5 Energy, Industry, and Pollution: Mercury, the Winged Messenger
The large expansion in the use of fossil fuels in the industrial era increased access to energy and supported economic growth. Yet, the burning of fossil fuels, especially coal, emits the hazardous substances they contain into the atmosphere. These substances include mercury, other heavy metals, sulfur, and carbon dioxide. Hazardous byproducts of combustion that travel via air currents harm populations both nearby and far away. Past regulatory efforts to mitigate air pollution reduced emissions of mercury and other harmful substances in some places. These efforts had measurable benefits for the environment and human health, and are sometimes hailed as local and regional regulatory successes. Challenges remain in other places, however, and for other pollutants such as carbon dioxide. Efforts to control mercury emissions and deposition illustrate that there can be tradeoffs between incremental actions with local and regional benefits and efforts that seek to address the fundamental activities that ultimately produce pollution. However, interventions aimed toward incremental changes are not always in conflict with those that aim to fundamentally restructure underlying processes. In some cases, these two approaches can be synergistic.
The US Environmental Protection Agency (EPA) began measuring mercury in Steubenville, Ohio, in 2002. Data there showed elevated levels of mercury in rainfall, and nearly 80 percent of this mercury was estimated to come from nearby coal-fired power plants (Keeler et al. 2006). Researchers and pollution-control advocates have referred to places like Steubenville, where local sources cause elevated mercury deposition, as “hot spots” (Beusse et al. 2006). This was not the first time that Steubenville became known for its severe pollution. A group of Harvard University researchers began to study the effects of air pollution in Steubenville and five other locations in the United States in 1974. These sites were the so-called six cities in a groundbreaking study that sought to quantify the mortality risks of inhaled fine particulate matter. The researchers found that risk of death in Steubenville, the city among the six with the worst air quality, was 26 percent higher than in the least polluted city in the study (Dockery et al. 1993). Steubenville was called a “test lab” for dirty air and its impact on human health and the environment (Barringer 2006).
Air quality has improved in Steubenville and many other US cities since the 1970s, but air pollutants remain a problem. Researchers analyzed changes in air pollution in the six cities in a follow-up study in the early 2000s, and found that concentrations of fine particulate matter in Steubenville had nearly halved (Laden et al. 2006). Current pollution levels, including mercury deposition, are nevertheless still elevated. Steubenville’s air quality improvement is also embedded in a larger socio-economic context. The city’s predominant industries in the 1970s were coal mining and steel production; economic stagnation and declines in industrial production beginning in the 1980s deeply affected its residents. In 2006, one resident wondered aloud to a New York Times reporter whether cleaner air was worth the trade-offs (Barringer 2006). As mercury emissions from US coal burning have declined, decreasing deposition from domestic sources has in part been offset by increases in mercury coming from elsewhere. In the early 2000s, scientists at the top of Mount Bachelor in Oregon measured mercury in air transported across the Pacific Ocean that had chemical signals characteristic of industrial air pollution from China (Weiss-Penzias et al. 2006).
The story of air pollution in Steubenville is not unique to Ohio or the United States. As we highlight in the chapter title with a reference to the god Mercury as an airborne messenger, atmospheric mercury emissions from industrial point sources in many regions of the world connect geographically distant places across the globe. Like Steubenville, many cities in other industrialized countries have suffered pollution problems and undergone difficult economic transitions as once-dominating and prosperous industries closed down. Contemporary atmospheric mercury emissions from large stationary point sources mostly occur in developing countries, and a large fraction of these emissions results from burning coal to produce energy and industrial goods. Industrialization and rapid economic development in many developing countries over the last few decades, in particular in urban areas in China and India, led to increased emissions of mercury and other air pollutants. Fossil fuel–based energy production is at the same time coming under growing regulatory scrutiny worldwide due to its effect on local air quality as well as its influence on global climate change.
Contributions from local and distant emission sources to mercury deposition in any given region have varied in the past, and will continue to change. These variations result from the adoption of pollution prevention laws, the application of pollution control technology, transnational economic forces, and changes in weather patterns and climate conditions. The relative contribution of nearby and faraway sources is also a matter of definition. The statistic that 80 percent of mercury deposition in Steubenville in the early 2000s came from nearby sources is contingent on how “nearby” is defined, whether on scales of tens or hundreds or thousands of kilometers. Regardless of the definition, it is clear that long-range atmospheric transport is important to mercury deposition. For example, for the region of the United States that includes Ohio, Illinois, Indiana, Michigan, and Wisconsin, one study from the early 2010s estimated that 25 percent of mercury deposition came from sources beyond those states, located either in other US states or in other countries of the world (Lin et al. 2012).
We focus in this chapter on atmospheric mercury emissions from coal burning and other major point sources, and on the development of strategies to mitigate these emissions. In the section on system components, we discuss the human, technical, and environmental components that influence emissions from point sources to the atmosphere, and the institutions and knowledge that affect the amount of mercury emitted to air. We trace how industrial activities lead to pollution, examine how pollution controls influence mercury emissions, and address the transport and fate of mercury in the atmosphere in the section on interactions. Our discussion of interventions explains how governments have mandated, and the private sector has applied, new pollution abatement technology for mercury without, in most cases, making fundamental changes to the energy and production processes that result in mercury emissions. In the final section on insights, we discuss how atmospheric transport and socio-economic factors connect mercury from local to global scales, how reductions in mercury emissions have come largely from incremental changes, and why cross-scale action is important when further addressing mercury emissions to air.
System Components
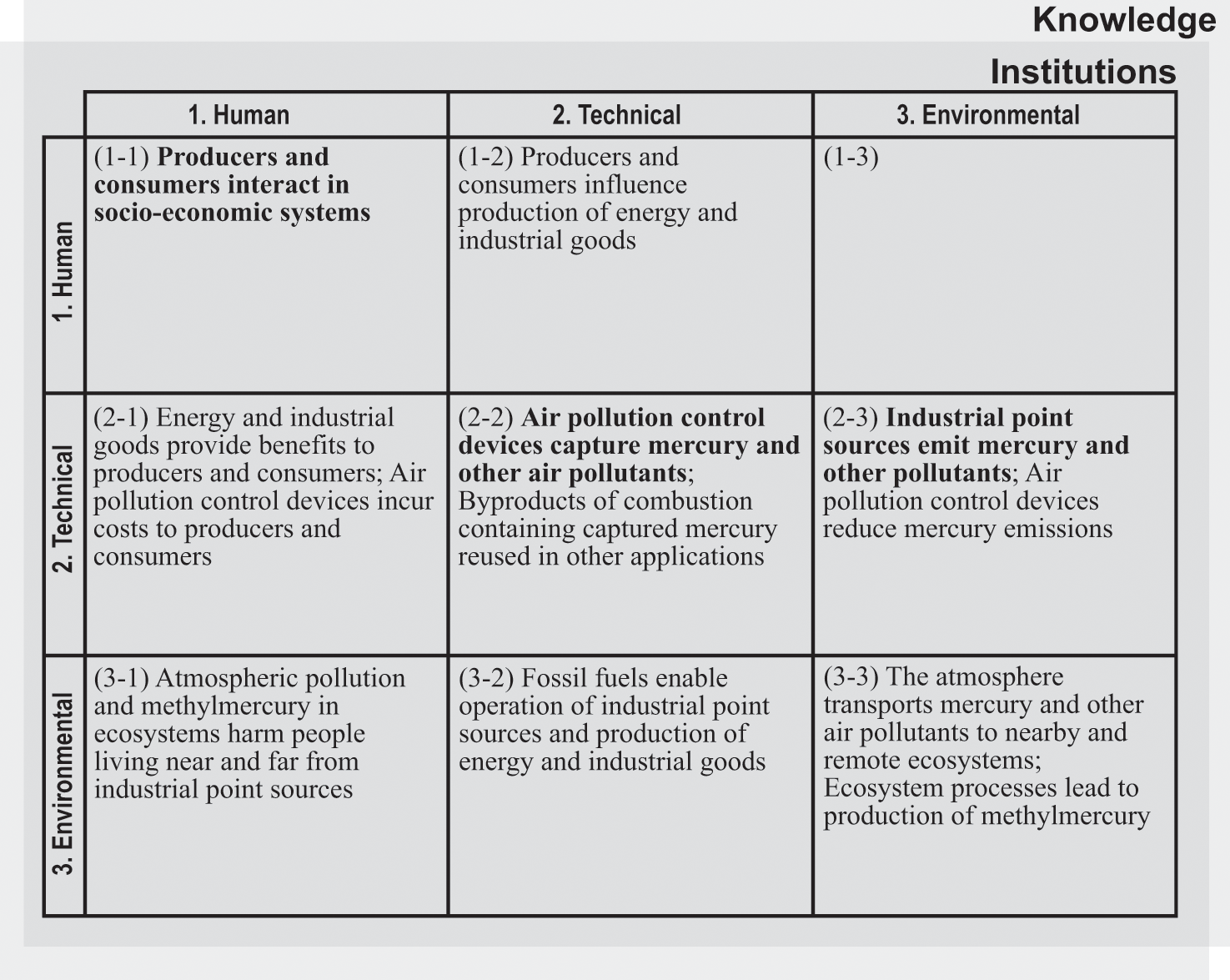
Much of the mercury that has been historically emitted to the atmosphere by human activities has come from industrial point sources. Industrial energy production based on fossil fuels contributed greatly to human development by providing an expanded supply of energy to billions of people. This had many societal benefits, but also led to sharply reduced air quality and ecological damages, including from mercury emissions. Other industrial activities producing a range of different products similarly provided material benefits to individuals and modern societies while causing much harmful pollution. Mercury pollution from contemporary industrial sources continues to be a global problem, but with important regional variations in emission levels. Figure 5.1 shows the human, technical, environmental, institutional, and knowledge components in the atmospheric system for mercury.

Components in the atmospheric system for mercury (referenced in the text in italic type).
People have mined coal in geological storage and used this extracted coal as an energy source for thousands of years. During most of this time, coal burning was small in scale. This could cause major indoor air quality problems, but contributed relatively little to atmospheric pollution worldwide. The Industrial Revolution led to a massive increase in coal use and associated mercury emissions—enough to increase mercury emissions from coal burning by 100-fold over the past 150 years (Streets et al. 2018). Present-day coal burning and mercury emissions to the air in many countries all over the world are deeply embedded in a long-standing fossil fuel–based economy: coal burning has been essential to the production of energy and industrial goods during the past three centuries, and people play important roles as producers and consumers of energy and industrial goods. Often, markets for energy and goods cross multiple spatial scales, and are influenced by forces of supply and demand. Many markets for energy are domestic or regional, and shape the prevalence of coal and other energy sources in the energy mix. Markets for goods have become more global over time given increased trade across international borders and continents.
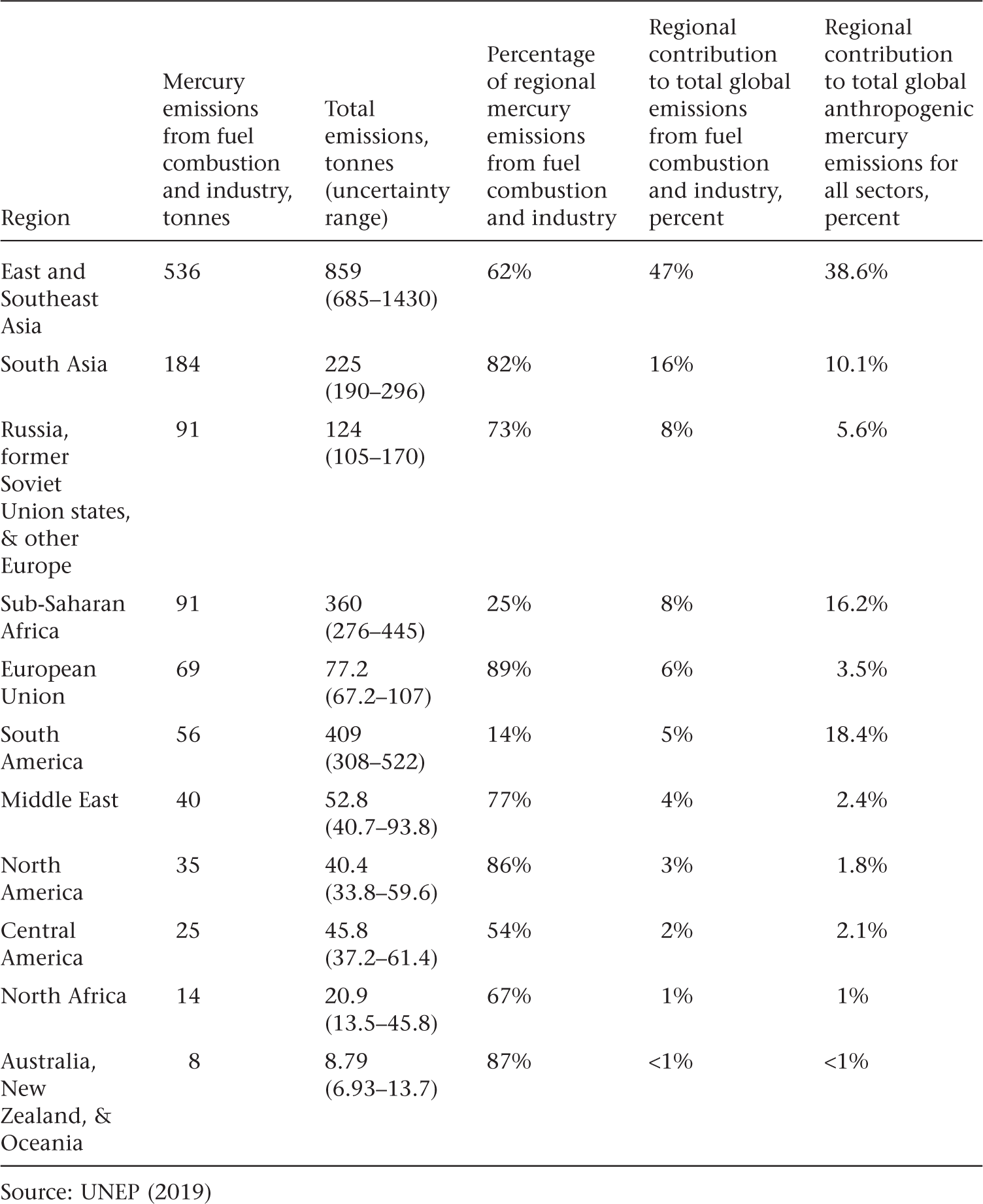
Present-day anthropogenic sources make up roughly a third of total mercury emissions to the atmosphere, as noted in chapter 3, with the remainder coming from natural sources and reemission of historically mobilized mercury (legacy mercury). Human activities emitted approximately 2,500 tonnes of mercury to air globally in 2015, according to the most recent global mercury assessment (UNEP 2019). Table 5.1 summarizes total mercury emissions to air by region, the fraction contributed by fuel combustion and industry in each region, and each region’s contribution to global emissions. Artisanal and small-scale gold mining (ASGM), which we discuss further in chapter 7, is thought to be the largest source globally, at 38 percent of total emissions. Stationary coal combustion contributed 21 percent of total emissions. Other types of industrial point sources of mercury emissions are also important, including production of non-ferrous metals such as copper, zinc, and lead (15 percent), cement production (11 percent), and production of ferrous metals (iron and steel) (2 percent). Emissions from mercury-added product waste contributed 7 percent, with other sources such as biomass and large-scale gold production contributing the remaining 6 percent. Emissions from some smaller sectors were not quantifiable.
Mercury Emissions to Air by Region for 2015
One study estimates that 38,000 tonnes of mercury has been mobilized over all time through coal combustion (Streets et al. 2018). The vast majority of that mercury has entered the environment after 1850, with 70 percent being emitted to the atmosphere. In the early 1970s, a back-of-the-envelope calculation—made by multiplying the average value of mercury in coal by the total amount of coal burned—estimated that some 3,000 tonnes of mercury were being emitted globally to the atmosphere by coal burning each year (Joensuu 1971). This estimate was too large by an order of magnitude because of errors in the average value of mercury in coal: different kinds of coal contain varying trace amounts of mercury (although this variability does not generally track with particular types of coal or regional origin). More recent estimates suggest that the amount of mercury emitted to the atmosphere from coal combustion in 1970 was between 300 and 400 tonnes (Streets et al. 2018). Estimates still vary about the amount of mercury emitted from coal for the present day—an estimate for 2010 quoted an uncertainty range of 221 to 1,473 tonnes (Streets et al. 2018).
Atmospheric emissions of mercury and other air pollutants affect humans and the environment both near and far away from sources. Mercury in air from nearby industrial point sources does not reach levels that typically cause health impacts from inhalation, but other air pollutants from the same sources can and do. Some forms of mercury remain in the atmosphere only a short time, and deposit to ecosystems near emission sources; other forms travel long distances through the air before undergoing chemical reactions and depositing to ecosystems far from emission sources. A fraction of the mercury deposited both locally and far from point sources is converted into methylmercury and accumulates in aquatic food webs. People’s health is affected by eating fish with elevated methylmercury concentrations, including people living near industrial point sources and people living far from industrial point sources. Air pollution control devices prevent varying amounts of mercury and other pollutants from point sources from entering into air. Many of these are end-of-pipe technologies that capture mercury emissions right before they would be emitted (Mukherjee et al. 2008).
Scientific knowledge about mercury concentrations in the atmosphere, the chemical behavior of different mercury compounds, and the propensity for long-range transport of different mercury compounds has developed over time. Some of the first measurements of atmospheric concentrations of mercury date back to the 1960s, when researchers detected high concentrations of mercury in the air in urban areas, such as around San Francisco (Williston 1968). These elevated mercury concentrations often coincided with high levels of other air pollutants. The first routine atmospheric mercury measurements began at a station in 1980 in Rörvik, Sweden (Iverfeldt 1991). Researchers at other sites began to measure atmospheric mercury concentrations in the 1990s, including at Wank Mountain in Germany in 1990, Cape Point in South Africa and Alert in Canada in 1995, and Mace Head in Ireland in 1996 (Slemr et al. 2003). Increasingly accurate measurements from these stations helped to document and determine regional and global atmospheric transport patterns of mercury and trends over time.
Today, atmospheric mercury monitoring stations exist in all regions of the world (Sprovieri et al. 2016). However, measurement data remain sparse in several regions, especially in the tropics and in the Southern Hemisphere (Obrist et al. 2018). Analytical techniques could be improved, specifically for measuring different forms of mercury in the atmosphere (Jaffe et al. 2014). The exact chemical composition of some of these forms of atmospheric mercury also remains unknown. Several measurement networks generate knowledge about mercury deposition, with most sites in the United States and Europe (Lindberg et al. 2007; Prestbo and Gay 2009). Historical records of mercury levels in environmental archives such as lake sediment cores are used to understand atmospheric deposition in the past (Biester et al. 2007). Measurements of mercury isotopes (atoms of mercury with different numbers of neutrons) are increasing in accuracy, and can help identify different sources of natural and anthropogenic mercury emissions as well as environmental processes such as chemical transformations in the atmosphere (Blum et al. 2014).
Institutions such as national and local laws and regulations address mercury emissions from various point sources. International air pollution agreements started to tackle mercury emissions in the 1980s. The Convention on Long-Range Transboundary Air Pollution (CLRTAP), originally developed in the 1970s to reduce pollution from sulfur dioxide and associated acid rain issues, began to consider mercury in the late 1980s (Selin and Selin 2006). Efforts under the Global Mercury Partnership have not only prompted increased awareness of mercury emissions, but also generated detailed technological information on control options for power generation and other major atmospheric sources. International expert groups, working under the Global Mercury Partnership and the Minamata Convention, develop and update technical guidelines for pollution controls. These guidelines communicate knowledge about techniques for air pollution control, including options for mercury controls and associated costs, and technical conditions and economic feasibility influence their application in different countries and situations (UNEP 2016).
Interactions
Mercury emissions from stationary sources to air are distributed widely in the environment. Figure 5.2 shows interactions in the atmospheric system for mercury: we have selected three interactions in the matrix (the items in bold type in boxes 1-1, 2-3, and 2-2) to focus on in this section; we then trace the pathways that influence these interactions, which we summarize in figure 5.3 (where the bold boxes correspond to the selected interactions). First, producers and consumers interact in socio-economic systems (box 1-1), producing energy and industrial goods that benefit society, and at the same time lead to emissions of mercury and other air pollutants (boxes 1-2, 2-1, 3-2, and 2-3). Second, industrial point sources emit mercury and other pollutants (box 2-3), and this mercury travels through the atmosphere and deposits in both nearby and remote ecosystems, where some is transformed into methylmercury that harms people (boxes 3-3 and 3-1). Third, air pollution control devices capture mercury and other air pollutants (box 2-2), affecting both emissions and costs to producers and consumers, while some of the captured mercury can be reused in other applications where it may be emitted to the atmosphere (boxes 2-3, 2-2, and 2-1).
Interaction matrix for the atmospheric system for mercury.
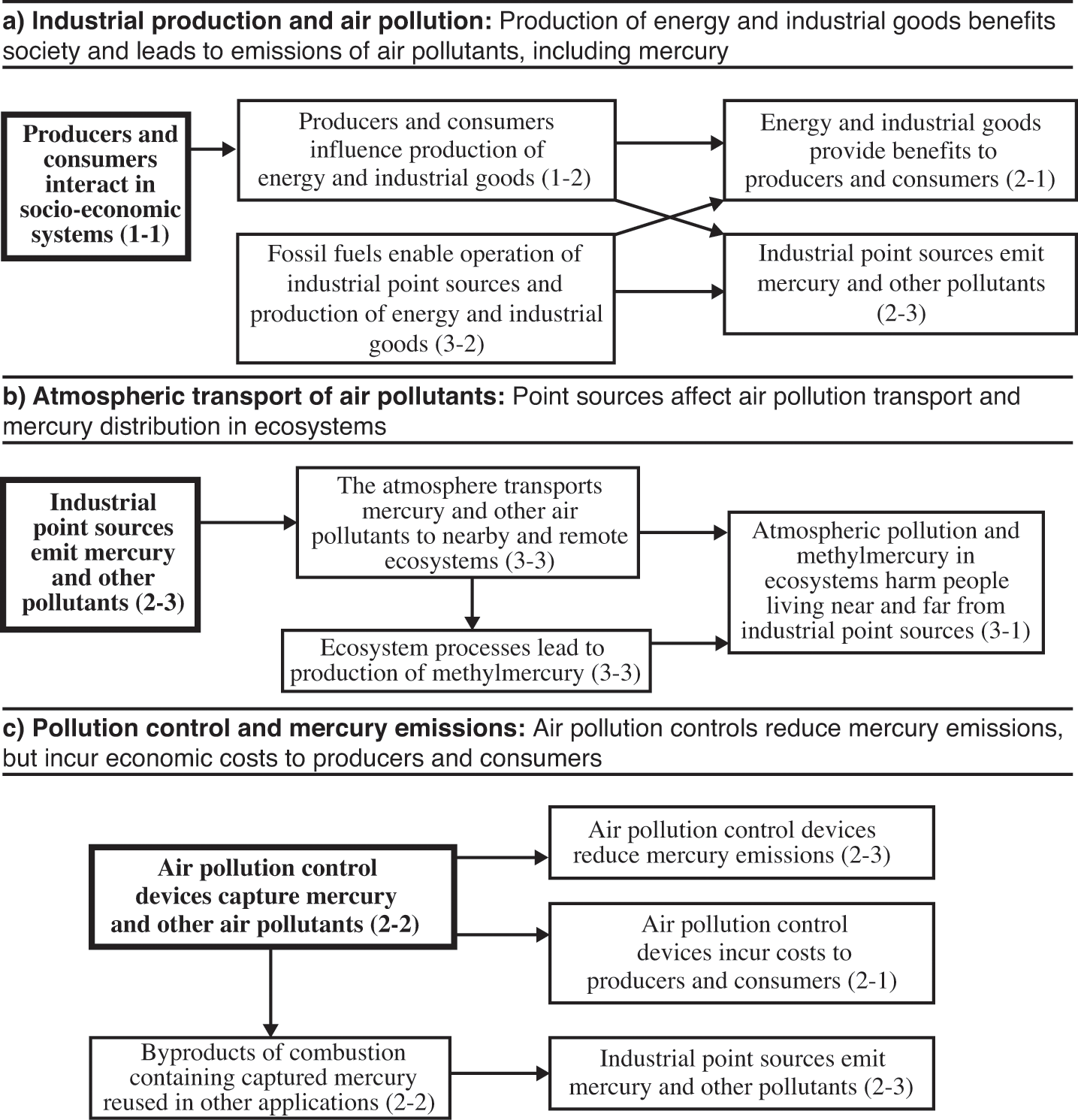
Pathways of interactions in the atmospheric system for mercury. Bold box indicates focal interaction for each subsection.
Industrial Production and Air Pollution
Industrial production and air pollution originate with producers and consumers interacting in socio-economic systems (box 1-1). The industrial era marked the beginning of a dramatic expansion of production, consumption, and trade worldwide, which accelerated further after 1950 (Steffen et al. 2007). These interactions, through supply and demand, influence the production of energy and industrial goods (box 1-2). Between 1850 and 2017, global energy production increased by almost a factor of 20 (Ritchie and Roser 2019). During the same period, world gross domestic product (GDP)—the monetary value of goods and services in the economy—increased by a factor of more than 50 (Roser 2019). Energy and industrial goods provide benefits to producers and consumers (box 2-1). Increased production raised material standards of living and enabled dramatic improvements in health and educational attainment. But the beneficial impacts of these changes were not evenly distributed globally: the Global North industrialized earlier and faster than did much of the Global South.
The availability and extensive use of fossil fuels, especially relatively cheap coal, enabled the operation of industrial point sources and production of energy and industrial goods (box 3-2). Changes in production in different regions over time altered the location and quantity of coal use (Streets et al. 2018). Coal use in Western Europe peaked in the 1950s, and declined thereafter. In the United States, coal use increased from the 1970s to the 1990s, but declined in the 2000s. Most of the growth in coal consumption since the 1960s was in developing countries, with dramatic increases particularly in China. These trends are related to patterns of industrialization and a shift in energy-intensive production away from North America and Europe to other rapidly industrializing regions, mainly Asia. Global coal-fired power capacity doubled between 2000 and 2018, to 2,000 gigawatts (Carbon Brief 2018). After declining from 2014 to 2016, coal production increased again in 2017 (International Energy Agency 2018). Coal’s share of the world’s energy mix was 27 percent in 2016 (International Energy Agency 2018). There are an estimated 1.1 trillion tonnes of identified coal reserves worldwide, which translates into another 150 years of coal use at current production rates (World Coal Association 2019).
Industrial point sources emit mercury and other pollutants (box 2-3). Because of an early expansion of coal-based manufacturing and energy generation, point sources in Europe and North America were the dominant contributors of mercury emissions to air from 1850 to the late 1900s (Streets et al. 2018). By 2015, however, the European Union (EU) and North America contributed only 6 percent and 2 percent, respectively, to global mercury emissions from these sources (see table 5.1). Instead, most mercury emissions from fuel combustion and other industrial point sources came from East, Southeast, and South Asia (62 percent), where China and India, by far, are the largest emitters. North and Sub-Saharan Africa were responsible for 9 percent, and Russia and other countries formerly part of the Soviet Union contributed 8 percent. Some mercury emissions are related to the production of exported goods. One estimate is that 33 percent of China’s mercury emissions and releases for the year 2010 were a result of production for export (Hui et al. 2016). Thus, the decline in North American and EU mercury emissions has been offset by increases elsewhere that are in part driven by consumption in these two regions.
Atmospheric Transport of Air Pollutants
Industrial point sources emit mercury and other air pollutants (box 2-3). The atmosphere transports air pollutants to nearby and remote ecosystems (box 3-3), where they harm people living near and far from industrial point sources (box 3-1). The influence of local air pollution on human health has long been recognized. For example, coal use in London and other British cities in the nineteenth century led to frequent episodes of dense “fog”—essentially coal smoke that turned the sky ash grey (Fouquet 2011). Extreme pollution events in cities such as London and Donora, Pennsylvania, in the 1940s and 1950s led to cardiovascular and respiratory problems and mortalities (Davis 2002; Jacobs et al. 2018). Awareness of atmospheric long-range transport of pollutants expanded with the acid rain issue in the 1960s and the recognition that substances like sulfur can travel up to thousands of kilometers, crossing national borders (Odén 1967; Odén 1976). People can be exposed to air pollutants like fine particulate matter at dangerously high levels when they breathe air in urban areas, but, as noted above, mercury is not present in ambient air in concentrations that cause health concerns for exposure via inhalation, even downwind of industrial point sources.
As we explained in chapter 3, mercury from industrial point sources is emitted in different forms, and the properties of these different forms determine how far they can travel through the atmosphere. Coal-fired power plants emit both elemental and oxidized (more soluble) forms of mercury. Some oxidized mercury is attached to atmospheric particulate matter. Mercury in its elemental form can travel worldwide, but mercury in oxidized form, including the fraction on atmospheric particles, largely deposits near to sources. Experts are still uncertain about the proportion of these different kinds of mercury emitted from different sources: the fraction of mercury emitted in each form from an industrial point source depends on the characteristics of the fuel, operational procedures, and the control technologies applied (Muntean et al. 2018). Emitted mercury, depending on its form, transports via the atmosphere to nearby or remote ecosystems (box 3-3), where it is deposited. Ecosystem processes then lead to production of methylmercury (box 3-3). Methylmercury in ecosystems, in turn, harms people (box 3-1).
Mercury deposition to ecosystems originates from a combination of local and far away sources. But it was not possible for a long time to attribute measured mercury concentrations to source regions because sophisticated mercury transport models and data on emissions, transport, and deposition were unavailable. Researchers had advanced computer modeling capabilities and conducted enough measurement studies on mercury transport by the early 2000s to be able to get a clearer scientific picture of mercury atmospheric transportation and deposition patterns. Several of the initial studies attempted to connect emission sources to remote concentrations for the first time; some used atmospheric models to calculate the percentage of deposition resulting from domestic sources, particularly in the United States (Seigneur et al. 2004; Selin and Jacob 2008). Other studies calculated intercontinental and interregional transport for different regions, including between Asia and North America (Travnikov 2005; Corbitt et al. 2011). Researchers also used the fraction of different mercury isotopes in environmental measurements to differentiate some of the mercury coming from coal combustion versus other sources (Sherman et al. 2015).
The relative contribution of industrial sources in any given region to mercury deposition in the same region is both uncertain and variable. Nearly all mercury deposition in the Arctic comes from beyond the region, but there are also a few sources within the Arctic that contribute (UNEP and AMAP 2015). In contrast, some models suggest that over half of the mercury deposited in Europe came from within the region, although estimates differ (Kwon and Selin 2016). Estimates for Asia are that 23 to 55 percent of mercury deposition comes from sources within the region (UNEP and AMAP 2015). One study calculated that 80 percent of nationwide deposition of mercury in the United States originated from sources in other parts of the world (Selin and Jacob 2008). Variation exists within regions as well: the percentage of domestically emitted mercury depositing to areas of the northeastern United States could reach as high as 60 to 80 percent, though many other US areas predominantly receive deposition that originates from outside North America (Seigneur et al. 2004; Selin and Jacob 2008). Steubenville, the city whose story begins this chapter, is one of the US locations that receive a large amount of deposition from nearby sources.
Pollution Control and Mercury Emissions
The operation of air pollution control devices captures mercury and other pollutants before they are emitted to the atmosphere (box 2-2). These control devices on industrial point sources often exist in the form of end-of-pipe technologies. One of the simplest examples of an end-of-pipe pollution control technology dates back to at least ancient Egypt and uses fabric, such as a piece of cloth or a bag, to filter out particles from an effluent stream (Billings and Wilder 1970). Starting in the nineteenth century, another action to mitigate the impacts of air pollution from industrial sources in urban areas was to build taller and taller smokestacks that were high enough to route the emission out of industrializing and expanding cities (Fenger 2009). Although higher smokestacks mitigated some of the pollution’s local effects on air quality and human health, they simply shifted the impacts of pollutants to other geographical areas.
Air pollution control technologies have become more efficient and effective over time. Many of these are still end-of-pipe technologies. More modern fabric filters made of advanced materials designed to optimize airflow were applied in the twentieth century to control emissions of sulfur as well as lead and arsenic (Billings and Wilder 1970). Electrostatic precipitator technology, in which an electrical current is used to attract and capture particles, emerged in the late 1800s, but its first larger-scale applications on point sources were in the early twentieth century (Parker 1997). Other technologies chemically treat or “scrub” the flue gas produced by the burning of fossil fuels to reduce harmful emissions, such as emissions of sulfur dioxide or nitrogen oxides. Flue gas desulfurization, during which sulfur dioxide undergoes a chemical reaction to remove it from the flue gas before being emitted into the atmosphere, began to be applied at large scale in the early 1970s (Lefohn et al. 1999). Other air pollution control techniques that are not installed at the end-of-pipe include processes to wash the coal before it is burned (UNEP 2016). This is often done to reduce sulfur dioxide emissions or to increase combustion efficiency, but the washing can reduce mercury content as well. More recent technologies that control mercury specifically include activated carbon injection, a process that can capture 90 to 98 percent of mercury emissions (Srivastava et al. 2006).
Different air pollution control devices capture different forms of mercury, and this has implications for how far emitted mercury can travel in the atmosphere. Some control technologies, especially those applied earlier on for controlling other air pollutants, preferentially capture more soluble (oxidized) or particulate forms of mercury that tend to travel regionally. For example, control equipment that captures particulate matter such as fabric filters and electrostatic precipitators also reduces emissions of particle-associated mercury. Similarly, flue gas desulfurization captures mercury in its oxidized form. This was the case historically in North America, and is also the case in contemporary application of mercury controls in rapidly industrializing economies such as China and India (Giang et al. 2015). More recent controls such as activated carbon injection can control elemental mercury emissions, which travel globally. The performance of modern air pollution control devices in capturing mercury depends on several factors, including the type of coal, operating conditions such as temperature and air-to-fuel ratio, and other components affecting the chemical reactions that mercury undergoes in the burning process (UNEP 2016).
The application of air pollution control devices reduces mercury emissions (box 2-3). Atmospheric mercury emissions in the United States decreased by nearly 80 percent from 1990 to 2011 because of the implementation of air pollution control technologies (US Environmental Protection Agency 2015). In the EU, mercury emissions in 2016 were 71 percent lower than they were in 1990 (European Environment Agency 2018). Mercury emissions have not always risen and fallen proportionally with coal use over time. David G. Streets and colleagues (2018, 133) note that the level of mercury releases “at any given time is determined by competition between production growth and technology improvement.” For example, coal use increased in the United States in the 1970s and 1980s, but its mercury emissions stayed constant. Increases in the number and output of new point sources in recent years have nevertheless outpaced the installation of air pollution control devices: global anthropogenic mercury emissions rose 20 percent from 2010 to 2015 (UNEP 2019).
End-of-pipe controls prevent mercury emissions to the atmosphere, but they do not change the total amount of mercury removed from fossil fuel reserves. The fraction of mercury emitted to the air globally out of the total amount of mercury in burned coal (including power generation and industrial production driven by coal) dropped from 77 percent in 1950 to 55 percent in 2010 (Streets et al. 2018). Because the amount of emitted mercury went down, the amount of captured mercury increased. Byproducts of combustion that contain captured mercury are sometimes reused in other industrial applications (box 2-2). Mercury-containing fly ash from a coal-fired power plant, for example, can be later reused. When fly ash is heated to high temperatures as a component in making cement, mercury can be emitted to the atmosphere unless appropriate control technology is present in the cement factory (Wang et al. 2014). Thus, if byproducts are not properly disposed of or used in environmentally safe ways, at least some of the previously captured mercury can be emitted from these industrial point sources (box 2-3).
The installation of air pollution control devices incurs costs to producers and consumers (box 2-1). For producers, those include the fixed capital costs of the equipment itself as well as variable operating and maintenance costs once the equipment is installed. At least some of these increased production costs are passed on to consumers in the form of higher energy prices and more expensive goods. Government regulations can help drive down pollutant control costs by providing incentives for technological innovation. This was, for instance, the case for the US federal cap-and-trade system for sulfur dioxide (Schmalensee and Stavins 2013), which led to lower costs for air pollution controls that also reduced mercury emissions. Some people, like the Steubenville resident who wondered whether cleaning up the air was worth the cost to jobs, may view costs of pollution control as a tradeoff against their environmental and human health benefits. Several studies that have attempted to monetize the benefits of mercury controls, as mentioned in chapter 4, find those benefits to be substantial (Trasande et al. 2005; Bellanger et al. 2013; Giang and Selin 2016; Zhang et al. 2017). In the US, such benefits have been found to be greater than economic costs of mercury reductions (Sunderland et al. 2018).
Interventions
Interveners such as national and local governments, industries, and international bodies have taken a number of intentional actions to address atmospheric emissions of mercury at varying scales. Figure 5.4 highlights interventions in the atmospheric system for mercury. First, we discuss standards that regulate mercury emissions through technological and other approaches targeting pollution controls and mercury emissions; emission-control approaches include developing, mandating, and optimizing relevant technologies (boxes 2-2, 2-3, and 3-1). Second, we examine mercury controls involving industrial production, focusing on efforts to reduce dependence on fossil fuels (box 3-2).

Intervention matrix for the atmospheric system for mercury.
Laws and Regulations Addressing Mercury
Laws address atmospheric mercury emissions in different ways. Some of the earliest mercury-specific regulations set by national and local governments were ambient air and environmental quality standards (box 3-1). Ambient air quality standards identify a maximum permitted concentration of mercury in air, and environmental quality standards introduce a maximum allowable mercury concentration in other environmental media such as water, soil, or fish. But these regulatory efforts on industrial point sources were not the first ones that led to the reduction of mercury emissions. Initial efforts that reduced mercury emissions in many countries instead took the form of legislation and regulations that were primarily developed for the purpose of controlling other air pollutants. Although air pollution controls implemented during the 1800s and much of the 1900s aimed to reduce atmospheric emissions of sulfur dioxide and particulate matter from industrial sources, they also captured mercury, even though the capture was largely unintended. Governments began specific interventions to address atmospheric mercury emissions in the 1970s.
National and local governments have set air emission standards to regulate the amount of mercury emitted from a particular source (box 2-3). Regulatory approaches can take several different forms. Emission limit values (ELVs) set the maximum allowable amount of mercury (or another pollutant) that can be emitted from an individual point source. National and local governments have also mandated the use of air pollution control devices on specific sources (box 2-2). These control devices are then deployed by industries (box 2-2). One specific type of technology-based approach is setting best available techniques (BAT). BAT is often defined as the techniques most effective to prevent or reduce emissions and releases, taking into account both economic and technical considerations. The use of best environmental practices (BEP) is defined as the most appropriate combination of environmental control strategies and measures for reducing pollution. Applying BEP, for example, can help ensure the appropriate management of mercury captured as a byproduct of coal combustion so that it is not reemitted elsewhere.
Policies and regulatory standards that both indirectly and directly reduce mercury emissions can be, and have been, strengthened over time. ELVs can be lowered, and the suite of technologies that constitute BAT can be made more stringent. Correspondingly, and often prompted by regulatory actions, engineers and industry have developed and deployed new and more effective devices and techniques for emission controls. Combinations of these techniques applied together can lead to different rates of mercury capture, and the performance of emission controls for reducing mercury emissions can be enhanced (UNEP 2016). Multiple pollutants including mercury can also be captured simultaneously as part of a broader approach to controlling air pollution. Studies point out that there is not one single approach or best solution for mercury control from stationary sources such as coal-fired power plants; the choice depends in part on plant-specific characteristics (Sloss 2008).
The United States adopted some of the earliest policies that specifically addressed mercury emissions. Regulations under the 1970 US Clean Air Act named mercury, along with asbestos and beryllium, to an initial list of regulated hazardous air pollutants (National Emission Standards for Hazardous Air Pollutants 1973). The US EPA established emission limits in 1973 for two types of mercury emission sources—mercury ore processing facilities and mercury cell chlor-alkali plants—but it did not address emissions from coal burning. The formulation and implementation of regulatory standards at the time were hampered by lack of scientific knowledge on emission sources and levels. The 1973 regulation listing mercury as a hazardous air pollutant notes: “Current data on the environmental transport of mercury do not permit a clear assessment of the effect of mercury emissions into the atmosphere on the mercury content in the aquatic and terrestrial environments” (National Emission Standards for Hazardous Air Pollutants 1973, 8825). Few actions on atmospheric mercury emissions in the United States were driven by concerns about deposition until more scientific data emerged two decades later.
The United States strengthened its mercury emission controls in the 1990s and into the 2000s. The US Clean Air Act Amendments of 1990 mandated controls on mercury emissions from waste incinerators. However, language on regulating mercury emissions from power generation in an earlier draft of the amendments was dropped in favor of a clause that merely called for the EPA to conduct further study before issuing regulations (Lee 1991). The amendments require a Maximum Achievable Control Technology approach (a type of BAT standard) to hazardous air pollutants whereby the EPA determines a technology-based standard by averaging the best-performing 12 percent of facilities. Yet, the EPA initiated no regulatory action on mercury emissions from the power sector at that time. In contrast, states in New England took further action on mercury emissions, also working together with eastern Canadian provinces on an action plan on mercury dating back to 1997 (Selin and VanDeveer 2005). This plan set aggressive goals that went beyond national commitments in both the United States and Canada at the time, aiming for a 50 percent reduction in regional mercury emissions by 2003 and a 75 percent reduction by 2010, which were also achieved (Smith and Trip 2005; New England Governors and Eastern Canadian Premiers 2011).
Environmental groups used legal means in the 1990s to pressure the US federal government to adopt regulations on mercury emissions from coal-fired power plants. This included litigation by the Natural Resources Defense Council (NRDC) and other advocacy groups to force the EPA to control such emissions under the Clean Air Act (Walke 2011a). This litigation led the EPA, following a protracted process and a settlement agreement, to determine in 2000 that regulation of mercury emissions from power generation was “appropriate and necessary” (US Environmental Protection Agency 2000). The Clean Air Mercury Rule, introduced in 2005, was an effort by the George W. Bush administration to meet the requirement to control mercury emissions from coal-fired power plants. Rather than mandating technology-based control standards, the administration proposed a cap-and-trade scheme similar to that used to reduce emissions of sulfur and nitrogen oxides. The administration argued that this would result in a more economically efficient outcome, and its relatively weak requirements were crafted with strong influence by the power generation industry (Walke 2011b).
Many US states and environmental groups argued that differential applications of control technologies to power plants under the Clean Air Mercury Rule would create mercury “hot spots” in areas such as those around Steubenville, resulting in harm to people who lived near power plants. Shorter-lived forms of emitted mercury travel a distance comparable to sulfur dioxide, for which cap-and-trade approaches were successfully implemented, but the fact that methylmercury is a toxic substance that bioaccumulates in ecosystems made the “hot spot” issue an effective argument. The US Court of Appeals for the District of Columbia Circuit in 2008 vacated the Clean Air Mercury Rule, finding that it did not adequately control mercury emissions based on requirements under the Clean Air Act. Under President Barack Obama, the EPA in 2013 finalized new Mercury and Air Toxics Standards that set technology-based limits on coal-fired power plants. These standards survived repeated court challenges by industry groups, and utilities invested in pollution control measures to comply with them by 2015. In late 2018, the EPA under the administration of President Donald Trump proposed to roll back these standards. The EPA reversed the “appropriate and necessary” finding in spring 2020 (Friedman and Davenport 2020).
Canada introduced federal standards related to mercury emissions in 1996 with the adoption of national guidelines on the use of wastes, including mercury-containing wastes, as supplementary fuels in cement kilns (Government of Canada n.d.). Two years later, the federal government set mercury emission guidelines for cement kilns. The federal government in 2000 formulated technology-based control standards for mercury emissions from smelting and roasting processes in non-ferrous metal production, and ELVs for mercury emissions from waste incineration (Canadian Council of Ministers of the Environment 2000; Canadian Council of Ministers of the Environment 2019). The province of Manitoba had adopted its own standards on waste incinerators two years earlier. The federal government set nationwide limits and provincial caps on mercury emissions from coal-fired power plants in 2006, consisting of overall mercury emissions goals as well as requirements for technology-based controls on new sources (Canadian Council of Ministers of the Environment 2006). Canada took these measures shortly after the United States had introduced the Clean Air Mercury Rule in 2005.
The EU adopted its first regulation to address atmospheric mercury emissions in the late 1980s: a 1989 directive on new waste incineration plants set ELVs for mercury. This directive was superseded by stricter regulations in the 2000 Waste Incineration Directive. Like the United States, the EU did not take action on mercury emissions from power generation in the 1990s, aside from capturing mercury as a side effect of controls that were mandated for other pollutants. The EU’s Industrial Emissions Directive from 2010 introduced technology-based standards to control mercury emissions from coal-fired power plants together with emission limits for waste incineration plants and cement kilns. Germany was the only EU member state that had specific mercury emission limits for power generation sources prior to the 2010 directive, which it originally set in 2004 as part of its large combustion plant ordinance. These standards were further revised in 2013 (Mayer et al. 2014). The revised German standard, however, is still roughly five times less stringent than the current US standard (Lin et al. 2017).
Some countries outside of North America and Europe also began to set mercury emission limits starting in the 1990s. For example, in a 1999 air pollution control law, the Philippines set mercury emission limits from industrial sources (Republic of the Philippines 1999). China, by far the world’s largest user of coal, established a technology-based standard for mercury emissions from coal-fired power plants in 2011 that was set at the same level as the German standard (Chen et al. 2017; Lin et al. 2017). This emission standard was easily met by most Chinese power plants with basic emission controls, and more advanced air pollution controls for sulfur dioxide and nitrogen oxides capture an even greater percentage of mercury in coal (Sloss 2012). Indonesia introduced mercury-specific emission standards for coal-fired power plants starting in 2020, but these were set at a modest level. Benefits from these control measures will be dwarfed by an increased use of coal, however, if coal use increases as expected over the next decade.
Some countries recently strengthened their legislation on mercury emissions, while others continue to rely on capturing mercury as a result of controls on other pollutants. South Korea strengthened its national mercury emission limits in 2010 (Sloss 2012). These limits, however, are less stringent than both the German and US standards (Sloss 2012). Japan amended its air pollution control act in 2015 to include a mercury emission control system, including technology-based standards (Government of Japan n.d.). India, the world’s second largest user of coal, is increasing its coal capacity without mercury-specific emission controls. Similarly, South Africa relies on technology-based controls for particulate matter, sulfur dioxide, and nitrogen oxide to reduce mercury emissions. This may result in a capture of 6 to 13 percent of mercury emissions by 2026 compared to a 2011 to 2015 emissions baseline (Garnham and Langerman 2016). However, an increase in coal use could result in a net increase in mercury emissions in South Africa. Russia is another country that lacks mercury-specific controls on coal-fired power plants, but has standards for non-ferrous metals production.
International bodies have developed guidelines for the use of air pollution control devices (box 2-2). Some mercury air pollution controls are included in regional agreements covering North America and Europe. Canada, the United States, and Mexico, acting under the Commission for Environmental Cooperation that was set up as part of the implementation of the North American Free Trade Agreement (NAFTA), adopted a joint action plan on mercury in 1997 with general commitments to address pollution (Commission for Environmental Cooperation 1997). A second phase of the action plan, launched in 2000, set an aspirational goal to reduce national mercury emissions by 50 percent from 1990 levels by 2006 (Commission for Environmental Cooperation 2000). The US and Canada both exceeded this goal. The parties to CLRTAP adopted a protocol on heavy metals in 1998 that entered into force in 2003 (Selin and Selin 2006). Parties to the protocol, which was made more stringent in 2012, include the United States, Canada, the EU, and a few other European countries. The protocol targets mercury emissions from large stationary sources and mandates that parties reduce their mercury emissions below their levels in 1990 (or an alternative year between 1985 and 1995). It sets ELVs and stipulates that parties apply technology-based controls in the form of BAT.
The existence of national and regional regulations and the availability of effective end-of-pipe control technology facilitated the decision to include industrial atmospheric emission sources in the Minamata Convention. The Global Mercury Partnership built on this information to produce a summary of available control technology for power plants, informing treaty negotiations (Krishnakumar et al. 2012). The Minamata Convention mandates the application of controls on new and existing emission sources in five different categories: coal-fired power plants, coal-fired industrial boilers, non-ferrous metals production, waste incineration facilities, and cement production. The regulatory approach is similar to the one under the CLRTAP heavy metals protocol. Parties must apply BAT, BEP, or ELVs to new point sources to control emissions, and where feasible reduce them, no later than five years after they join. Parties must also control, and where feasible reduce, emissions from existing point sources through BAT, BEP, or ELVs, or a multi-pollutant control strategy no later than 10 years after the treaty becomes legally binding for them.
The Minamata Convention largely leaves it up to each party to define BAT, BEP, and ELVs for stationary sources based their own socio-economic and technical context, but the treaty’s Conference of the Parties (COP) is tasked to develop technical guidance documents to assist this process. This decision was based on the recognition that some parties had already set their own (and sometimes diverging) standards. It was also a way to allow developing countries to initially adopt standards that may not be as stringent as in industrialized countries. As a result, different “best available” domestic standards will capture varying amounts of mercury. Countries may also formulate standards using different metrics. For example, the United States currently uses performance metrics (lb/Btu), while others including China and the EU apply concentration limits (μg/m3). In addition, the language in the Minamata Convention regarding “control, and where feasible reduce” mercury emissions from stationary sources represents a compromise that allowed for increases in coal use—and may, even with stringent application of BAT, result in increasing mercury emissions globally. Increased waste incineration, cement production, and mining may also result in higher mercury emissions despite the introduction of stricter emission controls.
Industry resistance to mercury emission controls is strong in some countries, and lobbying by industry can influence government positions, including on joining the Minamata Convention. Australia’s decision so far to not ratify the Minamata Convention is attributed to opposition from the fossil fuel sector to stricter controls on mercury emissions (Bramwell et al. 2018). Australia lacks a federal standard on mercury emissions. Some Australian states have set no mercury-specific standards on coal-fired power plants while other states have only lax ones—some plants in New South Wales are allowed to emit 33 times more mercury than they would under the German and Chinese standards and 666 times more mercury than if they operated under the US standard (Environmental Justice Australia 2017). New Zealand, which similarly is not a party to the Minamata Convention, also has not set any mercury-specific emission standards. New Zealand’s largest energy retailer, Genesis Energy, announced in 2015 that it would close the country’s last two coal-fired power plants in 2018, but this decision was later revisited, and coal burning is expected to continue until at least 2025 and maybe longer (Coughlan 2018).
Scientific data show that stringent emission controls on industrial sources can reduce the environmental and human health burden of mercury emissions. Domestic and regional controls through the 1990s and 2000s reduced atmospheric mercury emissions, concentrations, and deposition near regulated point sources in North America. A study of mercury air concentrations from two sites in Vermont and New York found declines of a few percent per year from 1992 to 2014, with faster declines in the beginning and then slowing down or leveling off (Zhou et al. 2017). The study attributed these declines to reduced mercury emissions from regional sources, and correlations with declines in sulfur dioxide indicate that reductions in emissions from coal-fired power plants contributed to these reductions in atmospheric mercury concentrations. Declining emissions are also linked to reductions in mercury concentrations in fish (Hutcheson et al. 2014). Another study reported that mercury concentrations in rainfall declined overall between 1998 and 2005 in the northeastern United States (Butler et al. 2008). Much of this decline was attributed to the domestic controls on municipal waste incineration that were implemented in the 1990s.
Measurement studies carried out outside of North America also find declines in atmospheric mercury emissions, concentrations, and deposition in some regions starting in the 1980s, concurrently with the introduction of domestic and regional emission controls. Records from a few long-term stations in Europe show that atmospheric mercury concentrations declined substantially from 1980 to 1993 together with regional mercury emission reductions (Tørseth et al. 2012). Atmospheric concentrations at the monitoring site in Sweden with the longest time series of data declined about 60 percent between 1980 and the early 1990s (Tørseth et al. 2012). Another study showed a 7 percent yearly decline in atmospheric concentrations at Wank Mountain in southern Germany between 1990 and 1996 (Slemr and Scheel 1998). In addition, scientists have attributed measured decreases in atmospheric mercury concentration in the early 2010s in parts of China to declining mercury emissions from nearby domestic sources (Tang et al. 2018). Declines in mercury emissions also have longer-term benefits, as the amount of mercury available to remobilize and cycle later on is reduced (see chapter 3).
Trends in mercury concentrations and deposition at other sites, including those farther away from point sources, are less consistent. Measured mercury deposition in rainfall has increased at some sites—even in the United States where national emissions have declined—complicating interpretation of overall trends (Butler et al. 2008; Weiss-Penzias et al. 2016). Variations in weather patterns also affect trends in mercury deposition (Giang et al. 2018). Scientists debate whether observed atmospheric concentration trends in past decades at remote monitoring sites in North America and Europe—as opposed to those directly downwind of controlled sources—reflect regional or global emission declines (Zhang et al. 2016; Obrist et al. 2018). Global mercury emissions were relatively stable between 1980 and 2000, and increased slightly after that, but their regional distribution changed (Streets et al. 2017). The timescales of these changes are also affected by the cycling of historical emissions in the oceans (Soerensen et al. 2012). Some evidence suggests that concentrations in the Southern Hemisphere may have begun increasing again after 2007 (Martin et al. 2017). The more recently reported 20 percent increase in global emissions between 2010 and 2015, driven by emissions in Asia, may also affect future trends in atmospheric concentration and deposition.
Efforts to Reduce Dependence on Fossil Fuels
International bodies, national and local governments, and industries influence fossil fuel use in different ways (box 3-2). These types of interventions are often associated with efforts to address carbon dioxide emissions, such as those under the Paris Agreement. Substituting the underlying activity leading to pollution differs from efforts to reduce pollution that increase the efficiency of coal-fired power plants and other stationary sources or that apply end-of-pipe controls to capture emissions. Moving the world’s energy system away from fossil fuels—necessary from a climate change perspective—would prevent mercury mobilization from this source entirely, including air emissions and releases to land and water. It would also eliminate the need to properly dispose of captured mercury. Future mercury emissions from combustion and industrial production will largely be determined by how much coal is used, in Asia in particular, and the implementation of pollution controls in countries with the highest coal use (Streets et al. 2009). The benefits of climate policy for mercury pollution are thus likely to be largest in places that rely heavily on coal (Rafaj et al. 2014).
Few policy-makers so far have shown an appetite for linking mercury reduction efforts with the more contentious politics of fossil fuel reductions. China and India stressed the importance of coal burning for economic development during the Minamata Convention negotiations, and India linked coal use to its push for greater electrification. Negotiators from the United States and the EU who were pushing for action on atmospheric emissions also noted the continuing need for coal burning in some places. Moving away from coal completely would eliminate its associated mobilization of mercury, but state-of-the-art, end-of-pipe technologies can reduce atmospheric mercury emissions by 90 percent or more. China, India, and Indonesia are projected to significantly increase their use of coal from today’s levels despite their climate change commitments under the Paris Agreement (Edenhofer et al. 2018). In these and other countries, the control technologies that address mercury can be implemented far more cheaply than large-scale changes to a fossil fuel–free energy supply. But provisions under the Paris Agreement and the Minamata Convention can be synergistic, as they can result in reductions of mercury from different sectors (Mulvaney et al. 2020).
Mandates to apply end-of-pipe technologies can either extend or shorten the lifetime of polluting sources. With less environmental burden due to the application of pollution control technology, more modern coal-fired power plants may remain in the energy system for longer than they otherwise would. In China, for example, new coal-fired power plants are being built that accommodate state-of-the-art, end-of-pipe pollutant control technology to capture mercury and other air pollutants (but not carbon dioxide), reducing reasons to close them down. In contrast, technologies can shorten the lifetime of some, especially older, power plants. In the run-up to the Mercury and Air Toxics Standards taking effect in 2015 in the United States, anticipation of future regulatory actions reportedly prompted the retirement of several older coal plants, whose operators cited the cost of compliance with federal regulations as a reason for their premature closure (Storrow 2017). Mercury standards are also thought to have been a factor in the closure of coal-fired power plants in Canada, or their conversion to other fuels such as natural gas or wood (Sloss 2012).
Insights
The story of air pollution in Steubenville at the beginning of this chapter illustrates both the ability and difficulty of addressing mercury emissions to the atmosphere from the large point sources that are integral to industrial and energy production in contemporary societies. In this section, we draw insights from the interactions and interventions that characterize the atmospheric system for mercury. First, atmospheric mercury connects places across the world not only through its environmental transport but also through connections facilitated by socio-economic factors and institutions. Second, actions to address mercury have largely involved incremental transitions, but these have had substantial benefits for human well-being in particular places. Finally, efforts to govern mercury emissions should consider their multi-scale, complex nature.
Systems Analysis for Sustainability
Understanding how mercury emissions to air disperse in the environment and impact human well-being requires paying attention to local as well as long-range dynamics. Mercury from industrial point sources is emitted in different forms, which can deposit either nearby or far away. Mercury emissions that deposit regionally in the near term can be remobilized and cycle further through the environment, later affecting other populations far away in time and space. End-of-pipe control technologies change both the total amount of mercury emitted and the relative fraction of emissions in shorter- and longer-lived forms. Mercury captured in air pollution control technology creates mercury storage needs, and this mercury can be further distributed when using coal-combustion byproducts in industrial processes. The introduction of stricter mercury-specific pollution controls primarily in North America and the EU beginning in the late 1900s resulted in emission reductions. In contrast, in Asia, mercury emissions from coal burning and industrial production continue to rise.
A systems perspective on atmospheric mercury emissions from industrial point sources should take into account how the locations and levels of such emissions have changed over time, as the provision of energy and consumer goods has increased worldwide. Interventions to reduce mercury emissions in North America and Europe have accomplished their goals through setting technology-based emission controls from large point sources. This allowed the production of energy and consumer goods to continue providing benefits to human well-being, without altering underlying processes of coal burning and industrial manufacturing. Governments in other regions of the world to date have intervened less to target mercury emissions from industrial point sources. This allows mercury emissions to continue in these regions, and also shifts the relative importance of different regions for global emissions. Mercury emissions between 2010 and 2015 increased in all regions outside North America and Europe, but Asian mercury emissions are particularly high. China has introduced mercury-specific emission controls, but India has yet to move in this direction.
Mercury travels globally, but global average concentrations alone do not reflect the harms posed by mercury to the environment and human well-being. This is because the different forms of mercury emitted to the atmosphere from large point sources can either disperse globally or deposit regionally and locally. Ecosystems downwind from coal-fired power plants and other industrial sources may experience high levels of mercury deposition, leading to exposure to nearby populations. These “hot spots” of mercury emissions and deposition are a political issue of importance to human health and the environment in many regions of the world. Trends in environmental concentration and deposition of mercury track national and global emission trends in some places, but not in other locations. Even in the United States, where national mercury emissions declined dramatically over the past three decades, there remain places where measured mercury deposition increased during the 2000s. As a result, studies of environmental and human health impacts of mercury emissions from large point sources must consider local, national, regional, and global trends in emissions and deposition simultaneously.
Sustainability Definitions and Transitions
The underlying production and consumption processes resulting in atmospheric mercury emissions lead both to benefits and harms to human well-being that have been valued differently in different places over time. This complicates efforts to define what constitutes progress toward greater sustainability. The production of fossil fuel–based energy and industrial goods has had substantial material and health benefits to people for over 250 years. At the same time, mercury that was emitted into the atmosphere through these processes has damaged human health and the environment, and created a legacy of long-lasting environmental pollution. The burning of fossil fuels and industrial manufacturing, and associated mercury emissions, continue decades after mercury was identified as a major air pollutant. Some governments and private sector actors have attempted to reduce the amount of mercury that is emitted into the atmosphere together with other air pollutants, but governments in other countries do not view mercury emissions to be dangerous enough to warrant the economic costs of mandating pollution controls. As a result of this and other factors, the negative impacts of mercury emissions are unevenly distributed across the planet and influence future generations.
Most national and regional action on mercury emissions from large point sources have relied on an incremental introduction of stricter pollution control technologies rather than on seeking shifts in underlying activities of coal burning and industrial production. Such incremental transitions toward reducing mercury emissions facilitated the continued burning of fossil fuel and industrial manufacturing with fewer environmental and public health burdens. These actions had substantial benefits in regions that took action, but mercury remains a global problem. The Global Mercury Partnership and the Minamata Convention largely codify this vision of a technology-based approach to addressing atmospheric mercury emissions at the global level. Neither the Global Mercury Partnership nor the Minamata Convention has encouraged the deeper systemic change of rapidly phasing out the use of fossil fuels. Pollution prevention has reduced the quantity of mercury that would otherwise have entered the atmosphere, with both near-term and long-term benefits, but the limits of a technology-based approach to controlling mercury emissions are evident by the fact that global mercury emissions still grew in the early 2010s.
The atmospheric mercury system shows that incremental, technology-based change and long-term systemic change can be both conflicting and synergistic, sometimes simultaneously. Technology-based approaches can, under some conditions, lead to more fundamental change. Mercury standards in the United States and Canada accelerated the closing of at least some coal-fired power plants that were too old or unprofitable to warrant the addition of new end-of-pipe technology. This illustrates how dynamics that lead to gradual progress toward sustainability and to more disruptive change can be simultaneously present, and potentially reinforcing. In the near-term, however, there is a potential for tension between policies that support incremental change and those that call for quicker and more radical change. The introduction of emission standards and control technology on industrial sources can mitigate mercury emissions and other local pollution problems, but the resultant longer operability of modern coal-fired power plants can leave the longer-range effects and broader implications of carbon dioxide emissions on climate change largely unaddressed.
Sustainability Governance
Efforts to reduce atmospheric mercury emissions rely on creating and adapting institutions that fit the multi-scale nature of the problem. Addressing only national and regional mercury emissions—illustrated by government-led actions in North America and Europe in the 1990s and early 2000s—can lead to emission reductions, but both regions still have problems with mercury deposition from the long-range transport of mercury emissions from sources located elsewhere. This shows the importance of a global governance approach to controlling mercury emissions. The Minamata Convention covers mercury emissions globally, but also attempts to balance its global scope with regional and national flexibility in formulating air pollution laws and setting mercury emission standards. It requires all parties to control, and where feasible, reduce their mercury emissions from certain categories of point sources, but it is largely left to the individual parties to define their own standards and approaches to mercury and multi-pollutant controls. International cooperation helps diffuse knowledge about regulatory practices and different air pollution control techniques.
A range of regulatory strategies can reduce mercury emissions. Multi-pollutant control technology can increase the efficiency of pollutant capture and reduce implementation costs. Some end-of-pipe technologies allow countries to control mercury emissions using strategies that are primarily designed to address other air pollutants such as fine particulate matter. Taking advantage of material synergies can be politically attractive in some cases, but not in others. Reductions in mercury emissions from industrial point sources during the twentieth century came as an unintended side effect of efforts to reduce emissions of other pollutants. But links between addressing mercury emissions from coal-fired power plants and the more politically contentious issue of climate change mitigation create governance challenges. Reducing mercury emissions from industrial sources is both technically feasible and politically attractive in many countries across the world, but phasing out coal is less so. Countries decided during the negotiations of the Minamata Convention that it was preferable to have a treaty that merely mandates end-of-pipe measures for large stationary sources without any further restrictions on coal burning than to have no mercury treaty at all.
It is difficult to assess the ultimate effectiveness of governance efforts to protect the environment and human health from mercury emissions from large point sources. Mercury emissions from these sources can decrease due to factors other than regulatory controls, including market-driven changes in coal burning and industrial manufacturing. Variations in weather patterns and ecosystem processes furthermore make interpreting mercury concentration trends in the environment difficult. This affects the ability to monitor the impacts of implementation and compliance with pollution standards. Mercury captured by pollution control technology can also later be discharged into the environment through reuse in other industrial manufacturing processes unless there are proper standards in place. In the absence of a large societal shift away from fossil fuels—whether driven by economic factors in energy markets, by concerns about air pollution and climate change, or by a combination of those factors and concerns—the greater application of technology-based controls addressing mercury emissions as mandated by the Minamata Convention remains critical to protecting human well-being.
Mercury, the winged messenger god, conveys a mixed message in terms of efforts to control mercury emissions and deposition. Industrialization resulted in emissions of mercury to the atmosphere from coal burning and other point sources. Some early efforts to address other pollutants from these sources also reduced mercury emissions, but governments have taken specific actions targeting mercury emissions only over the past few decades. Regulatory efforts to control emissions of mercury and other harmful pollutants to date have focused on technology-based approaches that have both near-term and long-term environmental and human health benefits. Mercury emissions have declined in North America and Europe, but emissions have grown elsewhere, particularly in Asia. The geographical origin of emissions and their local impacts have thus shifted, but atmospheric transport carries much mercury across borders. Future policy efforts that focus more aggressively on eliminating fossil fuel use by restructuring energy systems could ultimately prevent industrial mercury emissions at their source.