1
“Why turn CO2 into stone?” the email asked. Because humanity had already emitted so much carbon “that we have to physically remove it from the atmosphere to keep global warming at safe levels.” I immediately signed on, becoming a so-called “pioneer.” Every month, the company sent me another email—“your subscription will renew soon and you will continue to turn CO2 emissions into stone”—before billing my credit card. After a year of this, I decided it was time to visit my emissions, an admittedly reckless move that swelled my emissions still further.
Though Climeworks is based in Switzerland, its air-into-rock operation is situated in southern Iceland. Once I got to Reykjavík, I rented a car and drove east along Route 1, the ring road that circles the country. After about ten minutes, I was clear of the city. After about twenty, I was beyond the suburbs, racing across an ancient lava field.
Iceland is essentially all lava field. It sits atop the Mid-Atlantic Ridge, and, as the Atlantic Ocean widens, it’s being pulled in opposite directions. Running diagonally across the country is a seam lined with active volcanoes. I was headed toward a spot near the seam—a three-hundred-megawatt geothermal plant known as the Hellisheiði Power Station. The landscape looked as if it had been paved by giants and then abandoned. There were no trees or bushes, just clumps of grass and moss. Squarish black boulders lay jumbled in heaps.
When I arrived at the gate of the plant, the whole place seemed to be steaming and the air stank of sulfur. Soon a cute little car drove up, painted in bright orange. Out of it climbed Edda Aradóttir, a managing director at Reykjavík Energy, which owns the power station. Aradóttir is blond and bespectacled, with a round face and long hair that she was wearing pinned back. She handed me a hard hat and put one on herself.
As power stations go, geothermal plants are “clean.” Instead of burning fossil fuels, they rely on steam or superheated water pumped from underground, which is why they tend to be sited in volcanically active areas. Still, as Aradóttir explained to me, they, too, produce emissions. With the superheated water inevitably come unwanted gases, like hydrogen sulfide (responsible for the stink) and carbon dioxide. Indeed, pre-Anthropocene, volcanoes were the atmosphere’s chief source of CO2.
About a decade ago, Reykjavík Energy came up with a plan to make its clean energy even cleaner. Instead of allowing the carbon dioxide to escape into the air, the Hellisheiði plant would capture the gas and dissolve it in water. Then the mixture—basically, high-pressure club soda—would be injected back underground. Calculations done by Aradóttir and others suggested that deep beneath the surface, the CO2 would react with the volcanic rock and mineralize.
“We know that rocks, they store CO2,” she told me. “They’re actually one of the biggest reservoirs of carbon on earth. The idea is to imitate and accelerate this process to fight global climate change.”
Aradóttir opened the gate, and we drove in the little orange car to the back of the power station. It was a breezy day in late spring, and the steam rising from the pipes and cooling towers seemed unable to make up its mind which way to blow. We paused at a large metal-clad outbuilding attached to a structure resembling a rocket launcher. A sign on the building said: Steinrunnið Gróðurhúsaloft, which was translated as “greenhouse gas petrified.” Aradóttir told me that the rocket launcher was where the power station’s CO2 was separated from other geothermal gases and prepared for injection. We drove on a bit farther and came to what looked like an outsized air conditioner stuck onto a shipping container. A sign on the container said: Úr Lausu Lofti, or “out of thin air.”
This, Aradóttir said, was the Climeworks machine that was scrubbing my emissions—really, just a fraction of my emissions—from the atmosphere. The machine, formally known as a direct air capture unit, suddenly started to hum. “Oh, the cycle just started,” she said. “Lucky us!
“At the beginning of the cycle, the equipment sucks in air,” she went on. “The CO2 sticks to specific chemicals inside the capture unit. We heat up the chemicals and that releases the CO2.” This CO2—the Climeworks CO2—is then added to the club-soda mixture from the power plant as it makes its way to the injection site.
Even without any help, most of the carbon dioxide humans have emitted would eventually turn to stone, via a natural process known as chemical weathering. But “eventually” here means hundreds of thousands of years, and who has time to wait for nature? At Hellisheiði, Aradóttir and her colleagues were speeding up the chemical reactions by several orders of magnitude. A process that would ordinarily take millennia to unfold was being compressed into a matter of months.
Arádottir had brought along a rock core to show me the end result. The core, which was roughly two feet long and a couple of inches in diameter, was the dark color of the lava fields. But the black rock—basalt—was pocked with little holes, and these holes were filled with a chalky white compound—calcium carbonate. The white deposits represented, if not my own emissions, then at least somebody’s.

When, exactly, people began altering the atmosphere is a matter of debate. According to one theory, the process got under way eight or nine thousand years ago, before the dawn of recorded history, when wheat was domesticated in the Middle East and rice in Asia. Early farmers set to clearing land for agriculture, and as they chopped and burned their way through the forests, carbon dioxide was released. The quantities involved were comparatively small, but, according to advocates of this theory, known as the “early Anthropocene hypothesis,” the effect was fortuitous. Owing to natural cycles, CO2 levels should have been falling during this period. Human intervention kept them more or less constant.
“The start of the switchover from control of climate by nature to control by humans occurred several thousand years ago,” William Ruddiman, a professor emeritus at the University of Virginia and the most prominent proponent of an “early Anthropocene,” has written.
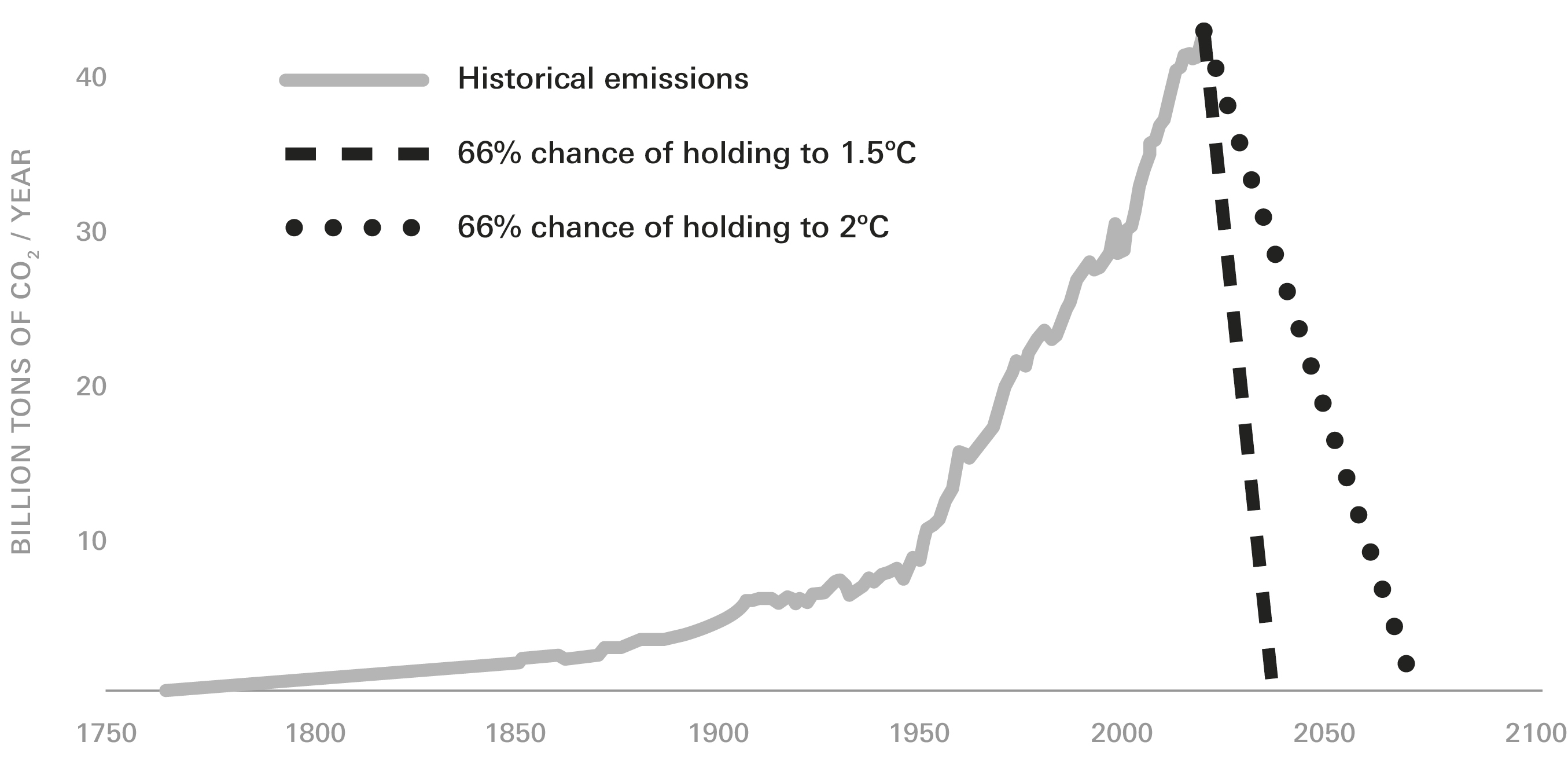
According to a second, more widely held view, the switchover only really started in the late-eighteenth century, after the Scottish engineer James Watt designed a new kind of steam engine. Watt’s engine, it’s often said, anachronistically, “kick-started” the Industrial Revolution. As water power gave way to steam power, CO2 emissions began to rise, at first slowly, then vertiginously. In 1776, the first year Watt marketed his invention, humans emitted some fifteen million tons of CO2. By 1800, that figure had risen to thirty million tons. By 1850 it had increased to two hundred million tons a year and by 1900 to almost two billion. Now, the figure is close to forty billion tons annually. So much have we altered the atmosphere that one out of every three molecules of CO2 loose in the air today was put there by people.
Thanks to this intervention, average global temperatures have, since Watt’s day, risen by 1.1° Celsius (2° Fahrenheit). This has led to a variety of increasingly unhappy consequences. Droughts are growing deeper, storms fiercer, heat waves deadlier. Wildfire season is getting longer and the fires more intense. The rate of sea-level rise is accelerating. A recent study in the journal Nature reported that, since the 1990s, melt off of Antarctica has increased threefold. Another recent study predicted that most atolls will, in another few decades, become uninhabitable; this includes entire nations, like the Maldives and the Marshall Islands. To paraphrase J. R. McNeill paraphrasing Marx, “Men make their own climate, but they do not make it just as they please.”
No one can say exactly how hot the world can get before out-and-out disaster—the inundation of a populous country like Bangladesh, say, or the collapse of crucial ecosystems like coral reefs—becomes inevitable. Officially, the threshold of catastrophe is an average global temperature rise of 2°C (3.6°F). Virtually every nation signed on to this figure at a round of climate negotiations held in Cancún in 2010.
Meeting in Paris in 2015, world leaders had second thoughts. The two-degree threshold, they decided, was too high. The signatories of the Paris Agreement committed themselves to “holding the increase in the global average temperature to well below 2°C…and pursuing efforts to limit the temperature increase to 1.5°C.”
In either case, the math is punishing. To stay under 2°C, global emissions would have to fall nearly to zero within the next several decades. To stave off 1.5°C, they’d have to drop most of the way toward zero within a single decade. This would entail, for starters: revamping agricultural systems, transforming manufacturing, scrapping gasoline- and diesel-powered vehicles, and replacing most of the world’s power plants.
Carbon dioxide removal offers a way to change the math. Extract large amounts of CO2 from the atmosphere and “negative emissions” could, conceivably, balance out the positive variety. It might even be feasible to cross the threshold of catastrophe and then suck enough carbon out of the air to keep calamity at bay, a situation that’s become known as “overshoot.”
If anyone can be said to have invented “negative emissions,” it’s a German-born physicist named Klaus Lackner. Lackner, who’s now in his late sixties, is a trim man with dark eyes and a prominent forehead. He works at Arizona State University, in Tempe, and I met up with him one day at his office there. The office was almost entirely bare, except for a few New Yorker cartoons on the theme of nerd-dom, which, Lackner told me, his wife had cut out for him. In one of the cartoons, a couple of scientists stand in front of an enormous whiteboard covered in equations. “The math is right,” the first scientist says. “It’s just in poor taste.”
Lackner has lived in the United States for most of his adult life. In the late 1970s, he moved to Pasadena to study with George Zweig, one of the discoverers of quarks, and a few years later, he moved to the Los Alamos National Laboratory, to do research on fusion. “Some of the work was classified,” he told me, “some of it not.”
Fusion is the process that powers the stars and, closer to home, thermonuclear bombs. When Lackner was at Los Alamos, it was being touted as the energy source of the future. A fusion reactor could generate essentially limitless quantities of carbon-free power from isotopes of hydrogen. Lackner became convinced that a fusion reactor was, at a minimum, decades away. Decades later, it’s generally agreed that a workable reactor is still decades away.
“I realized, probably earlier than most, that the claims of the demise of fossil fuels were greatly exaggerated,” Lackner told me.
One evening in the early 1990s, Lackner was having a beer with a friend, Christopher Wendt, who’s also a physicist. The two got to wondering why, as Lackner put it to me, “nobody’s doing these really crazy, big things anymore.” This led to more questions and more conversations (and possibly also more beers).
The two came up with their own “crazy, big” idea, which, they decided, wasn’t really so crazy. A few years after the original conversation, they produced an equation-dense paper in which they argued that self-replicating machines could satisfy the world’s energy needs, and, more or less at the same time, clean up the mess humans had created by burning fossil fuels. They called the machines “auxons,” from the Greek αυξάνω, meaning “grow.” The auxons would be powered by solar panels and, as they multiplied, they’d produce more solar panels, which they’d assemble using elements, like silicon and aluminum, extracted from ordinary dirt. The expanding collection of panels would produce ever more power, at a rate that would increase exponentially. An array covering three hundred eighty-six thousand square miles, an area as large as Nigeria but, as Lackner and Wendt noted, “smaller than many deserts,” could meet all the globe’s electricity demands many times over.
This same array could also be put to use scrubbing carbon. A Nigeria-sized solar farm would, they calculated, be sufficient to remove all the carbon dioxide emitted by humans up to that point. Ideally, the CO2 would be converted to rock, much the same way my emissions had been converted in Iceland. Only instead of little pockets of calcium carbonate, there’d be whole countries’ worth of it—enough to cover Venezuela in a layer a foot and a half deep. (Where this rock would go, the two did not specify.)
More years went by. Lackner let the auxon idea slide. But he found himself more and more interested in negative emissions.
“Sometimes by thinking through this extreme endpoint you learn a lot,” he told me. He began giving talks and writing papers on the subject. Humanity, he said, was going to have to find a way to pull carbon out of the air. Some of his fellow scientists decided he was nuts, others that he was a visionary. “Klaus is, in fact, a genius,” Julio Friedmann, a former deputy energy secretary who now works at Columbia University, told me.
In the mid-2000s, Lackner pitched a plan for developing a carbon-sucking technology to Gary Comer, a founder of Lands’ End. Comer brought to the meeting his investment adviser, who quipped that what Lackner was looking for wasn’t so much venture capital as “adventure capital.” Nevertheless, Comer put up $5 million. The company got as far as building a small prototype, but just as it was looking for new investors, the financial crisis of 2008 hit.
“Our timing was exquisite,” Lackner told me. Unable to raise more funds, the company folded. Meanwhile, fossil-fuel consumption continued to rise, and along with it, CO2 levels. Lackner came to believe that, unwittingly, humanity had already committed itself to carbon dioxide removal.
“I think that we’re in a very uncomfortable situation,” he told me. “I would argue that if technologies to pull CO2 out of the environment fail, then we’re in deep trouble.”
Lackner founded the Center for Negative Carbon Emissions at ASU in 2014. Most of the equipment he dreams up is put together in a workshop a few blocks from his office. After we had chatted for a while, we walked over there.
In the workshop, an engineer was tinkering with what looked like the guts of a foldout couch. Where, in the living-room version, there would have been a mattress, in this one was an elaborate array of plastic ribbons. Embedded in each ribbon was a powder made from thousands upon thousands of tiny amber-colored beads. The beads, Lackner explained, were composed of a resin normally used in water treatment and could be purchased by the truckload. When dry, the powder made from the beads would absorb carbon dioxide. When wet, it would release it. The idea behind the couch-like arrangement was to expose the ribbons to Arizona’s thirsty air, then fold the device into a sealed container filled with water. The CO2 that had been captured in the dry phase would be released in the wet phase; it could then be piped out of the container and the whole process restarted, the couch folding and unfolding over and over again.
Lackner told me he’d calculated that an apparatus the size of a semi-trailer could remove a ton of carbon dioxide per day, or three hundred and sixty-five tons a year. Since global emissions are now running around forty billion tons a year, he observed, “if you built a hundred million trailer-sized units,” you could more or less keep up. He acknowledged the hundred-million figure sounded daunting. But, he noted, the iPhone has only been around since 2007, and there are now almost a billion in use. “We are still very early in this game,” he said.
The way Lackner sees things, the key to avoiding “deep trouble” is thinking differently. “We need to change the paradigm,” he told me. Carbon dioxide, in his view, should be regarded much the same way we look at sewage. We don’t expect people to stop producing waste. “Rewarding people for going to the bathroom less would be nonsensical,” Lackner has observed. At the same time, we don’t let them shit on the sidewalk. One of the reasons we’ve had such trouble addressing the carbon problem, he contends, is the issue has acquired an ethical charge. To the extent that emissions are seen as bad, emitters become guilty.
“Such a moral stance makes virtually everyone a sinner and makes hypocrites out of many who are concerned about climate change but still partake in the benefits of modernity,” he has written. Shifting the paradigm, he thinks, will shift the conversation. Yes, people have fundamentally altered the atmosphere. And, yes, this is likely to lead to all sorts of dreadful consequences. But people are ingenious. They come up with crazy, big ideas, and sometimes these actually work.
During the first few months of 2020, a vast, unsupervised experiment took place. As the coronavirus raged, billions of people were ordered to stay home. At the peak of the lockdown, in April, global CO2 emissions were down an estimated seventeen percent compared with the comparable period the previous year.
This drop—the largest recorded ever—was immediately followed by a new high. In May 2020, carbon dioxide levels in the atmosphere set a record of 417.1 parts per million.
Declining emissions and rising atmospheric concentrations point to a stubborn fact about carbon dioxide: once it’s in the air, it stays there. How long, exactly, is a complicated question; for all intents and purposes, though, CO2 emissions are cumulative. The comparison that’s often made is to a bathtub. So long as the tap is running, a stoppered tub will continue to fill. Turn the tap down, and the tub will still keep filling, just more slowly.
To extend the analogy, it could be said that the 2°C tub is approaching capacity and that the tub for 1.5°C is all-but-overflowing. This is why the carbon math is so difficult. Cutting emissions is at once absolutely essential and insufficient. Were we to halve emissions—a step that would entail rebuilding much of the world’s infrastructure—CO2 levels wouldn’t drop; they’d simply rise less quickly.
Then there’s the issue of equity. Since carbon emissions are cumulative, those most to blame for climate change are those who’ve emitted the most over time. With just four percent of the world’s population, the United States is responsible for almost thirty percent of aggregate emissions. The countries of the European Union, with about seven percent of the globe’s population, have produced about twenty-two percent of aggregate emissions. For China, home to roughly eighteen percent of the globe’s population, the figure is thirteen percent. India, which is expected soon to overtake China as the world’s most populous nation, is responsible for about three percent. All the nations of Africa and all the nations of South America put together are responsible for less than six percent.
To get to zero, everyone would have to stop emitting—not only Americans and Europeans and Chinese, but also Indians and Africans and South Americans. But asking countries that have contributed almost nothing to the problem to swear off carbon because other countries have already produced way, way too much of it is grossly unfair. It’s also geopolitically untenable. For this reason, international climate agreements have always been based on the premise of “common but differentiated responsibilities.” Under the Paris accord, developed countries are supposed to “lead by undertaking economy-wide absolute emission reduction targets,” while developing countries are called on, more hazily, to enhance their “mitigation efforts.”
All of which makes negative emissions—as an idea at least—irresistible. The extent to which humanity is already counting on them is illustrated by the latest report of the Intergovernmental Panel on Climate Change, which was published in the run-up to Paris. To peer into the future, the IPCC relies on computer models that represent the world’s economic and energy systems as a tangle of equations. The output of these models is then translated into figures that climate scientists can use to forecast how much temperatures are going to rise. For its report, the IPCC considered more than a thousand scenarios. The majority of these led to temperature increases beyond the official 2°C disaster threshold, and some led to warming of more than 5°C (9° Fahrenheit). Just a hundred and sixteen scenarios were consistent with holding warming under 2°C, and of these, a hundred and one involved negative emissions. Following Paris, the IPCC produced another report, based on the 1.5°C threshold. All of the scenarios consistent with that goal relied on negative emissions.
“I think what the IPCC really is saying is, ‘We tried lots and lots of scenarios,’ ” Klaus Lackner told me. “ ‘And, of the scenarios which stayed safe, virtually every one needed some magic touch of negative emissions. If we didn’t do that, we ran into a brick wall.’ ”
Climeworks, the company I paid to bury my emissions in Iceland, was founded by two college friends, Christoph Gebald and Jan Wurzbacher. “We met on the first day of starting university,” Wurzbacher recalled. “I think we asked each other in the first week, ‘Hey, what do you want to do?’ And I said, ‘Well, I want to found my own company.’ ” The pair ended up splitting a single graduate school stipend; each worked half-time on his PhD and half-time on getting their company off the ground.

Like Lackner, the two initially faced a lot of skepticism. What the duo was trying to do, they were told, was a distraction. If people thought there was some way to draw carbon dioxide out of the atmosphere, they’d just emit even more of it. “People were fighting us, saying, ‘Well, guys, you shouldn’t be doing that,’ ” Wurzbacher told me. “But we were always stubborn.”
Wurzbacher, who’s now in his mid-thirties, is reed-thin, with a boyish mop of dark hair. I met with him at Climeworks’ headquarters in Zurich, which houses both the company’s offices and its metalworking shop. The place had some of the vibe of a tech start-up and some of the vibe of a bike store.
“Taking CO2 out of a gas stream, that’s not rocket science,” Wurzbacher told me. “And it’s also not new. People have filtered CO2 out of gas streams for the last fifty years, just for other applications.” On submarines, for instance, the carbon dioxide the crew breathes out has to be sucked from the air; otherwise, it will build up to dangerous levels.
But it’s one thing to be able to pull carbon out of the air and quite another to be able to pull this off at scale. Burning fossil fuels generates energy. Capturing CO2 from the air requires energy. So long as this energy comes from burning fossil fuels, it will add to the carbon that has to be captured.
A second major challenge is disposal. Once captured, CO2 has to go somewhere, and where it goes has to be secure. “The good thing about basaltic rock is it’s so easy to explain,” Wurzbacher observed. “If someone asks you, ‘Hey, but is it really safe?’ the answer is very simple: within two years it’s stone, one kilometer underground. Period.” suitable underground storage sites aren’t rare, but they aren’t common, either, meaning that, should large-scale capture plants ever be built, they’ll either have to be located in places with the right geology or the CO2 will have to be shipped long distances.
Finally, there’s the issue of cost. Pulling CO2 from the air takes money. Right now, a lot of money. Climeworks charges $1,000 a ton to turn subscribers’ emissions to stone. I used up my allotment of twelve hundred pounds to fly one-way to Reykjavík, leaving all the rest of my emissions, including those from my return trip and my flight to Switzerland, floating loose. Wurzbacher assured me that, as more capture units went up, the price would come down; within a decade or so, he predicted, it would fall to around $100 per ton. Were emissions taxed at a comparable rate, then the math could work out: Basically, a ton extracted would be a ton that could avoid the tax. But who’s going to spend that when carbon can still be dumped in the air for free? Even at $100 a ton, burying a billion tons of CO2—a small percentage of the world’s annual output—would run to $100 billion.*
“Maybe we are too early,” Wurzbacher mused, when I asked whether the world was prepared to pay for direct air capture. “Maybe we’re just right. Maybe we’re too late. No one knows.”
Just as there are lots of ways to add CO2 to the air, there are lots of ways—potentially—to remove it.
A technique known as “enhanced weathering” is a sort of upside-down version of the project I toured at the Hellisheiði Power Station. Instead of injecting CO2 deep into rock, the idea is to bring the rock up to the surface to meet the CO2. Basalt could be mined, crushed, and then spread over croplands in hot, humid parts of the world. The crushed stone would react with carbon dioxide, drawing it out of the air. Alternatively, it’s been proposed that olivine, a greenish mineral that’s common in volcanic rock, could be ground up and dissolved in the oceans. This would induce the seas to absorb more CO2 and, as an added benefit, combat ocean acidification.
Another family of negative-emissions technologies, or NETs, takes its cue from biology. Plants absorb carbon dioxide while they’re growing; then, when they rot, they return that CO2 to the air. Grow a new forest and it will draw down carbon until it reaches maturity. A recent study by Swiss researchers estimated that planting a trillion trees could remove two hundred billion tons of carbon from the atmosphere over the next several decades. Other researchers argued that this figure overstated the case by a factor of ten or even more. Nevertheless, they observed, the capacity of new forests to sequester carbon was “still substantial.”
To deal with the rot problem, all sorts of preservation techniques have been proposed. One entails cutting down mature trees and burying them in trenches; in the absence of oxygen, the trees’ decay—and the release of CO2—would be forestalled. Another scheme involves collecting crop residues, like cornstalks, and dumping them deep into the ocean; in the dark, cold depths, the waste material would decay very gradually or perhaps not at all. As strange as these ideas may sound, they, too, take their inspiration from nature. In the Carboniferous period, vast quantities of plant material got flooded and buried. The eventual result was coal, which, had it been left in the ground, would have held on to its carbon more or less forever.
Reforestation, when combined with underground injection, yields a technique that’s become known as BECCS (pronounced “becks”), short for “bioenergy with carbon capture and storage.” The models employed by the IPCC are extremely partial to BECCS, which offers negative emissions and electrical power at the same time—a have-your-cake-and-eat-it-too arrangement that, in climate-math terms, is tough to beat.
With BECCS the idea is to plant trees (or some other crop) that can pull carbon from the air. The trees are then burned to produce electricity and the resulting CO2 is captured from the smokestack and shoved underground. (The world’s first BECCS pilot project launched in 2019, at a power plant in northern England that runs off wood pellets.)
With all of these alternatives, the challenge is much the same as with direct air capture: scale. Ning Zeng is a professor at the University of Maryland and the author of the “wood harvest and storage” concept. He has calculated that to sequester five billion tons of carbon per year, ten million tree-burial trenches, each the size of an Olympic swimming pool, would be required. “Assuming it takes a crew of ten people (with machinery) one week to dig a trench,” he has written, “two hundred thousand crews (two million workers) and sets of machinery would be needed.”
According to a recent study by a team of German scientists, to remove a billion tons of CO2 through “enhanced weathering,” approximately three billion tons of basalt would have to be mined, crushed, and transported. “While this is a very large amount” of rock to mine, grind, and ship, the authors noted, it is less than global coal production, which totals some eight billion tons per year.
For the trillion-tree project, something on the order of 3.5 million square miles of new forest would be needed. That’s an expanse of woods roughly the size of the United States, including Alaska. Take that much arable land out of production and millions could be pushed toward starvation. As  O. Táíwò, a professor at Georgetown, put it recently, there’s a danger of moving “two steps backward in justice for every gigaton step forward.” But it’s not clear that using uncultivated land would be any safer. Trees are dark, so if, say, tundra were converted to forest, it would increase the amount of energy being absorbed by the earth, thus contributing to global warming and defeating the purpose. One way around this problem might be to genetically engineer lighter-colored trees, using CRISPR. So far as I know, no one has yet proposed this, but it seems only a matter of time.
O. Táíwò, a professor at Georgetown, put it recently, there’s a danger of moving “two steps backward in justice for every gigaton step forward.” But it’s not clear that using uncultivated land would be any safer. Trees are dark, so if, say, tundra were converted to forest, it would increase the amount of energy being absorbed by the earth, thus contributing to global warming and defeating the purpose. One way around this problem might be to genetically engineer lighter-colored trees, using CRISPR. So far as I know, no one has yet proposed this, but it seems only a matter of time.
A couple of years before Climeworks launched its “pioneer” program in Iceland, the company opened its first direct-air-capture operation, atop a garbage incinerator in Switzerland. “Climeworks makes history,” the company declared.
One afternoon while I was in Zurich, I went to visit the “history-making” operation with Climeworks’ communications manager, Louise Charles. We took a train and then a bus out to the town of Hinwil, about twenty miles southeast of the city. As we walked up the access road to the incinerator, a huge box of a building with a candy-striped smokestack, a truck rolled by filled with rubbish. In the entrance hall, we paused to admire a series of artworks, also made of rubbish. Several men were seated before video monitors that displayed more rubbish. We signed the visitors’ log and took a service elevator up to the top floor.
On the roof of the incinerator were eighteen capture units just like the one at the Hellisheiði plant. These were arranged in three rows, which were stacked one above the other, like children’s blocks. A metal placard, aimed at visiting school groups, explained the Climeworks operation in pictures. It showed a garbage truck pulling up to the incinerator, which was depicted with little flames inside. One pipe, labeled Waste Heat, led from the flames to the stack of capture units. (Using waste heat from the incinerator allows Climeworks to sidestep the it-takes-emissions-to-catch-emissions trap.) A second pipe, labeled Concentrated CO2, led from the units to a greenhouse filled with floating vegetables.

From the roof, I could see in the distance the actual greenhouses where the CO2 was headed. Charles had arranged for us to tour those as well, but she’d recently had knee surgery and was hobbling painfully, so I walked over alone. I was met at the entrance by the manager of the complex, Paul Ruser. Without Charles to translate, we had to make do with a hodgepodge of English and German.
Ruser told me—or at least I think he told me—that the greenhouses covered an area of eleven acres: an entire farm, under glass. Outside, it was sweater weather; inside, it was summertime. Bumblebees, which had been imported in boxes, buzzed around groggily. Twelve-foot-tall cucumber vines rose out of small bricks of potting soil. The cucumbers—a miniature variety the Swiss call Snack-Gurken—had just been picked and were piled high in bins. Ruser pointed out a black plastic tube running along the floor. This, he explained, was carrying CO2 from the Climeworks units.
“All plants need CO2,” Ruser observed. “And if you supply more to them, they become stronger.” Eggplants in particular, he said, thrive on lots of carbon dioxide; for their sake, he might crank the level way up, to as much as a thousand parts per million—more than double the level in the outside world. He needed to be careful, though. He was paying Climeworks for the piped-in CO2, so he had to make every molecule count: “I have to figure out the level that’s going to be profitable.”
Carbon dioxide removal may be essential; it’s already built into the calculations of the IPCC. Under the current order, however, it’s also economically infeasible. How do you go about creating a $100 billion industry for a product no one wants to buy? The eggplants and the Snack-Gurken represented an admittedly jury-rigged solution. By selling its CO2 to the greenhouses, Climeworks had secured a revenue stream to underwrite its capture units. The catch was that the captured carbon was only briefly being captured. Whoever snacked on the Snack-Gurken would liberate the CO2 that had gone into producing them.
From more little bricks of dirt, cherry tomato plants stretched to the roof in helical coils. The tomatoes, just a day or two from harvest, were perfect, in that greenhouse tomato-y sort of way. Ruser picked a couple and handed them to me. The burning trash, the acres of glass, the boxes of bumblebees, the vegetables raised on chemicals and captured CO2—was it all totally cool or totally crazy? I paused for a second, then popped the tomatoes into my mouth.