
5
Body and Mind:
the influence of hormones
We are all aware that older people acquire wrinkles and have more fragile bones. We know that men in their seventies have weaker muscles than 40-year-olds. But do we know why that is? In fact, it’s all down to hormones.
The word ‘hormone’ comes from the Greek horm, meaning ‘impetus’. Hormones are chemical substances produced by the body and transported by the bloodstream to tissues and organs, stimulating or slowing their activity. One example is insulin, which regulates the concentration of blood sugar. It ensures that cells in the body take up glucose, reducing the levels in the blood.
As we grow older, the body’s production and delivery of many major hormones diminishes. The most important of these are growth hormone and the sex hormones: oestrogens in women and testosterone in men. In women, the decline in production of oestrogens is called menopause, starting on average at the age of 50 and lasting about five years. A well-known symptom is what are called ‘hot flashes’. These sudden feelings of heat in the upper body can be so overwhelming that sufferers will open all the windows in the middle of winter. A British actress about to be interviewed by The Daily Telegraph in a hotel lobby asked for the interview to take place outside on a bench in a bitter autumn wind — because of her hot flashes.
Other common symptoms of menopause include night sweats, bladder problems, dry skin, irritability, and fatigue. Expectations can also aggravate symptoms. Research has shown that women who have a negative attitude to menopause earlier in life often experience more symptoms when it actually arrives. Women in Eastern cultures, where it is less widely regarded as a problematic period, report fewer unpleasant symptoms than women in the West.
What is less well known is that hormonal changes in older men are also noticeable: this is sometimes called andropause or male menopause. Symptoms experienced by both men and women include reduced energy levels, mood swings, and diminished libido. Andropause is less severe than menopause. This is understandable: in women the whole hormonal reproductive system gradually shuts down, while in men there is only a slow decline in production. Incidentally, as with many other signs of ageing, not everyone experiences problems.
HORMONAL CHANGES
We don’t often associate sex hormones with our brains. Nevertheless, declining hormone levels have an unfavourable effect on our mental abilities. When Pam, a businesswoman in her mid-fifties, entered menopause, she worried for a time that she was suffering from early-onset dementia. Sometimes she couldn’t even think of a simple word such as ‘cat’. She had just started a course on mortgages (she was a successful financial adviser to banks) but noticed that she wasn’t absorbing or retaining any of the information. When she signed up for the course, she thought it would be a cakewalk, but in the end she didn’t take the final exam.
A study conducted at the University of California of over 2000 women showed that Pam is not alone. Memory problems increase early in menopause. Many women process information more slowly in this period. Mostly, this is not an alarming decline — they can still find their way around town, for instance — but they notice that their thought processes are fuzzier than before. The good news is that a year after their last menstruation (when menopause is over), memory, concentration, and the ability to learn will improve to some extent and will be less of a problem than during menopause. But first, let’s take a look at how these hormones influence the brain.
OESTROGENS AND MENOPAUSAL SYMPTOMS
Oestrogens are known as the ‘female hormones’. In fact, men also produce these hormones, but oestrogen levels are much higher in women. The production cycle of oestrogens and many other hormones resembles the path of a boomerang: it begins in the brain, and eventually returns to the brain. The hypothalamus begins the process. The signal to start secreting oestrogens passes a couple of intervening stations, including the pituitary gland, before arriving in the ovaries (figure 16).

FIGURE 16. After receiving a signal from the brain (hypothalamus), the ovaries begin to produce oestrogens.
In a woman’s fertile years, her hormone levels rise and fall. Over the course of the menstrual cycle, the amount of available oestrogens fluctuates from low during actual menstruation to high around ovulation, when the egg is released. These hormones play a crucial role in female sexual development during puberty. They control the cycle and are responsible for breast enlargement and distribution of deposits of fatty tissue in hips, breasts, and thighs. But oestrogens have many more functions. Thanks to their positive effect on fat levels in the blood and their role in widening blood vessels, they protect against cardiovascular disease. They also help make bones strong. When oestrogen production ends after menopause, women run a much higher risk of osteoporosis (‘brittle bones’) than men.
But oestrogens affect brain function as well, as shown by the influence of oestrogen levels on mood in women. This is confirmed by direct biological evidence: oestrogen receptors are located in various parts of the brain, including in major structures such as the hippocampus. Like neurotransmitters, hormones attach themselves to the neuron cell wall in order to do their work.
Which brain functions are affected by oestrogens? Oestrogen receptors are mainly found in the areas of the brain concerned with memory, as well as those that regulate mood, emotion, and stress: the amygdala, hippocampus, and the cingulate gyrus (an arc-shaped structure in the centre of the brain). These hormones also affect serotonin, a chemical messenger that modulates mood. Low serotonin levels can lead to depression.
For women who suffer from severe menopausal symptoms, prescribing oestrogens would seem an obvious answer. And this was in fact the practice for many years. Research has shown that it had a positive effect in reducing hot flashes and improving sleep, mood, and general wellbeing. Even memory improved in some cases. Studies using brain scans established that prescribing oestrogens after menopause helps activate the prefrontal lobe, improving working memory. Unfortunately, this type of hormone therapy has serious side effects: in some women, the risk of developing certain forms of cancer (ovarian cancer, for instance) is increased. Oestrogens promote the growth of tumour cells. What’s more, the therapy increases the risk of cardiovascular disease.
For all these reasons, doctors and scientists have been searching for other substances with the positive effects of oestrogens but not the negative. Phytoestrogens are a natural candidate, both literally and figuratively speaking. These compounds, obtained from plants, have the same structure as oestrogens and would appear to have the same effects. However, they do not promote the growth of tumour cells and have no significant side effects.
The soybean plant contains abundant quantities of phytoestrogens. So does eating lots of soy relieve menopausal symptoms? This would seem to be the case. In Asian countries, for example, where soy is an important part of the diet, women usually have fewer symptoms, both mental and physical. Though that is a very interesting fact, it is doesn’t necessarily mean that this is the result of eating soy. Other, cultural differences may be implicated. Yet the assumed link with soy is not unreasonable: animal research has demonstrated that the oestrogens in soy can have biologically comparable effects to those of mammalian oestrogens.
A number of studies have shown that eating soy can slightly reduce hot flashes in women who suffer severely from them. The best way to find out if soy products can also have a beneficial effect on memory and concentration would be to take a group of women in menopause who eat soy daily and compare them with a control group receiving a placebo. After a certain amount of time has elapsed, researchers see whether the women in the first group indeed suffer fewer symptoms. This is what a team led by Professor Yvonne van der Schouw at the University Medical Centre Utrecht did. Together with Professor Edward de Haan, I was responsible for the choice of neuropsychological tests and interpretation of the scores for this study. The subjects were 202 women in menopause, assigned on a random basis to the soy group or the placebo group. In the first group, the women consumed a soy preparation, consisting of a powder that could be added to drinks or to food, every day for a year. The placebo also consisted of a powder, this time containing milk proteins but no active ingredient.[1]
Neuropsychological tests were administered before and after the trial year, assessing attention, memory, reasoning, and the ability to plan and switch focus. Other tests measured bone density and blood fats (such as cholesterol), areas where oestrogens also have a beneficial effect. The researchers eagerly awaited the results of all these analyses: would the phytoestrogens in the soy preparation improve cognitive function? Disappointingly, the answer was no. In neither group was any improvement measured, either in cognitive function or bone density and fat levels. In 2004, the results of the study were reported in JAMA, the authoritative journal of the American Medical Association. Later research, including a Chinese study of 191 women who took either soy or a placebo for six months, also failed to demonstrate any improvement in memory and other cognitive functions. Recently, the absence of any effect from phytoestrogens was confirmed once again, in a study conducted at the University of Miami in which nearly 250 women took part. One group took pills on a daily basis containing twice the amount of soy normally consumed by people who eat a soy-rich diet. The other group took a placebo. The researchers found no improvement in bone density, quality of sleep, or the frequency of hot flashes. Unfortunately, they did not measure cognitive function.
Thus, phytoestrogens have not lived up to their early promise. I would add, though, that the most recent studies also question the effectiveness of regular oestrogen therapy. This, too, does not seem to bring about a long-term improvement in mental capacities, perhaps because hormone levels are being raised artificially. The oestrogens produced by the body before menopause are released into the bloodstream in a different manner, spread more evenly over the day, but with peaks and troughs. It is also possible that the number and function of oestrogen receptors declines after menopause as a result of diminished oestrogen production, and that this is the reason oestrogen therapy has less effect.
TESTOSTERONE AND COGNITIVE FUNCTIONS
Testosterone quite often gets a bad press. Almost everyone knows that it is a male hormone, abused by some athletes, particularly bodybuilders, and associated with aggression and annoying behaviour. If teenage boys are loud and insolent or get into fights, we put it down to excess testosterone. The same goes for macho behaviour.

FIGURE 17. Test of spatial insight: is the figure on the right the same as the one on the left? Answers: A, Yes; B, Yes; C, No
It’s true that testosterone is the most important male sex hormone. It builds muscle and may in some cases have an influence on whether someone actually makes use of those muscles. But that’s not the whole story. Testosterone is also important for skin and bones, a healthy sense of competition, the male sex drive, and the production of sperm cells. It’s a very ‘physical’ hormone. Like oestrogen, it illustrates the close relationship between mind and body. Testosterone influences mental capacities such as memory and concentration. If you measure the testosterone in the blood of 100 men and then ask them to take a number of neuropsychological tests, you’ll see a correlation between the amount of testosterone and performance: men with more testosterone score better, mostly where spatial insight is concerned. One way of testing spatial insight is to ask people to decide whether two abstract figures drawn from different perspectives are the same (figure 17).
To answer the question, people rotate the figure in their minds so they can see if it is the same as the figure in the second drawing. On average, men are better at this than women, while men with a lot of testosterone score better than men with less.
Just as men produce little oestrogen, women produce little testosterone. To test the influence of testosterone on cognitive functions, a single testosterone pill was given to young women in a number of studies to see if their performance changed. If women were to take such a pill every day for a long period of time, they would develop male characteristics such as increased facial hair and deepening of the voice. But a single pill is harmless. In a study we conducted with psychologist Dr Jack van Honk, a specialist in the influence of hormones on human behaviour, we examined the effect of a single testosterone pill on spatial insight. This improved in the female participants after they ingested the pill, compared with those who took a placebo. This shows that testosterone can have a powerful influence on cognitive functions, something that other studies have also demonstrated. In the laboratory, we used to joke that it might be handy for our wives and girlfriends to take the testosterone pill before reading maps in the car while on holiday.
From about the age 50, testosterone production in men starts to decline: andropause begins. Although hormone levels diminish less radically than they do in women, this can still have serious effects, ranging from a loss of muscle mass and strength, and reduced sex drive (erection problems, for instance), to sleeplessness, fatigue, and depression. But is this accompanied by a decline in cognitive functions? Dr Majon Muller researched this question at the University Medical Centre Utrecht in a study involving 400 men between the ages of 40 and 80 (100 subjects for each decade). I worked on the neuropsychological part of the study. A link was established between testosterone levels in the blood (without any testosterone being administered), and memory performance and speed of information processing.

FIGURE 18. Inverted U-shaped curve reflecting the relationship between testosterone levels and cognitive functions in men aged between 40 and 70. Men with very low and very high levels perform less well in neuropsychological testing than men who have intermediate levels. X-axis: testosterone levels; y-axis: scores in neuropsychological testing.
What was remarkable about this result was the fact that the relationship was curvilinear: it had the shape of an inverted U (see figure 18). A curvilinear relationship indicates that there is an optimal level (in the middle of the inverted U). A linear relation (that is, the higher the better) was only found in the oldest group, the men between 70 and 80.
For men in this age group, whose testosterone levels are generally low, the rule of thumb is that the more testosterone they have, the better their memory and speed of processing. This raises the question of whether giving them testosterone would lead to improved cognitive functions, a hypothesis that was also investigated by researchers at the same institute, with whom I worked.[2] Again, we used a range of neuropsychological tests to map memory performance, processing speed, and cognitive flexibility. Because the study centred on testosterone, we added an extra test of spatial insight using the same objects as in figure 17. The participants were a group of 236 men between the ages of 60 and 80 with low testosterone levels. This was a requirement for participation, since it provided a biological reason for supplementing the hormone. For six months, the participants took a twice-daily pill containing testosterone or a placebo. There was no greater improvement in cognitive skills in the group that took the testosterone compared with the group that took the placebo. The men in the testosterone group displayed a decrease in fatty tissue but no increase in muscle strength.
So it seems that increasing low testosterone levels in older men does not automatically result in improved memory or concentration. We found no improvement even in spatial insight, which is more closely linked to testosterone levels in younger people than memory and concentration are. It is of course possible that six months is too short a period for changes in cognitive functions to occur, though many substances and hormones produce changes within a few weeks. Ingesting pills results in temporary increases in hormone levels that persist for several hours afterwards. This is different from natural hormone levels, which may have an impact on the pills’ effectiveness, or even result in a lack of effect. Testosterone plasters and gels that can produce a more stable increase in hormone levels have recently come onto the market. Though more research is needed, there are currently no indications that these have a positive influence on cognitive function.
Another question that deserves further study is whether testosterone might lead to improved cognitive skills in people who score low on neuropsychological tests. In our study, the participants scored just above average for their age while their testosterone levels were lower than average. This meant two things: first, that there was plenty of room for improvement; but second, that they had no greater memory and concentration problems than their peers. It is possible that people who do have these problems would benefit from hormone treatment. The aim of this type of treatment is to improve a clearly reduced cognitive ability, such as poor memory.
Commercial organisations go further and play on fears of growing older and a general decline in bodily functions. In line with the worship of youth in America (and increasingly in Europe), the American age-management physician Dr Jeffry Life advocates the use of hormones to ensure a well-muscled body in your seventies. He is himself the living proof of his theories. Though born in 1938, he boasts a tanned body, heavily muscled arms, a broad chest, and a six-pack. People who see the before-and-after photos he uses to advertise his business often think that the images have been photoshopped — that the head of a 70-year-old has been placed on the body of a 30-year-old Olympic athlete. But Dr Life is real; even his name is real. He transformed his body through working out at the gym, a special diet, and treatments with testosterone and growth hormone. You may wonder, of course, if it is a good thing to fight off the natural ageing process in this way. In any event, the usefulness of hormone therapy has not been scientifically proven, especially when it comes to brain function. And although it can help you develop a more highly muscled body, it can also have unwanted side effects. The use of testosterone may activate latent prostate cancer. And the risk of side effects is even greater when it comes to growth hormone, perhaps the most important hormone that declines as we grow older and a major contributor to the symptoms of ageing.
A MYSTERIOUS HORMONE
The 17th-century French philosopher René Descartes is famous for his statement ‘Cogito ergo sum’ (I think, therefore I am) and for his take on the concept of dualism, which holds that body and mind are two separate things. He argued that body and mind interact through the pineal gland, a small organ located near the centre of the brain between the two hemispheres. If Descartes were alive today, he would probably have regarded growth hormone as the link between mind and body. This is the most ‘physical’ hormone we produce, but it nevertheless has an enormous influence on our minds. Faced with our current knowledge of this hormone, Descartes might even have come to doubt the theory of dualism.
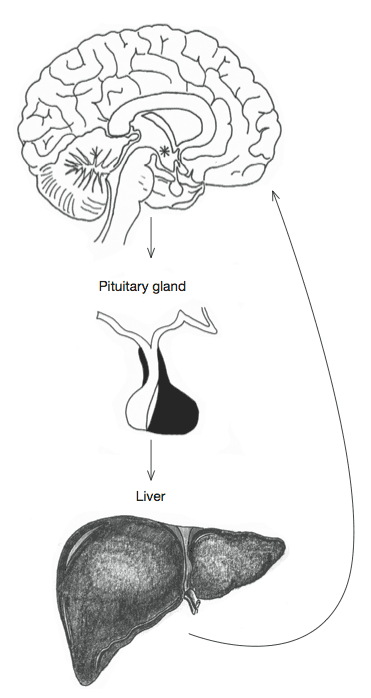
FIGURE 19. The hypothalamus stimulates the release of growth hormone by the pituitary gland, which in turn signals to the liver to start producing insulin-like growth factor (IGF), which has a beneficial effect on the brain.
As its name suggests, growth hormone is what makes the body grow from infancy into adulthood. People with too little remain small; people with too much can grow to well over six feet tall. But it continues to play a major role throughout our lives. It encourages the formation of fat-free tissue in the body, which is healthy. It helps to renew skin and hair, and to repair damaged tissue. Less well known is the fact that this natural hormone has a positive influence on brain function. People with high levels of growth hormone feel better than those with low levels: they literally and figuratively feel more comfortable in their own skin. We have only known for about 20 years that growth-hormone levels correlate with scores in memory, concentration, and thinking skills. Before we look at that more closely, let’s look at how growth hormone actually works.
As in the case of sex hormones, the signal to start producing growth hormone is sent by the hypothalamus to the pituitary gland on the underside of the brain. Cells in the pituitary produce and release growth hormone in a series of irregular pulsations into the bloodstream. Release peaks about an hour after the onset of sleep, which is one of the reasons why this stage of sleep, characterised by deep sleep, is so important. The levels of growth hormone in the body decline as we age, halving every ten years after adulthood. Its effect on organs and tissues is mediated by an intriguing hormone related to insulin and therefore called ‘insulin-like growth factor’ (IGF), the last piece in the domino effect initiated by the hypothalamus (see figure 19). It is difficult to reliably measure growth-hormone levels in the blood because the pituitary releases it in the irregular pulsations described above. Levels of IGF, on the other hand, are much more stable. And because IGF is the chief slave of growth hormone, obeying its orders to bind with cells in tissue, many studies simply measure IGF as an indication of the activity of growth hormone. The remarkable thing about IGF is that it works throughout the body, including the brain. This is unusual because most substances elsewhere in the body cannot cross what is called the blood–brain barrier. But IGF is involved in the growth of brain cells and the dendrites formed when we learn new information. It is also essential to the repair of damaged brain tissue.
I first heard of IGF in 1997 when I was doing my final project for my psychology degree. I was supervised by Dr Hans Koppeschaar, a specialist in internal medicine at the University Medical Centre Utrecht. His fascination with the possible influence of IGF on the brain was infectious, and I, too, became interested in this mysterious hormone. Both IGF and cognitive function decline with ageing, and our question was whether there was a connection between the two.[3] There were two reasons for investigating this only in men: first, because there may be differences between men and women in the way growth hormone works; and second, because we wanted to see if our findings were in line with the single previous study of IGF and cognitive function, an American study using older men.
The men in our study were between 65 and 76 years of age. The results showed a relationship between IGF levels and performance on two tests: one measuring processing speed; the other, cognitive flexibility. The more IGF a participant had in his blood, the better he scored on the tests. The earlier American study had also found a link between IGF and cognitive flexibility. Later research, using much larger groups, has confirmed that higher IGF values in healthy older people are associated with better cognitive performance. Researchers in Amsterdam, for example, found that people with the lowest IGF values in a group of over 1,300 participants (aged between 65 and 88) had the lowest scores on speed-of-processing tests. This was true of both men and women. The low IGF values also proved to be linked to reduced processing speed three years later.
The fact that growth hormone and IGF affect cognitive abilities did not come as a complete surprise. It had already been discovered that people with extremely low levels of growth hormone perform less well on neuropsychological tests, particularly those measuring memory and concentration. And it has recently been established that people who produce extremely high levels of growth hormone (a disease known as acromegaly) suffer from memory problems. As is the case with testosterone, both too little and too much of this hormone appears to have a negative effect on the brain.
It might seem logical to administer extra hormones to people with low levels to increase those levels, but there are risks involved. Extra growth hormone encourages the growth of any cancer cells that might be present but are as yet undetected. And that risk is higher for older people. That is why little research has been done in this area. In fact, only three studies have been published: one in which growth hormone was given to older people with low hormone levels, another where IGF was given to a group of older women, and a third in which the substance released by the pituitary gland to stimulate growth hormone secretion (known as growth-hormone-releasing factor or GHRF) was administered. The first two studies showed no improvement in cognitive function, though the study using GHRF did. Growth hormone works by setting off a chain reaction in the body, and intervening as early as possible in the chain (GHRF comes in at the start of the chain reaction) might achieve the best results.
Caution with regard to administering growth hormone to older people is justified. In my view, further research should focus on people with memory problems or mild cognitive decline because their needs are more compelling: to halt the progress of Alzheimer’s at an early stage.
IMPORTANT INSIGHTS