SCIENCE
The world of science is so vast and expanding that to condense it into 30 pages seems like a futile experiment. Every school system teaches the topic differently, so what may seem familiar and commonplace to one person can remain a mystery to others. Consider this chapter the foundation on which you can build.
Biology
The term biology comes from the Greek, meaning study of life; therefore, this field of learning concerns plants and animals and how the human body works.
• PHOTOSYNTHESIS
This is the process by which plants convert carbon dioxide and water into the carbohydrates they need for growth, using energy that they absorb from light (hence, the photo element). Light is absorbed into the plant by the green pigment called chlorophyll, stored mainly in the leaves, which provides the green color of so many plants. In fact, plants need only the hydrogen element from water (H2O), so photosynthesis releases oxygen back into the atmosphere, enabling the rest of us to breathe.
• THE STRUCTURE OF A PLANT
The flower contains the plant’s reproductive organs. The stigma, style, and ovary make up the carpel, which contains the female cells; if a flower has more than one carpel, these combine to form the pistil. The male organ is called the stamen and consists of an anther that contains the pollen sacs and is supported on a filament. Most plants self-pollinate, but some, such as certain hollies and the kiwifruit, require a male and female plant of the same species in order to reproduce.
The leaves enable the plant to feed and breathe. They contain the chlorophyll that is essential to photosynthesis, which absorbs light. Leaves also contain pores (stoma), through which gases and water are absorbed and released back into the atmosphere. The shape of the leaf reflects the plant’s needs: big, broad leaves are designed to absorb maximum light; the fleshy, succulent leaves of a cactus store water in case of drought.
The stem is the plant’s support and the conduit between roots, leaves, and plants. It contains phloem, a tissue that transports food within the plant; and xylem, which principally transports water. It is the xylem that hardens to form the trunks of trees and shrubs.
The roots anchor the plant in the ground and absorb nutrients and water from the soil. A tap root system has a single main root; a fibrous system has—well, lots of fibers. In root vegetables, such as turnips and carrots, the vegetable part is, in fact, a swollen root. Adventitious roots are less common; the name means coming from the outside, and these roots grow in unusual places, such as from the stem.
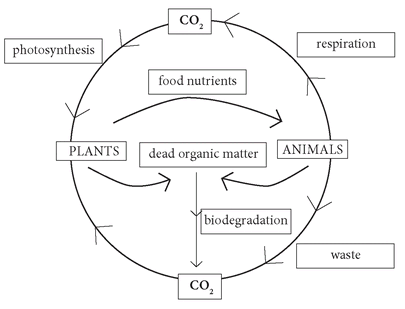
• THE CARBON CYCLE
The process by which carbon (in the form of carbon dioxide) is absorbed from the atmosphere during photosynthesis and is then transferred from one organism to another and eventually released back into the atmosphere is known as the carbon cycle. For example, a plant takes in carbon dioxide; the plant is eaten by a herbivorous animal, which is in turn eaten by a carnivore; when the animal dies, its rotting body releases carbon dioxide. Alternatively, the herbivorous animal excretes its waste, which also degrades to give off carbon dioxide.
This provides a smooth transition from plants to the human body.
• CHROMOSOMES
A normal human body has 46 chromosomes composed of 22 matched pairs and two sex chromosomes. Half of each pair, along with a single sex chromosome, is found in the sperm. The other half is in the egg. Fusion of the two creates the human embryo. Sex chromosomes are of two types, called X (female) and Y (male). The egg always contains an X chromosome, so the sex of the embryo is determined by whether a sperm is carrying an X or Y chromosome. Other chromosomes dictate other genetic factors, such as hair and eye color.
Chromosomes are made up of DNA, RNA, and protein.
DNA stands for deoxyribonucleic acid and is fundamental to the organization and functioning of living cells. It consists of the famous “double helix” (identified by the scientists Crick and Watson in 1953), with two strands coiled around each other. When the strands of a helix separate, each provides a template for the synthesis of an identical strand, containing the same genetic information. This enables normal growth, cell repair, and the production of cells that will turn into the next generation—which is why humans produce babies rather than tiger cubs, and why tigers produce tiger cubs rather than roses.
RNA stands for ribonucleic acid, which occurs as a single strand and contains different sugars and bases but is otherwise structurally similar to DNA. It’s vital to the synthesis of…
Proteins, which fulfill many important roles in a living organism—they are involved in the makeup of tissue; the properties of muscles; and the functioning of hormones, the immune system, and the digestive system, to name a few. They are manufactured within cells using information conveyed by the DNA and RNA.
• THE SKELETAL SYSTEM
The human skeleton is made up of more than 200 bones, held together by fibrous tissue called ligaments, and linked at the joints. Joints allow varying degrees of movement from none (between the bones that make up the skull) through some (the hinge joints at the elbow and knee) to lots (the ball-and-socket joints at the hip and shoulder).
The principal bones of the body, starting at the top and working down, are:
• cranium: skull
• spine: made up of 26 smaller bones called vertebrae
• clavicle: collar bone
• scapula: shoulder blade
• humerus: upper arm
• radius and ulna: lower arm—the radius is the broader one on the thumb side, the ulna the narrower one on the little finger side
• carpus: a collective name for the bones of the wrist, individually known as carpals
• metacarpus: ditto for the five long bones of the hand
• phalanges: fingers
• sacrum: actually a fusion of five vertebrae attached to the
• hip bone
• coccyx: tail bone, a fusion of the lowest four vertebrae
• femur: thigh bone
• patella: knee cap
• tibia and fibula: lower leg—the tibia is the broader one that runs down toward the big toe; the fibula the narrower one that runs toward the little toe
• tarsus: a collective name for the bones of the ankle and heel, individually known as tarsals
• metatarsus: ditto for the five long bones of the foot
• phalanges: toes
• THE CIRCULATORY SYSTEM
Blood is the body’s transportation system—everything from oxygen to hormones is transported around the body in the bloodstream, and its waste products, from carbon dioxide to urea, are carried away for disposal.
In order for blood to do its job, it needs to be pumped around, and that is the primary purpose of the heart. The heart is two pumps, each consisting of two chambers—an auricle and a ventricle—with a valve in between. The left side of the heart receives oxygen-rich blood from the lungs and forces it throughout the body; the right side receives the oxygen-depleted blood and returns it to the lungs to be re-oxygenated. (Oxygen, of course, comes into the lungs in the air that we breathe, and without it the cells in the body would die.)
All this requires a well-organized system of blood vessels. These are divided into arteries, which are strong and muscular and carry fast-flowing blood away from the heart, and veins, which are weaker and more sluggish and bring it back. The principal artery, the aorta, divides into smaller arteries and arterioles. Smaller veins are called venules, and really tiny blood vessels—whether veins or arteries—are called capillaries.
The exception to the useful mnemonia—arteries go away—is the pulmonary artery—the one that goes from the lung to the heart. The pulmonary vein runs from the heart to the lungs. Therefore, the truth is that the arteries simply carry the oxygen-rich blood.
Blood has four major components:
• red blood cells, which carry hemoglobin, made up of heme (an iron-containing pigment) and globin (a protein) (This combines with oxygen to form oxyhemoglobin, the means by which oxygen is transported throughout the body. Oxyhemoglobin also gives the blood its red color, which is why arterial blood is bright red; venous blood, having deposited oxygen in cells all over the body, has a bluish tinge.)
• white blood cells, or leukocytes, which fight infection
• platelets, which are necessary for the clotting process
• plasma, the liquid that makes the blood… well, liquid
• THE DIGESTIVE SYSTEM
The digestive process is divided into four parts:
• ingestion: eating food
• digestion: breaking the food down into constituent parts
• absorption: extracting nutrients from the food
• elimination: disposing of waste
Once you swallow food or drink, it enters the esophagus, or gullet, and passes (through a process of muscular contraction called peristalsis) into the stomach. From there it continues into the small intestine (comprising the duodenum, jejunum, and ileum), where digested food is absorbed into the bloodstream. The whole process is helped by the secretion of enzymes. One of the effects of the digestion of protein (which enters the body via meat, fish, eggs, etc.) is the release of amino acids, which are the building blocks of the protein the body needs for all sorts of different purposes.
Anything undigested after this stage passes into the colon (the beginning of the large intestine), where water is extracted from it. What remains are the feces, which pass through the rectum and out of the body via the anus.
Organs encountered along the way include:
• the liver, which in adult life often copes with our alcohol intake, but which has many more functions to do with digestion and keeping the blood healthy
• the gall bladder, which stores bile, needed in the digestion of fats
• the pancreas, which secretes various enzymes and the hormones insulin and glucagon, which regulate levels of blood sugar
• the kidneys, which control the amount of salt and water in the blood. (Excess fluid containing waste products is filtered through the kidneys down to the bladder and leaves the body in the form of urine.)
• THE RESPIRATORY SYSTEM
Air passes into the body through the trachea or windpipe via the mouth and nose. With the help of contractions from the diaphragm, which is a large muscle extending across the bottom of the rib cage, it is carried down into the lungs via two smaller tubes, called bronchi, which then split into even smaller bronchioles. Inside the lungs are lots of little air sacs, or alveoli. Within the alveoli oxygen is extracted from the air, absorbed into the bloodstream, and carried off to the heart via the pulmonary artery. The pulmonary vein brings “used” blood back to the alveoli, and the process is reversed as we breathe out air that now has a high carbon dioxide content.
• THE NERVOUS SYSTEM
The brain, spinal cord, and nerves make up perhaps the most important and intricately complex system in the human body. The nervous system essentially controls all the other systems in your body. It is what allows you to remember things, or at least remember that you used to know something. It tells your muscles and organs what to do and how to do it. The three interconnected parts of the nervous system are:
• the central nervous system, composed of the brain and spinal cord, which sends nerve impulses and analyzes information from the sense organs (eyes, ears, nose, mouth, skin, etc.). These organs enable you to see, touch, taste, hear, and feel.
• the peripheral nervous system, which includes the craniospinal nerves, a vast network of nerves that extends from your brain and spinal cord to all parts of your body and carries signals back and forth. It carries nerve impulses from the central nervous system to the muscles and glands.
• the autonomic nervous system (ANS), which regulates involuntary actions, such as pulse rate and digestion. The ANS is broken into the sympathetic nervous system (fight or flight), the parasympathetic nervous system (rest so you can digest), and the enteric nervous system (the digestive system’s personal messenger).
However, no discussion of the nervous system is complete without those trusty neurons, the nerve cells that send and carry the signals throughout your body. A neuron consists of a main cell body with a long nerve fiber, called an axon, branching from it. Electrical signals pass from axon to axon through small gaps called synapses. In order to do this, these electrical signals turn into chemical ones, called neurotransmitters. In fact, right now the neurons in the temporal lobe of your brain (which interprets language), your frontal lobe (which involves reasoning), and your occipital lobe (which controls sight) are firing away!
Chemistry
This is the study of elements and compounds and the reactions they undergo—which is a definition that surely cries out for a few more definitions.
atom: the smallest particle in an element that can take part in a chemical reaction, made up of a nucleus, which is containing positively charged protons and neutral neutrons; and a number of electrons, which are negatively charged particles that orbit the nucleus. Each atom normally has the same number of protons and electrons, leaving it with a neutral charge. The movement of electrons is responsible for most commonly observed chemical, electrical, or magnetic reactions. If an atom loses or gains an electron, it becomes either positively or negatively charged and is known as an ion.
element: a substance that cannot be decomposed into a simpler substance by a chemical process. Groups of elements come together to form a compound. So, for example, a combination of the element hydrogen (H) and the element oxygen (O) can form the compound water (H2O).
mole: also known as Avogadro’s number or Avogadro’s constant, a mole contains the same number of particles as there are in 12 g of carbon-12 atoms—that is, 6.022 x 1023 particles. Carbon has three naturally occurring isotopes (forms of the same substance with different numbers of neutrons), and one of these is carbon-12.
molecule: the smallest particle of a compound that can exist independently and retain its properties. So in the previous example, the smallest imaginable quantity of hydrogen and oxygen joined together in the right conditions and right proportions will still produce a molecule of water. Only when the hydrogen and oxygen are chemically separated again do they lose the properties that make them water and return to being atoms of hydrogen and oxygen.
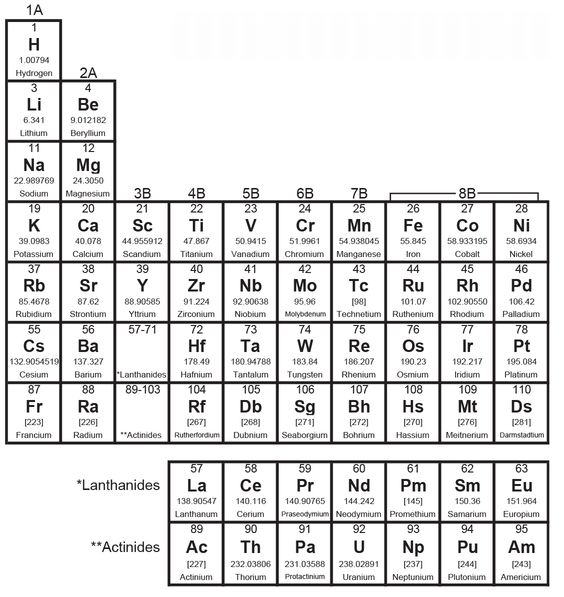
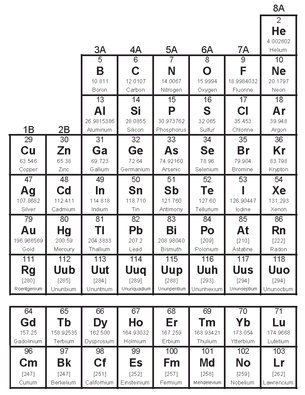
• THE PERIODIC TABLE OF THE ELEMENTS
The periodic table was first devised in 1889 by the Russian chemist Dmitri Mendeleev. When putting the table together, Mendeleev realized there were gaps between some of the elements. Based on this, he predicted that some elements had yet to be discovered.
The table arranges the elements in ascending order of atomic number (the number of protons that each possesses) in such a way that the vertical columns contain groups or families with similar chemical properties. The horizontal rows represent periods, with the most electropositive (an alkali metal) on the left and the so-called inert gases on the right, and the whole thing proves that “the chemical properties of the elements are periodic functions of their atomic weights”—or, in other words, that similar properties in an element recur at regular intervals.
PERIODIC TABLE OF THE ELEMENTS
The elements are traditionally designated by a one-, two-, or three-letter abbreviation, as you can see in the table, and there are 118 of them. The table above lists 103; listed below them are elements 104 through 118. From 93 upward the elements don’t occur naturally but have been synthesized in particle accelerators. The last few are recent achievements, and they have temporary names based on their atomic numbers. Element 117, which will be called Ununseptium, hasn’t been synthesized yet, but scientists are working on it. The lanthanides and actinides are usually separated from the rest of the table, as shown above, because—unlike the other rows—they have similar properties as you read across.
104 Rutherfordium Rf
105 Dubnium Db
106 Seaborgium Sg
107 Bohrium Bh
108 Hassium Hs
109 Meitnerium Mt
110 Darmstadtium Ds
111 Roentgenium Rg
112 Ununbium Uun
113 Ununtrium Uut
114 Ununquadium Uuq
115 Ununpentium Uup
116 Ununhexium Uuh
117 Ununseptium Uus
118 Ununoctium Uuo
• ACIDS, BASES, AND SALTS
An acid is a substance (often sour and corrosive) that contains hydrogen atoms that, when dissolved in water, dissociate into ions and may be replaced by metals to form a salt.
A base is a compound that combines with an acid to form a salt plus water. Bases that are soluble in water are called alkalis. Many bases are oxides (so their formula ends in O, possibly with a little number after it) or hydroxides (OH).
A salt is a (usually crystalline) solid compound formed from the combination of an acid and a base by the replacement of hydrogen ions in the acid by positive ions in the base.
For example, combine sulphuric acid with the base cupric oxide in the right conditions and you have copper sulphate (that lovely bright blue stuff) and water:
H2SO4 + CuO → CuSO4 + H2O.
In a school lab you test whether a substance is an acid or a base with litmus paper. Acids turn litmus red; bases turn it blue. Serious scientists use the pH—potential of hydrogen—which is measured by sensors and electrodes and such. Pure water has a pH of 7, with anything less considered acidic and anything higher alkaline. Gardeners use this as a way of testing soil; you also sometimes see the pH listed on shampoo bottles.
Another term you might remember—and one worth mentioning here—is valency, which means the number of atoms of hydrogen that an atom or group displaces when forming a compound. Hydrogen has a valency of 1 and oxygen a valency of 2, which is why the formula for water is H2O and not just HO—because you need two atoms of hydrogen to “match” one of oxygen. Copper can have either of two valencies, which is why the one mentioned a moment ago is called cupric oxide, not just copper oxide. There’s also cuprous oxide, CuO2.
• OXIDATION
Oxidation is a commonly quoted chemical reaction, and the most common example of it is rust. In fact, anything that reacts when it comes into contact with oxygen is being subjected to oxidation: The green coating on an old copper coin is the result of oxidation; the browning of fruit is caused by oxygen burning away at the stuff that is released when you peel off the protective skin. Rust is, strictly speaking, the oxide that forms on iron or steel. Stainless steel doesn’t rust, because it is protected by a layer of chromium, which doesn’t react to oxygen in the same way.
• DIFFUSION AND OSMOSIS
Molecules are constantly in motion and tend to move from regions where they are in higher concentration to regions where they are less concentrated—a process known as diffusion. Diffusion can occur in gases, in liquids, or through solids.
Osmosis is a form of diffusion that is specific to the movement of water. Water moves through a selectively permeable membrane (that is, one that lets some types of molecules through but not others) from a place where there is a higher concentration of water to one where it is lower.
In any form of diffusion, when the molecules are evenly distributed throughout a space, they have reached equilibrium.
• BOILING AND FREEZING POINTS
If the temperature is low enough, every known substance except helium becomes a solid. The temperature at which this happens is called its freezing point. Above its freezing point a substance is a liquid. At the other end of the scale, if the temperature is high enough, it becomes a gas, and this is called the boiling point.
Solid is the only state in which a substance retains its shape; a liquid assumes the shape of its container but does not necessarily fill it; a gas expands to fill the space available.
Take water, for instance. In its solid state, it is ice and retains its shape—whether ice cube, icicle, or iceberg—until the temperature rises sufficiently for it to melt and become liquid (water). If you take a tray of melted ice cubes and pour the water into a pan, it will take the shape of the container—that is, spread out to cover the bottom—but it may only come a certain distance up the side. If, however, you then turn on the heat under the pan, put a lid on it, and boil the water, it will turn into gas (steam), fill the pan completely, and probably seep out under the lid as well.
Nonscientists commonly measure temperature according to one of two scales: Celsius and Fahrenheit, both named after the people who invented them. Celsius was also once called centigrade, from the Latin for one hundred degrees.
The freezing point of water is 0°C, and its boiling point is 100°C. The equivalent in Fahrenheit is 32°F and 212°F. This means that the difference between freezing and boiling is 100°C and 180°F (212 - 32).
To convert Celsius to Fahrenheit, you need to divide by 100 and multiply by 180, which can also be expressed as multiplying by 1.8, or
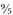
. Then, because the freezing point of water is 32°F, not 0°F, you need to add 32:
15°C x 1.8 = 27; 27 + 32 = 59°F.
To reverse the process, first deduct 32 from your Fahrenheit temperature, then divide by
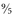
(or multiply by
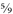
; it’s the same thing):
104°F - 32 = 72; 72 x
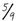
= 40°C.
This works for any temperature above freezing.
There are two other scales used by scientists—the Réaumur and the Kelvin. According to René Antoine Ferchault de Réaumur, water freezes at 0° and boils at 80°. Kelvin is interesting because he invented the concept of absolute zero, a temperature at which particles cease to have any energy—so a scientific impossibility, although in the laboratory, scientists have achieved temperatures within a millionth of a degree of it. Absolute zero is 0°K, or -273.15°C, which is very, very cold. Imagine how much energy you would have at that temperature.
Physics
Physics deals with the properties and interactions of matter and energy, but its theories are constantly being redefined as physicists discover new things.
• OPTICS
Optics is all about light and there are several terms that may ring a bell.
Remember “The angle of incidence equals the angle of reflection”? You probably do. But do you remember what it means? Well, the angle of incidence is the angle at which light hits a surface; with specular (mirrorlike) reflection the light is reflected at the same angle. If the surface is rough, you get diffuse reflection, which means that the light bounces off in all directions.
Light may also pass through a medium—such as glass or water—and be refracted (change direction). This is because of the difference in the velocity with which light passes through the two different media (say, air and water), which is measured by the refractive index.
• CONDUCTION, CONVECTION, AND RADIATION
There are three ways in which heat is transferred:
Conduction can occur in solids, liquids, or gases and means (more or less) that a cool thing is warmed up by coming into contact with a hot thing. The different levels of conductivity in metals are reflected in their uses in anything from the science lab to kitchenware: Copper, for example, is highly conductive, and therefore it works well for fast cooking (although it may react with certain foods, which is why copper-bottomed pans are often lined with tin); whereas cast iron heats slowly but then cooks evenly.
Convection occurs in liquids and gases and is the basis of the principle that hot air rises. A hot liquid or gas is generally less dense than a cool one; as the hot particles rise, cooler ones rush in underneath to take their place. As the hot particles rise, they cool and come down again, and so on.
Radiation involves the energy that all objects, hot or cold, emit. It is the only one of the three that works in a vacuum and is how the sun’s rays manage to warm the Earth from such a far distance away.
Heat is not the only commodity that is transferred in these ways. There is also electrical conduction, mass convection (of which evaporation is an example), and electromagnetic radiation. So, strictly speaking, you should insert the words “heat” or “therma” in front of conduction, convection, and radiation if that is what you mean.
• PHYSICAL LAWS
Physics is based on properties that explain what matter and energy can or can’t do; without these interactions the universe would probably fall apart. From the observation of the interactions, laws were developed. Some of the physical processes and phenomena are revealed in this section. But a few definitions might help first.
Mass is the quantity of matter a body contains. Newton defined it more precisely by bringing in inertia, which is “a property of matter by which it continues in its existing state of rest or uniform motion in a straight line, unless that state is changed by an external force.” All this means is that a thing will sit still until you push it.
Force is calculated by multiplying mass by acceleration and concerns producing motion in a stationary body or changing the direction of a moving one.
Velocity is speed (the dictionary says, “measure of the rate of movement,” but most people call that speed) in a given direction.
Acceleration is the rate of increase in velocity.
Work is the exertion of force overcoming resistance (which might be electrical resistance, or it could be physical resistance, such as friction).
And, regardless of what anyone else may tell you, in this context a body is a thing. The dictionary says, “an object or substance that has three dimensions, a mass, and is distinguishable from surrounding objects.”
• THE LAWS OF THERMODYNAMICS
Thermodynamics is the study of heat and its relationship with other forms of energy, and it is important in the study of heat engines such as gas-driven motors and gas turbines.
The other key term here is entropy, which is defined as “a measure of the disorder of a system.” A solid has less entropy than a liquid, since the constituent particles in a solid are in a more ordered state. The flow of energy maintatins order and life. Entropy states the opposite. Entropy takes over when energy ceases.
If you have managed to follow along this far, then you are ready for the three laws of thermodynamics:
1. Energy can change from one form to another, but it can never be created or destroyed.
2. In all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will be less than that of the initial state.
3. As the thermodynamic temperature of a system approaches absolute zero, its entropy approaches zero.
The British scientist and author C. P. Snow came up with a great way of remembering the three laws:
1. You cannot win (you cannot get something for nothing, because matter and energy are conserved).
2. You cannot break even (you cannot return to the same energy state, because there is always an increase in disorder).
3. You cannot get out of the game (because absolute zero is unattainable).
Moving swiftly on.
• THE LAWS OF CONSERVATION OF ENERGY AND MASS
The most common of these laws states that energy in a closed system cannot be created or destroyed (it’s similar to the first law of thermodynamics), and nor can mass. At a more advanced level, similar laws apply to electric charge, linear momentum, and angular momentum, but most people never get that far.
• NEWTON’S THREE LAWS OF MOTION
1. A body remains at rest or moves with constant velocity in a straight line unless acted upon by a force.
2. The acceleration (a) of a body is proportional to the force (f) causing it: f = ma, where m is the mass of the body in question.
3. The action of a force always produces a reaction in the body, which is of equal magnitude but opposite in direction to the action.
Newton also came up with a law of gravity, which states that the force between two bodies is directly proportional to the product of their masses and inversely proportional to the square of the distance between them. The universal gravitational constant that makes this equation work is called G, and its value is 6.673 x 10-11 newton m2 per kg2.
However, Einstein’s general theory of relativity describes gravity more accurately.
• EINSTEIN’S THEORIES OF RELATIVITY
Before reviewing Einstein’s general theory of relativity, take a look at his special theory of relativity. Before Einstein—that is, until the start of the 20th century—it was believed that the speed of light relative to an observer could be calculated in the same way as the relative speed of any other two objects (such as two cars driving at different speeds). Einstein’s theory is based on the assumption that the speed of light in a vacuum is a constant (186,000 miles—or 2.998 x 108 m—per second), regardless if the observer is moving or at what speed. Furthermore, he suggested that as bodies increase in speed, they increase in mass and decrease in length (relative to the observer)—although this effect became noticeable only as objects neared the speed of light.
Relative to each observer, time moves at a slower rate. All this led him to the conclusion that mass and energy are two different aspects of the same thing, which led to the famous equation
where
E is energy,
m is mass, and
c is the velocity of light.
So, back to gravity. The special theory of relativity concerned motion in which there was no acceleration—that is, a constant speed. The general theory extended this to consider accelerated motion. According to this, gravity is a property of space and time that is “curved” by the presence of a mass. Einstein posited that the motion of the stars and planets was controlled by this curvature of space in the vicinity of matter, and that light was also bent by the gravitational field of a massive body. Subsequent experiments have shown him to be correct.
• ELECTRIC CURRENT
There are also a handful of laws to do with electricity. Here’s one of the more familiar:
Ohm’s law states that the current (I) flowing through an element in a circuit is directly proportional to the voltage drop or potential difference (V) across it: V = IR, where R means resistance—anything that gets in the way of the flow of current. What this means, more or less, is that the greater the resistance (measured in ohms), the greater the voltage (measured in volts) required to push the current (measured in amps) through it.
• EQUATIONS OF MOTION
These are basic equations that describe the motion of a body moving with constant acceleration.
A body moving with constant acceleration (a) starts with an initial velocity (u) and achieves a final velocity (v) in a time of t seconds, covering a total distance s. If you know any three of these components, you can decipher the other two.
Acceleration can be expressed as
Distance traveled (
s) is simply time multiplied by average speed:
These two equations—one for calculating acceleration and the other for calculating distance—are essentially all that is known here, but some other equations can be obtained by combining them.
For example, eliminate
v from both of them. The first equation can be recast as
(multiply everything by
t, then add
u to both sides) and the second as:
(multiply everything by 2, divide by t, and deduct u from both sides).
This may sound complicated, but the point is to produce an equation that defines
v. Just in case you want to calculate
v, you understand.… But you also now have two equations beginning “v=,” so you can put them together and deduce that:
which, after a bit of rearranging, is equivalent to
This looks a bit more impressive, but it’s not really telling you anything new.
Similarly, you could eliminate
u from each of our original equations, yielding:
Or eliminate
t from them both to show that:
So, to give an example, if a body traveling at 30 m/sec (
u) accelerates at 2 m/sec/sec (
a) for 10 sec (
t), it reaches a velocity (
v):
v = at + u = (2x10) + 30 = 50 meters per second
s = ut + ½at2 = (30 x 10)+(½ x 2 x 102)
= 300 + 100 = 400 meters.
Average speed is distance traveled (
s) divided by
t, which in this instance is
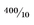
= 40 m/sec. Which sounds reasonable, because it starts at 30 and ends up at 50.
Apparently, this isn’t rocket science, unless you have a rate of acceleration equal to the force of gravity, in which case you are into the realm of projectiles and ballistics, which is, um, rocket science.


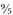 . Then, because the freezing point of water is 32°F, not 0°F, you need to add 32:
. Then, because the freezing point of water is 32°F, not 0°F, you need to add 32: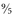 (or multiply by
(or multiply by 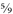 ; it’s the same thing):
; it’s the same thing):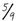 = 40°C.
= 40°C.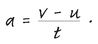
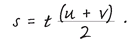
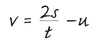
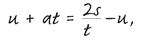
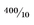 = 40 m/sec. Which sounds reasonable, because it starts at 30 and ends up at 50.
= 40 m/sec. Which sounds reasonable, because it starts at 30 and ends up at 50.