together with at least as much energy as was released in the decay process.
In the early stages of the big bang, protons, electrons, and neutrinos appeared first. Everything was so close together and moving fast enough to make some neutrons. But the expansion was so rapid that the making of neutrons ended quickly, leaving most matter in the form of protons, electrons, and neutrinos.
Besides making neutrons, the early universe was also hot and dense enough to cook up a few other nuclei. For instance, some protons and neutrons paired off to form the nucleus of a heavier isotope of hydrogen with mass number 2 called deuterium (denoted as D or H2). Two isotopes of helium also formed. The more common helium nucleus was the alpha particle (two protons and two neutrons or He4), but helium nuclei with only one neutron (He3) were also stable. Very small numbers of nuclei of other light elements also formed in the big bang. When the first burst of matter-creation ended, the nuclei picked up electrons. At that point, most matter in the universe consisted of hydrogen atoms and almost all the rest was helium atoms.

Star Power. Stars produce their energy by nuclear fusion, or combining light nuclei to make heavier ones. This diagram shows one fusion process that happens inside the Sun: deuterium-tritium, or D-T fusion. Both D and T are heavy isotopes of hydrogen with one proton. D has one neutron and T has two neutrons. They combine to form a nucleus of helium-4 and a free neutron. Because the total mass after the reaction is less than before, the extra mass becomes energy, which is carried off as light.
Once atoms formed, gravity could prevail over the more powerful electric force. Bare nuclei would repel each other electrically, but not neutral atoms. In some regions of the early universe, by pure chance, more atoms than average had formed. There, gravitational attraction began to draw the atoms together, slowly at first and then more rapidly. Eventually large numbers of atoms were coming together at high speed, sometimes heating up enough to strip away their electrons. Because of their high speed, not even electrical repulsion could stop the nuclei from getting close enough so that nuclear forces would take over. If circumstances were right, the nuclei would fuse, or join together.
Unlike the process that created neutrons from protons, electrons, and neutrinos in the early instants of the big bang, nuclear fusion events can sometimes release energy instead of absorbing it. That occurs when they make a particularly stable nucleus. For example, two deuterium nuclei (or deuterons) release energy when they fuse to form an alpha particle. Before and after the fusion event, there are two protons and two neutrons, but the alpha particle has less mass than the two deuterons. The difference in mass is released as energy.
Such fusion events are what powers stars such as the Sun, although the precise fusion reactions that take place are a bit more complicated than this example. Still, if gravity pulls enough hydrogen together in one place, fusion begins and continues until nearly all the protons have fused into alpha particles. While the star is “burning” its proton fuel, the outward pressure due to the heat counteracts the gravitational force that has been collapsing the matter.
When that fuel is finally used up, the collapse begins again, which raises the temperature of the star. Suddenly, there is enough energy to ignite other fusion events, such as the combination of three alpha particles into a carbon nucleus (C12), which may fuse with another alpha particle to make an oxygen nucleus (O16) at a still higher temperature. Those processes also release energy, but each step requires a higher temperature to ignite it. Depending on its size, each star stops all fusion at a different point, and becomes a slowing cooling cinder. For example, in about five or six billion years, our Sun will end up as a ball made mostly of hot carbon.
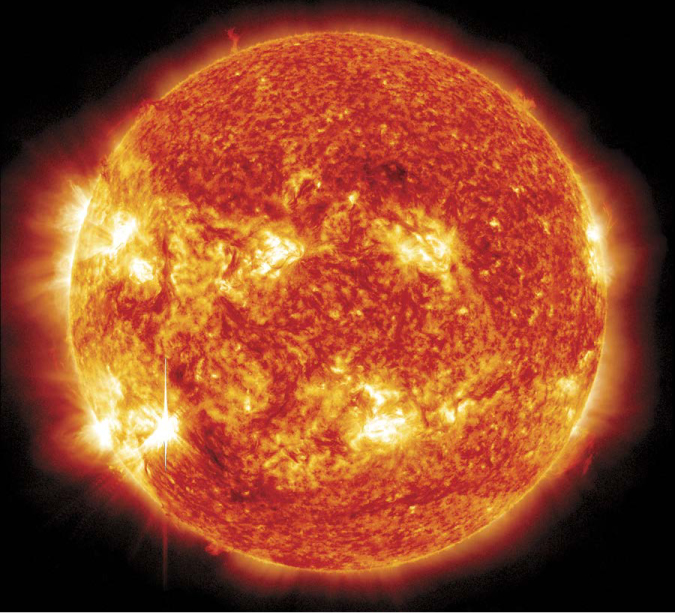
Seeing (Infra)Red. The Sun’s nuclear fusion furnace produces energy as electromagnetic waves of many wavelengths. This infrared image shows solar features that might not be apparent in visible light.
Larger stars follow other paths, but once a star is done fusing its nuclei into iron, additional fusion requires more energy than it produces. Iron’s atomic number is only 26. So where did Earth get all its elements with much larger numbers of protons? To make those elements requires a powerful event known as a supernova explosion, which occurs only for the largest stars. Those stars have so much mass that gravity squeezes their nuclei together until they explode with great violence. The event produces so much energy that it forces nuclei to fuse that would not ordinarily do so.

Seeds of Future Worlds. When a supernova such as this one explodes, its intense energy can create the heaviest known elements. Ordinary stars can only fuse nuclei up to the size of iron, atomic number 26. All of Earth’s heavier elements were made long ago in supernova explosions like this one. The formation of the Sun itself may have been triggered by a supernova explosion that sent a shock wave through a quiet cloud of dust, creating clumps of matter that then attracted each other to form a star and its planets.
It is sometimes thought that the products of fusion would all be unstable and blow apart as soon as the pressure is released. Many of them are, but some are like water in a deep well on top of a mountain. Once the water is in the well, you need a lot of energy to pump it out so it can run down to the valley. A few nuclei are radioactive—unstable but lasting a long time. They are like water in a shallower mountaintop well where severe storms come by from time to time. If you wait long enough, a storm is sure to strike and blow the water out.
Fusion Research and Technology
Understanding the nuclear processes in stars took a lot of research, and people involved in research often think about ways they can apply their discoveries. For instance, fusion energy has already been used in fearsome military weapons known as “hydrogen bombs” or “thermonuclear devices.” Might there be a way to make an electric power plant operating on the same principle? It would be like capturing the energy of the stars in a bottle.

Nuclear Fusion on Earth. The world’s most powerful weapons, called thermonuclear devices, create powerful bursts of nuclear fusion energy. These are many times more powerful than the bombs that used nuclear fission (splitting of heavy nuclei) that were dropped on Japan to end World War II. This image shows an American test explosion of a thermonuclear bomb. No aboveground American nuclear weapons tests have taken place since 1963.
Engineers have been trying to build fusion reactors for electric power for more than fifty years. The biggest problem has been finding a bottle to contain the super-hot gases. Since the fusing nuclei are electrically charged, they respond to magnetic fields, but even a slight instability is enough to disrupt the process before enough fusion energy is released. It takes electrical energy to make the powerful magnetic fields needed. So far, the most successful devices have only succeeded in producing more energy than it takes to run the reactor for a very short time, and at a great cost.
Another fusion power technique is called inertial confinement, and it avoids the need to create a powerful magnetic field. Instead, it blasts a pellet of hydrogen-rich material on all sides with a laser or similar intense energy source, hoping to create a situation that likens to the process by which material is drawn inward by a star’s gravity. The object is to create enough fusion reactions quickly before the heat blows the material outward again. It seems like a good idea, but so far it has been very difficult to achieve in practice.
Pieces of Protons
As scientists investigated subatomic particles and new forces, such as the strong and weak nuclear force, they have created machines that make beams of protons. These beams have a number of uses, including the treatment of some forms of cancer and the detection of very small amounts of certain elements in a sample of material. Probably the most interesting use is what happens when proton beams are accelerated to nearly the speed of light and then allowed to collide. The result is a shower of new and very unusual particles that reveal inner secrets of matter that might have surprised Ernest Rutherford even more than the discovery of the nucleus did.
One of those surprises is that, like atoms, protons and neutrons are not indivisible. They contain even smaller particles called quarks. Quarks stick together so tightly that they can never be detected separately, though scattering experiments can reveal their presence just as Rutherford’s experiments showed that atoms have nuclei.

Electricity from Fusion? Nuclear fusion promises to be an important source of electrical energy for the future. But despite years of research, it remains difficult to ignite a fusion reaction that can be contained and controlled. These German workers are building the main fusion chamber of the Wendelstein 7-X experimental fusion reactor in Greifswald, which was nearing completion in 2015.
There’s a lot you can learn about both quarks and the history of machines that create high-energy beams of protons or other particles. Fortunately, you don’t have to go far to do that. This series includes books about both quarks and the particle accelerators that led to their discovery.