One of the developments in recent years that really opened up scientists’ eyes to the possibility of life on other worlds was the realisation of just how adaptable and versatile life is, and the growing appreciation and understanding of the physical limits of organisms. Since the 1990s, a particular branch of microbiology has been gathering pace – the area of Extremophiles. In every corner of our planet in which we look, even in places barely survivable by humans, there is some form of life flourishing. It seems that once life gets going, it will fight to survive and, if needed, will adapt to fill almost any moist niche it comes across. With evidence for liquid water surfacing on many planets and moons in the Solar System, although in conditions beyond any that humanity can survive, organisms that have adapted to life in extreme conditions are taking centre stage. They are providing us with a template for finding life elsewhere and directing us to environments in which we would never have considered searching.
Understanding the range of current life on Earth and mapping it to current environments in the Solar System is simply the start as it lacks the element of time. Life on Earth was substantially alien when it arose around 4 billion years ago because the environments on Earth were so dramatically different. Similarly, the climatic conditions forecast for a billion or so years into the future are depressingly bleak for much of life as we currently know it, including ourselves. The range of what is possible is continually being stretched to incorporate new adaptations displayed by life in its bid for survival. Some organisms have created an extreme-living club, and you can only be a member if you can survive somewhere another form of life cannot.
Extreme-lovers
Terrestrial life, as defined in Chapter 2, is made up of carbon compounds and uses water as a solvent and as such has to abide by the limitations imposed by this chemistry. It manages to emerge and survive within boundaries such as the boiling and freezing points of water, the presence or absence of oxygen, extremes of acidity and alkalinity and all pHs in between. The range of tolerances to these are known for the most part for all cells, and the extent to which life can survive any combination of acidity, temperature and salinity determines the envelope of life, ranging from cold acidic waters in one corner to hot alkaline brines in the other. The complexity of eukaryotic cells (those with a nucleus, as found within us and all multicellular organisms) means that they are much more sensitive to perturbations of these three conditions. Therefore, the most extreme outer regions of the envelope of life are not dominated by complex eukaryotes but by the simpler prokaryotes. Any organism found thriving in these hostile and extreme environmental conditions on Earth is bestowed with the alias extremophile, literally meaning ‘extreme love’. When we hear the term extremophile, it immediately conjures up images of tiny microbial prokaryotes, yet the extremophilic taxonomic range spans all three domains, including multicellular sophisticated vertebrates. While adaptation to a single harsh habitat is already impressive, there are species that can survive a variety: the rare polyextremophiles. These organisms are exploiting an ecological niche for which they are uniquely adapted, and face little or no competition within it.
The Relativity of Extremity
What is extreme? Perhaps extreme is simply an opinion; whether an environment or an organism is extreme is determined by the eye of the beholder. It is clear to us humans that a heat-loving thermophile that dies at temperatures below 21°C (69.8°F), or a pressure-loving piezophile that finds the atmospheric pressure on the Earth’s surface too much to bear, is an extreme being in comparison to our own adaptations – but what determines an extremophily? Is it an evolutionary viewpoint? Does the earliest environment to contain life define what is considered normal? If life had arisen in a high-temperature, zero-oxygen hydrothermal vent, or around a cooler alkaline spring at the base of the earliest oceans, would that then be considered as normal, and every environment that has arisen since extreme? Or perhaps extremity is a physical state. All physical factors are on a continuum, and any changes in the conditions that make it difficult for organisms to function are therefore considered extreme. Extremophiles are extreme only in relation to the capabilities of other cells. To a bacterium living in the crushing pressures and high temperatures of an ocean-floor black smoker, we humans living at the level of atmospheric pressure present at the Earth’s surface – 1 atmosphere (1atm or 0.101 megapascals/MPa) – breathing oxygen and basking in moderate temperatures, are the extreme beings. Obviously, therefore, normal is relative to whoever is making the comparison, so from a human perspective a normal condition or environment refers to conditions in which humans could comfortably survive without artificial aid.
Must an extremophile actually love an extreme environment as its name suggests or can it merely tolerate it? And must an organism depend on these extreme situations for the entirety of its life cycle? An extreme-loving life form does not need to be completely smitten with the conditions it currently resides in. It may be an obligate organism that requires these particular extreme conditions to survive, or facultative – meaning it is not only able to tolerate certain extreme environs when necessary, but is also able to thrive in others at particular stages of its life cycle. The bacterium Deinococcus radiodurans, the present gold-medallist of radiation resistance, is widely regarded as an extremophile par excellence, yet retains its radiation superpower only as long as other extreme conditions are lacking; it is severely diminished under freezing or desiccating situations. Other examples are spores, seeds and eggs, which are all far more resistant to environmental extremes compared to their vegetative or animal forms. Similarly, trees, frogs, insects and fish shift their physiology as the seasons change, so they can tolerate remarkably low temperatures during the winter months.
The Extremes
Liquid water is the prerequisite of life on Earth and arguably will be the cornerstone of any life in our Solar System and beyond. Life also requires an input of energy but crucially must be able to control this energy as it courses through its system. It does this through redox chemistry, whereby the loss or gain of electrons between molecules, atoms or ions occurs through a process of oxidation or reduction. Redox chemistry is universal to life on Earth and as all life is based on carbon organic chemistry, it is assumed that such reactions must be allowed to operate for a life form to exist. In terms of extremophiles, they must either live within environments that adhere to these energy parameters or be able to guard against the hostile outside world in order to maintain these conditions within their cells. Life on Earth has been found enduring physical extremes (for example, temperature, radiation or pressure) and geochemical extremes (such as desiccation, salinity, pH, raised or absent oxygen levels or redox potential). It could also be argued that there are also biological extremes such as nutritional availability, and excesses or lack of population density, parasites and prey.
Temperature
More than 80 per cent of our planet, far from being a balmy paradise, exists at temperatures colder than 5°C (41°F) and, even more incredibly, these frigid places are inhabited. As such, the most well-known extremophiles are those that can work to adjust their thermostats, allowing them to live in the coldest, and hottest, places on the planet.
There are two top prizewinners among temperature-tolerant extremophiles. Thermophiles are heat-lovers, commonly found in hot springs and geysers the world over. As microorganisms cannot regulate their own temperature as animals do, they must instead adapt all of their cellular machinery to a particular set of operating conditions. Importantly, they have evolved reinforced proteins to hold themselves together against the violent shaking caused by thermal motion at high temperatures. If a protein shakes to such an extent that it loses its three-dimensional structure and becomes denatured, it loses its function and life cannot survive without working proteins. Temperatures approaching 100°C (212°F) normally cause this denaturation to occur in DNA and RNA as well as in proteins, and increase the ‘leakiness’ of cell membranes to lethal levels. Above 150°C (302°F), many organic molecules decompose entirely and chlorophyll degrades above 75°C (167°F), preventing photosynthesis from continuing within cyanobacteria and all plants, thereby leading to their death from starvation. The most hyperthermophilic organisms (extreme heat-lovers with maximum growth at temperatures over 80°C/176°F) are archaea. Among these, Pyrolobus fumarii is a chemolithoautotroph that obtains energy from the oxidation of inorganic compounds, and carbon from the fixation of carbon dioxide; it is capable of growing at temperatures of up to 113°C (235°F). Another archaean, dubbed Strain 121, is found growing quite happily at over 120°C (248°F). At these hotter temperatures, the solubility of gases such as oxygen and carbon dioxide decreases, so many hyperthermophilic organisms are also anaerobic (do not require or use oxygen). The more standard thermophiles are mainly found among the phototrophic bacteria, eubacteria and archaea. The eukaryotes, however, such as algae and fungi, are comparative wimps with an upper temperature limit of only around 60°C (140°F), while for vascular plants it is a pitiful 48°C (118°F), and for fish a relatively frigid 40°C (104°F). It is within this thermophilic group that the universal ancestor of life is commonly thought to have resided (see Chapter 4 for more on LUCA), and may be the type of life found on worlds closer to their stars than the Earth, or around the geysers of the gas giant moons.
The cold-lovers on the other hand, the psychrophiles, inhabit environs where the temperatures dip below 0°C (32°F). These communities have enzymes and membranes that are loosened so they can remain dynamic and keep the cells active even at temperatures down to –18°C (–0.4°F). Without this adaptation, as the temperature dropped, the cells’ contents would become rigid and inflexible, leaving them unable to fulfil their roles. Temperatures below freezing are a challenge for life as a consequence of the properties of its main solvent, water. Below 0°C (32°F), water freezes into ice crystals that can rip cell membranes apart and without liquid water, solution chemistry within cells stops. The freezing of intracellular water is almost always lethal to life. Despite this, many microbes and cells can be successfully preserved at –196°C (–320.8°F), which is the temperature of liquid nitrogen. In fact, human eggs in fertility clinics are preserved using this although they, together with microbes and cells, are not active at this temperature but held in suspended animation. Among animals, the Himalayan midge incredibly is still active at –18°C (–0.4°F), while the poor Antarctic icefish suffers from heat exhaustion above 4°C (39.2°F). Some of the most extreme psychrophiles reside inside solid icebergs within tiny channels of salty water that are kept liquid owing to their saltiness, and are killed at human body temperature. It is these extreme organisms that can survive the commonly lethal effects of the cold, which are of great interest in the search for life on the icy moons of Jupiter and Saturn.
In organisms more complex than microbes, it is perhaps their behaviour more than their biology that enables them to overcome the physical challenges of extreme temperature environments. As a defence mechanism, they can retreat from unfavourable conditions and relocate to a safer home. In the deserts of Earth, some animals have diurnal habits whereby they bury themselves in the more humid and wet layers beneath the surface to avoid the scorching Sun. In particular, the desert ants of the Sahara are among the most heat-tolerant species in the world and can be found sprinting across the scorching sands. They deliberately come out at the hottest point in the day, when surface temperatures are around 60°C (140°F), which, crucially, restricts their predators’ activities. The ants scavenge for the corpses of insects that have died of heat exposure and, although they are physically evolved to resist the high temperatures, could still die rapidly from heat shock themselves. They survive because they stay out for short periods and have long legs, enabling them to move quickly with as little contact with the sand as possible to prevent the heat from building up in their bodies. At the other end of the spectrum, some species of nematode worm in Antarctica can withstand the harsh cold temperatures and lack of water by producing antifreeze and drying themselves out, letting the wind blow them around until water is found again, i.e. sitting tight and waiting it out. The red flat bark beetle of northern Alaska is an excellent psychrophile; the formation of ice crystals in its internal fluids is the greatest threat to its survival, but the beetle produces antifreeze proteins that stop water molecules from grouping together. Their larvae have been found surviving at temperatures of –150°C (–238°F), for which the antifreeze proteins alone would not be enough. These beetles also deliberately dehydrate their internal tissues; internal water cannot freeze if it is not there any more!
pH
After temperature, the acidity or alkalinity of an environment affects life greatly. Acidity is rated on the pH scale, which measures the concentration of H+ ions (protons) in a solution. Low pH is an acidic environment, while high pH is an alkaline one; pH7 is neutral. Concentrations of protons and their movement from one area to another within a cell are a fundamental mechanism of energy transformation. Biological processes tend to favour the middle range of the pH spectrum, around pH7, and intracellular and environmental pH often falls in this range as well. Proteins commonly denature at exceptionally low pH conditions, yet this is where acidophiles are found thriving. Acidophiles are able to survive in highly acidic environments as they can protect the vital molecules inside their cells, such as DNA and proteins, from the high concentration of protons in their environment. These organisms work constantly to pump the excessive levels of protons back across their cell membranes to the outside, like a sailor trying to bail out a leaking ship. Many acidophiles can tolerate pH2 (about the acidity of lemon juice), and some as low as pH0. This is best characterised in the red alga Cyanidium caldarium, which has been discovered in nature at pHs as low as 0.5, although it grows most successfully in a laboratory at pH2–3. As well as acidophilic prokaryotes, eukaryotic life forms may also be active in environments lower than pH3, although many of these are acid tolerant rather than truly acidophilic and may grow equally well or even better in more neutral habitats. Most eukaryotic acidophiles are, however, still microbial and many yeasts and fungi can grow in acidic soils and peat bogs of pH3–5. A number of filamentous fungi has been found growing at above pH3, and protozoa within acidic, metal-rich waters of pH2–3. Acidophiles such as these could find quite an acceptable home in the acidic cloud decks of Venus.
Alkaliphiles on the other hand prefer a high pH (commonly 8.5–11) and alkaline environments, although they find it equally challenging. As with low pH, there is often a difference of two or more pH units between the internal and external milieu of the cell and alkaliphiles can struggle to generate energy with too few protons in their environment. If cells are to survive in an alkaline environment they must make their own cytoplasm more acidic to buffer the alkalinity and bring it closer to a comfortable neutral value. Representatives of all domains and kingdoms of life are able to tolerate pH as high as around 11, but perhaps the best understood are the alkaliphilic bacteria and archaea, such as Natronomonas pharaonis.
Water Availability and Salinity
Water possesses a number of properties such as a high melting and boiling point, a wide temperature range within which it remains a liquid, and it forms hydrogen bonds, which makes it essential for life. It makes up 95–99 per cent of the total molecules in invertebrates and a typical adult human cannot survive the loss of even 14 per cent of his or her water. As such, a lack of this life-giving fluid constitutes a pretty extreme environment. Organisms that can tolerate extreme water loss or desiccation enter anhydrobiosis, a state characterised by little intracellular water and no metabolic activity. A variety of organisms can become anhydrobiotic, including bacteria, yeast, fungi, plants, insects, tardigrades, mycophagous nematodes and the shrimp Artemia salina. A further variety of organisms use desiccation-resistant spores to survive dry periods as well as for dispersal, e.g. by the wind. Resurrection plants such as Craterostigma plantagineum are unusual in that the plant itself can survive desiccation and can even revive after several months in an air-dried state. Some can even survive a loss of chlorophyll (the pigment required for a plant to be able to photosynthesise). All of these organisms would be very well suited for survival on any planetary body further out from its star than the Earth, where liquid water is scarce.
Organisms can live in a wide range of salty environments, from essentially pure distilled water to completely saturated salt solutions. The latter, however, is much more problematic for life. Salt-loving halophiles grow in high-salt solutions including the Dead Sea, which is not actually all that dead! Very salty or briny water outside the semi-permeable membrane surrounding each cell risks drawing water out from the cell, leading to dehydration. Conversely, if the salt concentration of the environment outside is lower than that inside the cell, it could swell to bursting as water rushes in. This process is called osmosis. Some halophiles have modified their inner workings to cope with higher salt levels, keeping their insides balanced with the outside, and thus safe from osmosis. Others have taken a different approach and packed their cells with different solutes (chemicals dissolved in the fluid inside the cell) to produce an equally concentrated solution to guard against osmosis, while avoiding the issues of a briny interior. These cells protect their innards from becoming too dry or salty by keeping them agreeably sugary. With these adaptations, organisms are able to thrive in the high salt content of salt evaporation ponds at roughly 10 times the concentration of salt in the ocean. Most halophiles are archaeal and bacterial but humans, along with most plants and other vertebrates, cannot tolerate high-salt environments.
Radiation
Radiation, both ultraviolet (UV) and ionising, is particularly hazardous to life. It can damage every single biopolymer (long chains of molecules strung together, produced by living organisms), causing destruction of nucleic acids, proteins and lipids. Radiation is a huge problem beyond the Earth’s atmosphere, both within space itself and on worlds themselves lacking a protective atmosphere to shield life from incoming solar and cosmic radiation. As such, any life form that can survive high levels of radiation, or has the ability to recover from radiation damage, has a distinct advantage for survival. Because of the importance of keeping biopolymers intact and functional, organisms can avoid exposure by living underground or producing UV-attenuating pigments. However, because radiation damage cannot always be avoided, there are multiple mechanisms for DNA repair found in all organisms. Still, a few organisms stand out in their ability to handle radiation damage. The bacterium Deinococcus radiodurans, as mentioned earlier, is the reigning champion, having been found living within the cores of decommissioned nuclear power stations. It has the ability to withstand both ionising radiation (doses of up to 20 kilograys of gamma radiation) and UV radiation (doses of up to 1,000 joules per square metre) – levels that are 3,000 times higher than what is fatal for humans – but this extraordinary endurance is in fact thought to be a by-product of resistance to extreme desiccation.
Pressure
Life is sensitive to pressure, be it atmospheric (in the air), hydrostatic (underwater) or osmotic (within cells), since any form of pressure forces changes to the volume within cells. Pilots and divers must remain aware that rapid changes in pressure upon ascent can result in gases – generally nitrogen – coming out of solution in the blood to form gas bubbles and, if not treated, this can result in death. A similar situation occurs in microbes from the ocean floor that have gas-filled vacuoles. If they undergo decompression too rapidly, the vacuole expands and bursts, and they die. However, most microbes found in the deep ocean are able to grow at normal atmospheric pressure if decompression occurs gradually. Organisms that can survive in moderately high hydrostatic pressures, greater than the level of atmospheric pressure (1atm/0.101MPa) present at the Earth’s surface, are called piezophiles – the pressure-lovers, known until recently as barophiles (weight-lovers). The boiling point of water increases with pressure, so water at the bottom of the ocean remains liquid at up to 400°C (752°F). Piezophilic bacteria are found to grow at pressures up to 500atm (50MPa), and the most extreme piezophilic life at even greater pressures. To survive these high-pressure environments, cells have increased binding capacities of enzymes and extra fatty acids within cell membranes to help them retain their flexibility and motion. Piezophiles include microbes, invertebrates and fish. There is life under high hydrostatic pressure even in the deep trenches of the ocean, living at up to 1,091atm (110.6MPa), for example in the Mariana Trench, the world’s deepest sea floor at 10,898m (6.8 miles). Colwellia MT41 is a psychropiezophilic bacterium, a polyextremophile (cold- and pressure-loving) microorganism, found growing in the deep sea at 1,016atm (103MPa,) at only 8°C (46.4°F). Pressure may be a critical factor for higher animals and plants including ourselves, but neither the highest nor the lowest pressures encountered in the habitable parts of the Earth’s surface are obstacles to the establishment of microbial life. Low pressures are too rare on Earth for such communities to have evolved here, but that does not mean it would not be possible on other worlds. Earth’s pressure-lovers would be excellent organisms to colonise the base of the oceans on Europa or Enceladus, or to live deep underground on Mars.
Oxygen
The Earth has been anaerobic or oxygen-free throughout most of its history, so our reliance on oxygen is only a very recent phenomenon. Living organisms use energy released by respiration for their life processes and there are two types of respiration – aerobic (which needs oxygen) and anaerobic (which does not). Today, this need for oxygen is limited to only a handful of life forms on the Earth, so even though to us breathing oxygen is normal, we could be considered the odd ones out – the extremophiles. A huge variety of organisms are found to inhabit strictly anaerobic environments, where the mere presence of oxygen would be toxic. Currently, some bacteria and archaea use elements other than oxygen (such as nitrogen or sulphur) as the main source of their energy. However, a metabolism using oxygen is far more efficient, although this efficiency comes at a price. Molecular oxygen is highly reactive. The reduction of oxygen to water occurs during aerobic respiration in animals, and the reverse happens during oxygenic photosynthesis in plants, which creates hazardous chemically reactive molecules containing oxygen, particularly the hydroxyl radical (·OH). Without the superior generation of energy from aerobic over anaerobic respiration, it is unlikely that animals would have arisen owing to their high metabolic demands; cellular damage, or more specifically oxidative damage or stress caused by an excess of these oxygen-rich radicals, is the price we pay. Current thinking suggests that much, if not all, degenerative human disease involves oxidative damage. The need for oxygen for large and energy-intense organisms such as humans nonetheless outweighs these negative effects.
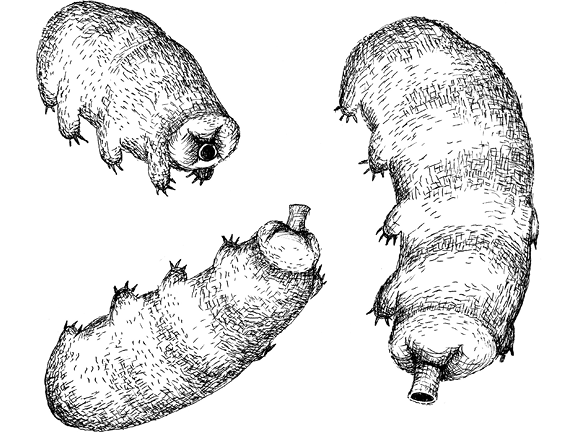
Nature’s Superheroes – the Water Bears
The epitome of a polyextremophile and the kings (or queens) of surviving extreme environments, tardigrades are incredibly endearing, eight-legged, all-but-indestructible and mainly microscopic animals. First named tardigrada from the Latin meaning ‘slow walker’, they are also known as water bears (a name I love, derived from their resemblance to eight-legged pandas) and even moss piglets (drawing comparisons to pygmy rhinoceroses and armadillos). Most tiny invertebrates dart about frantically but the water bears see no need for this; they shuffle along slowly, clambering across bits of debris, ambling around their habitats on pairs of short, stubby legs located under their bodies. The legs are outfitted with a number of hooked claws that resemble the talons of bears. Water bears have five body sections, including one that is obviously a head (with or without a pair of eyes) and are encased in a rugged yet flexible cuticle that must be shed as the organism grows. Animals generally grow by adding more cells or by making each cell larger. The water bears for the most part do the latter, as they must must break out of the cuticle in order to grow. All of this houses a nearly translucent, charismatic miniature creature only half a millimeter in length, about the size of the full stop at the end of this sentence.
These mostly microscopic aquatic animals can be seen with the naked eye in the right light and are found just about everywhere across the Earth, from the Arctic to the Equator, from freshwater droplets within garden moss to the salty deep ocean, and to the tops of forest canopies and the summits of mountains. The vast majority of water bears feed only on plant cells or bacteria, slicing them open with their dagger-like teeth and drinking their fluid contents. Others, however, are vicious predators. Moving incredibly fast on the first six legs, they employ their fourth pair to stand upright and attack prey with the rest of their claws – not unlike an actual bear.
The ubiquity of water bears is linked to their best-known feature, their survivorship – they have survived all five mass extinctions – quite possibly because of their strong determination to overcome a cacophony of spectacularly extreme conditions. This has earned them the title of the most extreme survivor of all, beating penguins in Antarctica, camels in the desert and the common cockroach. Only the land-dwelling water bears can boast this title however; marine and aquatic species appear to not have developed these superhero characteristics. All the survival adaptations water bears display were selected in response to their rapidly changing terrestrial-based micro-environments. Terrestrial water bears, for example, technically live on the land but actually reside within thin films of water. Moss and lichens, for example, provide sponge-like homes dissected by a myriad of small pockets of water for water bears to inhabit, but are always at risk of drying out. The water bears have two choices in this situation – die or adapt to new, drier environmental conditions. As such, terrestrial water bears have three basic conditions of life: active, anoxybiosis and cryptobiosis. When active they eat, grow, fight, reproduce, move and go about their normal daily routines. Anoxybiosis commences when a water bear finds itself in a low-oxygen environment. Prolonged asphyxia results in failure of the systems that regulate body water, causing the water bear to puff up like a newly-popped piece of popcorn and float around for a few days until it can resume active life. Cryptobiosis effectively resembles death and resurrection. It is a suspension of the water bear’s metabolism brought on by the loss of liquid water and extreme desiccation. As its surroundings lose water, the water bear dries up with them, losing up to 97 per cent of its body moisture, shrivelling into a structure about one-third its original size, called a tun. In this almost mummified state of anhydrobiosis – meaning life without water – this hardy creature can survive just about anything thrown at it. Water bears actually form tuns several times a year in nature simply by retracting their legs and head and curling into a ball, surrendering nearly all of their body’s water. The water bear in effect preserves itself by becoming a powder comprising the ingredients of life, held in suspended animation. When finally rehydrated by an adequate source of moisture, it returns to its active life in as little as a few minutes.
Water bears in their hibernating tun state have been experimentally subjected to temperatures far below freezing (down to –272.95°C/–459.31°F) and, once warmed and rehydrated, returned eagerly to active life. They have been boiled alive, exposed to 150°C (302°F), and still been revived. They have also been weighed down by nearly 400atm (40MPa) of pressure (equivalent to that felt at the base of the ocean) and exposed to superfluous concentrations of lethal gases, such as carbon monoxide, carbon dioxide, nitrogen and sulphur dioxide, and still they returned to life. How they can survive all of this remains something of a mystery. It may in part be linked to the fact they make excellent travellers. Water bear tuns are almost indistinguishable from dust grains in both appearance and size, and as such can float on the wind in a similar way to spores, pollen and seeds. Just like the latter, the tuns have a preference for where they land and many micro-environments will be unsuitable habitats for freshly arrived water bears. However, an unfortunately placed tun is able to wait for a change in conditions to something more favourable or to be picked up by the wind again and, with any luck, taken somewhere better. When the right watery conditions are finally found, life can begin again. Contributing to this success is the fact that many water bears are able to produce eggs without mating, and in a few cases are hermaphroditic, so able to self-fertilise. A lone water bear may thus be able to establish an entirely new population once it finds the right landing site. It appears they are creatures with few weaknesses; in fact, their only flaw is a vulnerability to mechanical damage when not in their protective tun phase – in other words, you can squash them!
Their near-indestructability may actually be written right into their DNA. Although still undergoing scrupulous investigation, recent sequencing of the water bear genome has revealed that a certain portion is of foreign origin. Potentially up to 17.5 per cent is made up of a mixture of around 6,000 genes from bacteria, archaea, fungi and even plants, which the water bear has absorbed into itself like a sponge, a process called horizontal gene transfer. This is not unusual within bacteria, which trade genes with each other as easily as we might swap emails, but these gene transfers are rarer in animals. A few other examples include ticks that have borrowed antibiotic-making genes from bacteria, aphids that have stolen colour genes from fungi, and wasps that have turned virus genes into biological weapons. One group of genes actually known as the Space Invaders has even repeatedly jumped between multiple organisms including lizards, frogs and rodents. Never has this new alien DNA, however, made up more than one per cent of the new, updated genome. The water bears are quite possibly the remarkable exception. How is this possible? It is thought that when they return to life after drying out in times of low water availability, their cells become sieve-like and molecules from the environment, including any nearby DNA, can enter. Since they are so good at repairing DNA damage, this patching-up ability seals in the new DNA and makes it part of the water bear genome. It has been found that the water bears can even switch on several of their borrowed genes, which in other organisms are involved in coping with stressful environments. Perhaps they owe at least part of their legendary durability to these genetic donations.
Most exciting for astrobiology is the water bear’s ability to appear ultimately unaffected by the rigours of space travel; they are the first multicellular animals to outlive exposure to the deadly conditions of the cosmic environment. In 2007, researchers in Europe launched an experiment on the European Space Agency’s BIOPAN 6/Foton-M3 mission that exposed tun-state water bears to the solar radiation, heat and vacuum of space, while orbiting the Earth at a distance of 260km (160 miles). When they returned to Earth and were given a little water, the animals soon began to move and feed, and over time grew and reproduced. They had survived an environment in which life as we previously knew it could not. Later in the summer of 2011, Project Biokis-Tardikiss, sponsored by the Italian Space Agency, ferried water bears yet again into space, this time on the US Space Shuttle Endeavor. Colonies were exposed to variable levels of apparently lethal ionising radiation but upon return to Earth showed a very high post-flight survival rate, apparently unaffected by the cosmic radiation or the microgravity. The water bears are, for sure, creatures that could survive on any number of worlds in the Solar System so long as evolution was able to progress as it did on Earth to allow them to come into being. Could we one day find similar microscopic animals lurking in pockets of liquid water on Mars or Europa?
Extremophiles from Space?
The theory of panspermia mentioned earlier says that reproductive bodies of living organisms can exist throughout the Universe and develop wherever the environment is favourable. This implies that conditions beneficial for the development of life prevailed at different locations in the Universe and at different times, and may be ultimately responsible for the advent of life on Earth. One of the major criticisms levelled at panspermia, however, is that living organisms could not survive their long journey to Earth, owing to exposure to the nutritional wasteland of space with its solar and galactic radiation, frigid temperatures and vacuum, let alone the fiery descent through the Earth’s atmosphere. The Long Duration Exposure Facility (LDEF) and BioPan space experiments sent halophiles into Earth orbit, showing that these salt-loving microbes, as well as water bears, can survive in space. This has led scientists to seriously reconsider the ability of living biological material to travel between celestial bodies, particularly focusing on extremophiles, which are those most likely to survive the trip.
After the water bears, of course, the most probable terrestrial organisms to survive conditions in space are microbes, which might feasibly be stored, protected and transported within comets or meteors. In the vastness of space, microgravity is not lethal to life forms, and the extreme cold and lack of liquid water are survivable by many. Transit times between the expulsion of a rock carrying microbes from its host body to its final destination cannot currently be estimated, however, so we cannot address adequately the nutritional needs of organisms during the journey. We can hypothesise that the exceedingly low metabolic rates resulting from the cosmic extremes of cold and desiccation would render nutritional needs almost non-existent. Thus, we are left with two potential show-stoppers, namely radiation and the space vacuum. Most damage to microbes exposed to space, if they were not protected by a comet or asteroid, would be due to UV radiation, especially in the short term, although heavy ionising radiation has a greater probability of being lethal. Although the data are controversial, Deinococcus radiodurans, our extremophilic radiation specialist, did not survive its several-month residency in space and its DNA had extensive unfixable breakages, while Chroococcidiopsis, a desiccation-tolerant cyanobacterium that on Earth lives within rocks, survived only 30 minutes when exposed to UV radiation similar to that experienced on Mars. Interestingly, the salt-loving halophiles Synechococcus and Haloarcula-G were shown to survive for two weeks in space as long as they had some rock or soil shielding them – and could probably last much longer.
The Solar System, and in fact the Universe, is an extremely hostile environment for life as we know it. Even the strongest organisms on the Earth, the extremophiles, would find it a challenge. Yet, knowing these tough organisms exist and have evolved survival strategies to endure within the most extreme environments of the Earth gives astrobiologists renewed hope that something similar, maybe even resembling a water bear itself, may have found a way to thrive in places previously thought inhospitable out in the cosmos. Suddenly the once barren, hostile Solar System has been lit up with biological possibilities, but where could they be hiding?