6 Assets and Liabilities: Mercury, God of Commerce
Those who study environmental science and governance are familiar with a historical evolution common to many substances: they are at first highly valued by society, then progressively seen as liabilities through their societal and environmental distribution, as their dangers to human and ecosystem health become increasingly appreciated. Useful properties of such substances, including mercury, supported scientific and technological advances and provided many other social and economic benefits to people and societies. However, some substances like mercury also caused severe human health damages in occupational settings and through their local and global dispersion in the environment. Changes in perceptions of hazardous substances, and societal responses to their dangers, occurred at different times and in different ways in places across the globe. Understanding the benefits and harms that stem from the intentional uses of hazardous substances is important for better managing societal uses of materials toward greater sustainability.
Daniel Gabriel Fahrenheit constructed the world’s first mercury thermometer in 1714 in Amsterdam. This groundbreaking invention was a significant improvement over existing, much less accurate, temperature-measuring devices. More than a century earlier, in the 1590s, Galileo Galilei had developed a thermoscope, or air thermometer, as a crude way to measure changes in ambient temperatures (Camuffo and Bertolin 2012). The thermoscope consisted of a glass tube that was submerged in water at one end; the rise and fall of the water line indicated relative atmospheric temperature differences. This construction of the thermoscope built on the fact that air expands when it is heated, a scientific discovery made by Philo of Byzantium in the third century BCE. Galileo was not able to measure temperatures against a fixed degree scale, however, and his rudimentary thermoscope was largely unreliable because its measurements varied with atmospheric pressure (Rasmussen 2012). The invention of the first modern thermometer is often credited to Santorio Santorio, who applied a simple measurement scale to Galileo’s thermoscope (Middleton 1966).
The introduction of fully sealed liquid-based thermometers using alcohol—developed beginning in the 1640s by several people including Evangelista Torricelli and the Grand Duke of Tuscany Ferdinand II—represented another important technological step forward (Wright and Mackowiak 2016). But these alcohol-based thermometers did not solve the fundamental problem: a lack of measurement precision and comparability. This all changed with the invention of the mercury-in-glass thermometer, which allowed Fahrenheit to conduct and record measurements with much more accuracy and detail. With his new thermometer, Fahrenheit was also able in 1724 to devise the first standardized scale to measure temperatures: the Fahrenheit scale where water freezes at +32 degrees and boils at +212 degrees. Using the new mercury thermometer, Anders Celsius in 1742 invented an alternative scale that reversed the hot and cold ends and set the water boiling point at 0 degrees and the water freezing point at +100 degrees. Proposals to invert this scale came a year later, resulting in the contemporary Celsius scale where water freezes at 0 degrees and boils at +100 degrees (Bolton 1900).
The story of the invention of the mercury thermometer is one example of how mercury has been an important component in a broad range of consumer products and production processes. Electronic thermometers are increasingly replacing the basic mercury thermometer, but the mercury thermometer was the dominant technology for nearly 300 years because of its simplicity, precision, and reliability. It was successfully used to advance scientific knowledge in meteorology and climatology, to develop new manufacturing techniques that required the ability to read accurate temperature measurements, and to diagnose and treat illnesses in patients all over the world. Many other commercial products and production methods also relied on the unique properties of mercury to provide a wide range of social and economic benefits. But as the chapter title, referring to Mercury, the god of commerce, indicates, societal attitudes about mercury fundamentally shifted over time, from seeing the element as a valuable asset that can be beneficially used, to viewing it as a liability whose intentional uses should be regulated and eventually phased out altogether.
Many governments have introduced increasingly strict controls on the use of mercury, both in consumer products and production processes. Their efforts to phase out mercury uses mirror other attempts to regulate the commercial use of toxic substances. Some of these regulated substances are naturally occurring elements like mercury, whereas others are synthesized in laboratories. Well-known examples include other heavy metals such as lead and cadmium and organic chemicals like DDT, PCBs, and CFCs. Plastics—a building block of modern consumer societies and now ubiquitous in landfills and oceans—are in the process of a similar transition. Other substances that societies currently place high value on—such as rare earth elements—might become the next generation of liabilities. Continuing uses of mercury and other hazardous substances may be warranted in some applications, at least in the short term, where decision-makers judge that societal benefits outweigh risks and liabilities. Ultimately, these decisions reflect broader issues of how societies identify and govern risks from materials, which vary in their utility as well as their hazard.
In this chapter, we examine intentional uses of mercury in products—excluding medicine, which we discussed in chapter 4—as well as in production processes, and societal efforts to reduce and eliminate such use. In the section on system components, we outline the human, technical, and environmental components that influence the use of mercury in products and processes, and the institutions and knowledge that surround these uses. We cover in the interactions section how the development of technologies for mercury-added products and processes led to social and economic benefits as well as negative environmental and human health consequences. We discuss efforts to reduce and eliminate mercury uses in products and processes, and address as well the negative consequences of related mercury discharges, in the section on interventions. In the final section on insights, we discuss how mercury’s benefits and costs to different populations were affected by system interactions over time, how largely incremental transitions reduced or eliminated mercury in products and processes, and how policy efforts can more effectively govern products and processes across scales.
System Components
Mercury that has been used in consumer products and production processes originated from two main sources: primary mercury mining and the reuse of mercury already in commerce. Of the 1 million tonnes of elemental mercury estimated to have been extracted from cinnabar and other ores since the year 1500, roughly half was used in a multitude of products and processes, while the other half was used in gold and silver mining (Hylander and Meili 2003). Much commercial mercury has been recycled and reused, sometimes multiple times, in other products and production processes. This means that some of the mercury in products and processes has cycled through society for long periods. The present-day main human, technical, environmental, institutional, and knowledge components for the products and processes system for mercury are summarized in figure 6.1.

Components in the products and processes system for mercury (referenced in the text in italic type).
Humans have been producers and consumers of mercury-added products and other goods made using mercury for millennia. Globally, annual use of mercury in commerce peaked in 1970, associated with a peak in annual mercury mining of 10,000 tonnes (Hylander and Meili 2003). Commercial mercury demand has declined since then, but mercury is still used in a variety of mercury-added products as well as other goods made using mercury. An estimated 2,040 to 3,600 tonnes of mercury were consumed in mercury-added products and production processes in 2015 (UNEP 2017). This estimate excludes mercury use in artisanal and small-scale gold mining (ASGM), which can also be considered a process-based use (we address that use separately in chapter 7). The term “mercury consumption” refers to the mercury content of all mercury-added products used, as well as the gross mercury input into all industrial processes during a given year. Production processes consumed 46 percent of this non-ASGM mercury; 44 percent was used in a variety of products, including batteries, measuring and control devices, lamps, and electrical and electronic devices; and the remaining 10 percent was used for dental amalgam.
Human uses of mercury in products and processes relied on the availability of mercury in geological reservoirs as well as knowledge about the properties of mercury and mercury-based product development and production techniques. Some of the earliest uses of cinnabar, connected to individual and societal beliefs, had religious and mystical connections in Asia, Africa, and Latin America (Mahdihassan 1985). Vermillion, a pigment made from grinding cinnabar powder, was applied to preserve human bones in graves from the Neolithic period in Spain in 3000 BCE (Martín-Gil et al. 1995). Cinnabar, which has a deep red hue, was also used as a coloring agent. Asian uses of “Chinese Red,” a cinnabar-based color for painting vases made from lacquer, go back at least to the first millennium BCE (Gettens et al. 1972). The Roman author Pliny the Elder (23–79 CE) noted that “the ancients” used to paint with cinnabar that was “adulterated by the agency of goats’ blood, or of bruised sorb-apples” (Pliny the Elder n.d., chapter 32: Quicksilver). Romans used mercury as an ingredient in makeup and other beauty products. For example, women used cinnabar as a rouge to heighten the color of their cheeks (Stewart 2014). People today use skin-lightening creams that contain mercury.
Alchemy, an ancient branch of natural philosophy and the forerunner of modern chemistry, focused on ways to improve, purify, or otherwise transform certain materials; mercury was central to alchemical concepts and work, and alchemists were the ones who gave the name quicksilver to mercury (Goldwater 1972). Attempts to unify the physical world linked the sun, the moon, and the five nearer planets to seven metals, including mercury, more than 2,000 years ago in Europe (Crosland 2004). Alchemy included a long-standing search for the philosopher’s stone—an elusive but potentially very valuable substance capable of turning base metals such as mercury into much more valuable gold (Goldwater 1972). Alchemical efforts continued at major universities well into the 1900s. As late as 1924, two chemists at Berlin Technical University claimed to have turned mercury into gold (Anonymous 1924). Alas, the search for the philosophers’ stone and efforts to change other metals into gold have proved unsuccessful.
Workers and employers in mercury-related sectors have used mercury and mercury compounds extensively. Mercury use in the silver-plating industry dates back at least to Roman times (Pliny the Elder n.d.). The use of mercury in production processes expanded beginning in the fourteenth century, first in Europe and then elsewhere (Svidén and Jonsson 2001). These early mercury uses included the production of mirrors and hats. Mercury use also grew in laboratories as science progressed. Industrial applications of mercury increased in the nineteenth century. Mercury fulminate was used in percussion and blasting caps starting in the 1800s. Mercury was a component of lamps beginning in the 1800s, and of batteries in the second half of the twentieth century. Mercury compounds were used in agricultural pesticides beginning in the 1910s (Novick 1969). In the 1900s, the pulp and paper industry added phenylmercury acetate to prevent the growth of fungi in the pulp during manufacturing, and to protect wood pulp during storage and shipment (Löfroth and Duffy 1969; Novick 1969). Phenylmercury was also applied as a biocide in indoor and outdoor paint (including in antifouling paint for ships).
The chemicals industry developed several different production processes that involved mercury to transform extracted fossil fuels such as coal and oil to make societally important high-volume chemicals. These included vinyl chloride monomer (VCM, a building block of plastic polyvinyl chloride, or PVC), acetaldehyde (an intermediate in the production of chemicals and plastics), and polyurethane (polymers used in many products). The chemicals industry also used mercury in processes that produced chlorine and alkali (caustic soda and sodium hydroxide used to make soap, textiles, and chemicals) from brine (sodium chloride solution). In the mid-twentieth century, mercury was used to make enriched lithium-6 for the development of thermonuclear weapons. In the nuclear power sector, lithium-7—a byproduct of mercury-based lithium-6 production—is a key component in the fluoride cooling systems of molten salt nuclear reactors developed in the 1950s.
Mercury that is no longer in commercial use either exists as mercury in stockpiles and landfills or has been discharged into the environment. Industrial point sources of mercury discharges have historically been a large source of releases of mercury to land and water as well as emissions to air. Some of the mercury releases first entered ecosystems near mercury uses. Mercury from industry, agriculture, and leaks and wastes from mercury-added products have combined to create a large number of contaminated sites. It is estimated that there are currently over 3,000 mercury-contaminated sites in different parts of the world (Kocman et al. 2013). Releases of mercury have created health risks to some people living near mercury discharges or contaminated sites. In addition, a portion of the discharged mercury that was used in products and processes has entered the atmosphere, where it can travel long distances before depositing in ecosystems far from mercury uses. As a result, people living far from mercury discharges are also exposed to mercury in the environment that originates from its use in products and processes.
Mercury’s use in a growing number of products and processes depended heavily on the development of knowledge of its properties. As markets for mercury-added products and goods made using mercury diffused these products and goods throughout societies, knowledge began over time to include information about negative environmental impacts from mercury discharges as well as health impacts from mercury exposure. The formulation of labor standards was facilitated by knowledge of health protection techniques for workers. Governments also adopted national and local laws and regulations on mercury uses and discharges. Uses of and human exposure to mercury were reduced through new knowledge of mercury-free product development and production techniques, especially since the 1970s. The development and commercialization of alternative products and processes that did not rely on the use of mercury have had a large impact on mercury markets by significantly reducing the demand for mercury. At the international level, the Global Mercury Partnership supports the further development and introduction of mercury-free alternatives, while the Minamata Convention mandates reductions and phaseouts of mercury use in products and processes.
Interactions
Intentional uses of mercury in consumer products and production processes have had both positive and negative influences on human well-being. Figure 6.2 shows interactions in the products and processes system for mercury: we have selected two interactions (the items in bold type in boxes 2-1 and 2-3) to focus on in this section; we then trace the pathways that influence them, which we summarize in figure 6.3 (where the bold boxes correspond to the selected interactions). First, mercury-added products and other goods using mercury provide benefits for producers and consumers but also harm them (box 2-1), and mercury uses affect and are affected by market dynamics and resource availability (boxes 3-2, 2-2, 1-1, and 1-2). Second, mercury-added products and industrial point sources discharge mercury into ecosystems (box 2-3), where it is transported and transformed into methylmercury and subsequently affects people (boxes 3-3, 3-1, and 1-3).

Interaction matrix for the products and processes system for mercury.

Pathways of interactions in the products and processes system for mercury. Bold boxes indicate focal interaction for each subsection.
Commercial Mercury Benefits and Harms
Mercury-added products and other goods made using mercury have provided benefits to as well as harmed people (box 2-1). Primary mining of cinnabar ores provided much of the mercury for early uses in products and processes (box 3-2). Mercury has been mined in all regions of the world, but much extracted mercury from cinnabar during preindustrial times came from a smaller number of mercury mines (see chapter 3). As the commercial demand for mercury in the chemicals industry and other sectors grew in industrial times, recycling of mercury from its original use became an increasingly important source of supply for both domestic use and trade. Mercury in production processes influences quantities of mercury in commerce and the amount of mercury stored in stockpiles (box 2-2). The gradual closing of previously large mercury mines coupled with the US and European Union (EU) elemental mercury export bans that were adopted in the 2000s made recycling and reuse a more important source of commercial mercury. At the same time, the amount of excess industrial mercury grew as industries switched to mercury-free manufacturing alternatives, which increased the amount of mercury in stockpiles in many countries.
Changes in the supply and demand for commercial mercury have been influenced by producers and consumers interacting in socio-economic systems (box 1-1). Producers and consumers for thousands of years have sold and bought mercury-added products and other goods made by using mercury (box 1-2). In addition to mercury’s use in traditional medicine (see chapter 4), ancient texts suggest that mercury was used in magico-religious rituals in China and India going back millennia. Mercury was important in Hinduism, including as a representative of the seed of Lord Shiva, and it was also discovered in Egyptian tombs (Masur 2011). Aristotle wrote in the fourth century BCE about the use of mercury in religious ceremonies (Nriagu 1979). In Caribbean religions with African roots such as Santeria, Palo, and Vodou, sprinkling mercury inside homes or mixing it into perfumes, lotions, or soap and water for ritualistic purposes is thought to bring good luck and ward off evil spirits (Wendroff 2005; Newby et al. 2006; Wexler 2016). Some of these practices continue in the Caribbean and in other countries where followers have relocated, including the United States (Yehle 2011).
Many early commercial mercury uses were associated with social status and affluence. Cinnabar imported from Spain and India was used in ancient Rome, where cinnabar-containing products were scarce and associated with prosperity (Stewart 2014). The expanded use of mercury in manufacturing that began in the fourteenth century not only increased the amount of mercury in commerce, but also solidified the metal’s links to prominence and wealth. Processes involving mercury were used to produce relatively expensive goods such as large mirrors and fur hats, whose ownership conferred social status, as only people with financial means could afford them. Mercury-based manufacturing techniques also provided economic advantages for producers. The technique of using mercuric nitrate in the fur-felting process in hat making, developed in France, was initially kept a trade secret because it helped French hat makers corner the market for a superior and highly desirable product. As the knowledge of how to use mercuric nitrate spread, many hat makers in other countries switched over to the new mercury-based production method (Svidén and Jonsson 2001).
Mercury and mercury-added products were important to the development of new scientific knowledge and capabilities that had societal benefits, particularly beginning in the seventeenth century. As Leonard J. Goldwater argues: “Without mercury some of the most significant advances in chemistry would have been delayed for years and possibly for centuries” (Goldwater 1972, 98). Experiments using mercury and mercury-added instruments facilitated the discovery of more than 20 chemical elements. In perhaps the most important of these discoveries, Joseph Priestly and Carl Wilhelm Scheele, in the mid-1770s, independently used mercury to identify oxygen—also referred to as “fire air”—which represented a major turning point in the history of modern science. Mercury-containing instruments also facilitated the development of dialysis and osmosis. The mercury thermometer, described at the beginning of this chapter, advanced both meteorology and medicine. The mercury barometer, invented by Evangelista Torricelli in 1643, greatly enhanced the ability to accurately measure atmospheric pressure (Middleton 1963; West 2013). In 1896, Scipione Riva-Rocci revolutionized medicine when he introduced the mercury-based sphygmomanometer for measuring blood pressure by wrapping an inflatable cuff around the upper arm (Roguin 2005).
Mercury continued to provide benefits to more modern societies. Mercury fulminate in blasting caps, including those invented by Alfred Nobel in the 1860s, helped build roads, bridges, and dams. Mercury was also a key component in the development of lighting technology. E. H. Jackson took out the first patent for a mercury-containing lamp in London in 1852, building on scientific discoveries that mercury could produce light when vaporized in an electric arc (Perkin 1911). Commercialization of this technology began in 1901 with a design by the engineer Peter Cooper Hewitt, whose company was acquired later by the General Electric Company (Cleveland and Morris 2014). Higher-intensity mercury lamps were introduced in 1934, and their efficiency and long life made the mercury-vapor lamp useful in public lighting, including for streets and highways (Freeman 1940). Mercury lamps were also used to light the large workspaces required for airplane factories and hangars (Anonymous 1945). Mercury was a key component in the first commercially viable fluorescent lamp, patented in 1926 (Cleveland and Morris 2014), and continues to be used in fluorescent bulbs and compact fluorescent lamps (CFLs). In addition, mercury was added to neon tubes to produce a blue light.
Mercury-containing batteries were first used in the 1940s. Samuel Ruben, the cofounder of Duracell, submitted a patent in 1945 for a new battery containing mercury (Ruben 1945). In zinc–mercuric oxide batteries, mercury is part of the electrode reaction (Naylor 2002). Using mercury allowed for the production of a dry cell battery that had a high capacity-to-volume ratio and could be stored under tropical conditions, criteria that were important to the US military during World War II (Ruben 1947). Mercury was also added to other kinds of batteries to enhance their performance. In small button-cell batteries, including alkaline manganese oxide batteries as well as zinc air and silver oxide batteries, mercury prevented the buildup of hydrogen gas from zinc corrosion (Northeast Waste Management Officials Association 2010).
The development of mercury-based production processes in the chemicals industry during the 1900s helped firms to produce more profitable products that protected human health and improved living standards. One example of such a process is in the production of chlorine and caustic soda (chlor-alkali production). It involves applying an electrical current to sodium chloride to separate positively charged sodium from negatively charged chlorine to make alkali. Initial technologies set up two reservoirs with opposite electrical charges separated by a permeable membrane—the so-called diaphragm-cell process (Crook and Mousavi 2016). In 1895, Hamilton Castner and Karl Kellner commercialized an alternative—the mercury-cell process—whereby sodium forms an amalgam with the mercury while the chlorine gas volatilizes (O’Brien et al. 2005). Mercury-based chlor-alkali technologies were more energy efficient than other techniques, and were widely adopted in Europe and Japan (Crook and Mousavi 2016). The use of chlorine benefited public health. Chlorination of London’s water supply in the early 1900s, for example, protected against water-borne diseases (MacKenzie 1945). The mercury-cell process also made it possible to produce a higher quality caustic soda, which beginning in the 1940s was used to make new popular kinds of synthetic fibers such as rayon (O’Brien et al. 2005).
The mercury-cell technology for chlor-alkali production gained ground in the United States as well, but the older diaphragm-cell process remained in use in the majority of US plants throughout the twentieth century. Masaru Yarime (2007) cites two main reasons for this. First, there were abundant brine wells in the United States that provided the raw material for chlorine and alkali in the liquid form used by the diaphragm-cell process; the mercury-cell process in contrast requires beginning with solid salt. Second, because energy was cheaper in the US compared with other regions, it was less advantageous for manufacturers to switch to the more energy-efficient mercury-cell technique. The diaphragm-cell process, however, relies on asbestos, another highly hazardous substance. The mercury-cell process was also used in a few other places, but in countries where chlorine production was developed later, it was more common to rely on a newer membrane-cell technology that needed neither mercury nor asbestos. There were roughly 75 plants in 40 countries that still used the mercury-cell process in 2015, accounting for 8 percent of the global chlorine production capacity of 60 million tonnes (UNEP 2017).
Mercury was applied in other chemical production processes as well. In VCM manufacturing, mercury is a catalyst for a reaction between acetylene and hydrochloric acid that produces the VCM, which is in turn used to make PVC. China is currently the world’s largest manufacturer of PVC, and its domestic use of PVC is increasing rapidly. A reported 85 percent of all VCM production in China as of 2014 still used a mercury-based production technique (UNEP 2017). Mercury-based technology was also adopted widely in the chemicals industry for the production of acetaldehyde, a technique that began in Germany in 1912 (Eckert et al. 2006). In this process, a sulfuric acid/mercury sulfate solution is used as a catalyst. The Chisso factory in Minamata used a slightly different method that was developed locally starting in 1932 (George 2001). The mercury-based technique that produced acetaldehyde from acetylene was the dominating production technique for acetaldehyde manufacturing until the early 1960s (Eckert et al. 2006). By the time of the negotiations of the Minamata Convention, there was no longer any known mercury-based manufacturing of acetaldehyde.
While some uses of mercury were beneficial, others caused harm. Many workers suffered, and the use of mercury-added products in some instances also harmed consumers through mercury exposure. In Iraq in 1956, 1960, and 1972, people used imported seeds treated with organic mercury compounds, which had been intended for planting, to make bread; thousands of people fell ill and hundreds died (Jalili and Abbasi 1961; Bakir et al. 1973; Rustam and Hamdi 1974). Other known fatalities from people eating mercury-treated seeds occurred in Pakistan in 1961 and 1969, in Guatemala from 1963 to 1965, and in Ghana in 1967 (Haq 1963; Bakir et al. 1973; Derban 1974). In the United States, three members of a New Mexico family were poisoned in 1969 after eating meat from hogs that had been fed methylmercury-treated seeds (Waldron 1970).
The use of mercury also had mixed societal implications in other ways. Many of the percussion and blasting caps containing mercury fulminate were put toward violent ends. Mercury was also used in the production of thermonuclear weapons. To make enriched lithium-6 for such weapons in the United States, Soviet Union, and China, mercury was run against a solution of lithium hydroxide in a column exchange process whereby lithium-6 accumulated in the mercury phase. Between 1950 and 1963, an estimated 11,000 tonnes of mercury—an amount more than four times greater than today’s global annual anthropogenic emissions—was used in Tennessee at the Oak Ridge National Laboratory in this process (Brooks and Southworth 2011). The United States stopped making lithium-6 in 1963, and the major mercury-based production of lithium-7 is currently in China and Russia (Mazur et al. 2014). Recent reports that North Korea acquired large quantities of mercury fueled speculation about the country’s capacity to produce thermonuclear weapons (Albright et al. 2017). In addition, chlorine made from mercury-based processes was used in chemical weapons production.
Commercial Mercury and the Environment
Many mercury-added products and industrial point sources have discharged mercury into ecosystems (box 2-3). Roughly three quarters of the mined mercury that was used in products and processes is estimated to have been discharged into the atmosphere, land, and water, or has ended up in landfills (Horowitz et al. 2014). But there are many global and local data uncertainties regarding mercury discharges into the environment from individual sources. The fate of many mercury-added products discarded for centuries all over the world is unknown, including their location in landfills. There are also large uncertainties about mercury discharges from individual point sources. One primary example of this was seen in Minamata. It is unclear how much methylmercury Chisso released into Minamata Bay and neighboring waters by the manufacturing of acetaldehyde and vinyl chloride, but the company claimed in 1972 that it lost roughly 82 tonnes of mercury between 1932 and 1971. One year later, the Japanese Ministry of International Trade and Industry calculated that it was more likely over 224 tonnes, while other estimates go as high as 600 tonnes (George 2001).
Ecosystem processes transport mercury and lead to the production of methylmercury (box 3-3). In turn, methylmercury in ecosystems and contaminated sites affects people (box 3-1). The industrial discharge by Chisso and its related damages to the environment and human health was not an isolated event. A similar case, also stemming from the use of mercury in acetaldehyde production, happened in Niigata, Japan, where the chemical company Showa Denko discharged methylmercury into the Agano River. Mercury poisoning in Niigata was detected in the fall of 1964. Fewer people were affected by Minamata disease in Niigata than in Minamata, but the discharges of methylmercury that began in 1936 and lasted until 1965 still caused much damage. The leadership at Showa Denko initially denied that it had caused the problem and deflected blame by coming up with alternative (false) explanations, including pesticides released from a 1964 earthquake (George 2001). In 1967, 13 victims of Minamata disease filed a suit against Showa Denko in Niigata District Court. In 1971, two years before the legal ruling against Chisso, the court found Showa Denko responsible for causing the outbreak of Minamata disease in Niigata (Funabashi 2006). By 1999, 690 cases of Minamata disease were recorded in Niigata (Eto 2000).
Industrial releases of mercury also caused damages in Ontario, Canada. The Dryden Chemicals company released an estimated 9 to 11 tonnes of mercury into the Wabigoon-English River system between 1962 and 1970 (Takeuchi et al. 1977). This mercury came from a chlor-alkali plant that used mercury cells to produce sodium hydroxide and chlorine for bleaching pulp at the nearby Dryden Paper Company. Chlor-alkali plants and paper mills were sometimes co-located for practical purposes. These releases caused an outbreak of Minamata disease in downstream communities of First Nations peoples in the Grassy Narrows and White Dog Reserves, where researchers found many symptoms in cats and humans that were similar to those previously documented in Minamata (Takeuchi et al. 1977; Harada et al. 2005; Takaoka et al. 2014). The provincial government in Ontario ordered Dryden to stop the mercury discharges in 1970, but it did not acknowledge the presence of Minamata disease (Mosa and Duffin 2017). A decision by the government in 1970 to close down all fishing significantly affected people for generations; similar to the fishers in Minamata, local indigenous groups depended on fish for both food and income (Jago 2018).
Producers and consumers have also intentionally discharged mercury-added products into ecosystems (box 1-3). Panogen and other liquid preparations of organic mercury compounds for use in agriculture against fungi were introduced in the 1940s. By 1950, the use of Panogen in Sweden was “as routine in farming as plowing” (Löfroth and Duffy 1969, 10). Swedish conservationists and ornithologists were among the first to notice fatalities in birds that had eaten treated seeds (Egan 2013). Panogen was also widely used in many other countries. A 1964 advertisement in the US publication The National Future Farmer, targeted toward young people in agricultural regions, highlighted the Morton Chemical Company’s efforts to educate farmers about the benefits of treating seeds with Panogen to prevent mold and encourage root and foliage development. It noted that farmers all over the world had been using Panogen for 25 years (Anonymous 1964). Direct release of mercury to ecosystems in these applications was both widespread and extensive. It was estimated that a total of 2,100 tonnes of mercury worldwide were used in agriculture in 1965 (Smart 1968).
Interventions
Different interveners have attempted to address problems stemming from mercury use in products and processes, including national and local governments, industries, experts, and international bodies. Figure 6.4 identifies the central interveners and interventions in the products and processes system for mercury. Two main categories of interventions have addressed commercial mercury use. First, some interventions have focused on reducing or eliminating the uses themselves (boxes 2-2 and 1-2). Second, other interventions have addressed mercury from an environmental perspective, by managing stockpiles, setting limits on discharges, and cleaning up contaminated sites (boxes 2-2, 2-3, and 3-3).
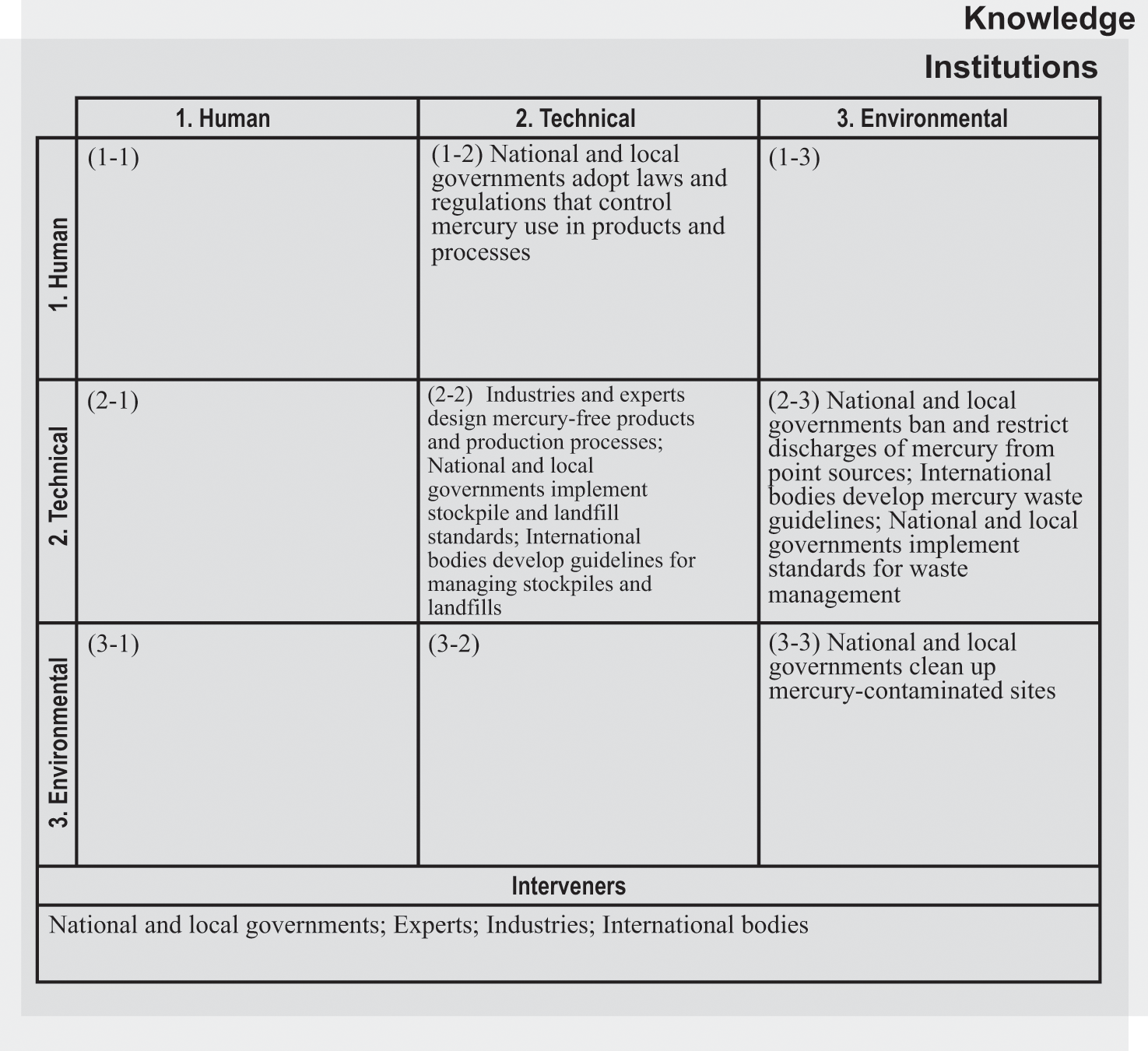
Intervention matrix for the products and processes system for mercury.
Reducing or Eliminating Commercial Mercury Uses
Many interventions that reduced or eliminated commercial mercury uses originated in the private sector, as industries and experts designed mercury-free products (box 2-2). Economic considerations, rather than environmental and health concerns, sometimes drove initiatives to either reduce the amount of mercury used or move to mercury-free alternatives in products. For example, making large mirrors using silver nitrate, a technique developed in the middle of the nineteenth century, was quicker and easier than using mercury to make them. It was not until around 1900, however, when technological advances made silver-backed mirrors more durable than mercury-containing mirrors, that they became competitive on the market (Hadsund 1993).
National and local governments have adopted laws and regulations that control mercury use in products (box 1-2). One of the earliest examples of controls on mercury-added products occurred when Swedish authorities banned Panogen and restricted the use of other mercury-containing pesticides in 1966. Their application quickly declined together with concentrations of mercury in food and the environment. Some use of less toxic mercury compounds nevertheless continued in Sweden, including Panogen Metox (methoxyethylmercuric acetate), which was not banned until 1988. In the United States, the Department of Agriculture in 1970 ordered that methylmercury seed treatments be taken off the market after the 1969 incident in which the New Mexico family was poisoned with Panogen was reported on television (Waldron 1970). After industry challenged this decision in court, an appellate court ruled that scientific evidence connecting Panogen to health impacts was insufficient, and that farmers had no economically viable substitute to mercury-treated seed. The US Environmental Protection Agency (EPA), however, issued a notice in 1972 that it intended to cancel registration for all mercury-treated pesticides, and it announced a ban on almost all such pesticides in 1976 (United Press International 1976).
Phaseouts of phenylmercury in paints involved both conflict and cooperation between governments and industry. The US EPA was forced to withdraw a notification to ban the use of phenylmercury in paints in 1976 (when mercury use in pesticides was prohibited) after industry successfully challenged the decision in court on the grounds that there were no available substitutes (Meier 1990). In 1990, the EPA reached a voluntary agreement with the National Paint and Coatings Association to cease manufacturing of mercury-containing paints. This decision followed a case in 1989 where a four-year-old boy in Michigan was hospitalized for several months with severe symptoms of acrodynia after the inside of his house was painted with mercury-containing paint (Agocs et al. 1990). This case gained much public attention and led authorities in Michigan and, shortly thereafter, the EPA to act. Japan banned the use of mercury compounds in paints in 1980 (UNEP/FAO 1996). Phenylmercury was used in paint production in Europe at least into the late 2000s (European Chemicals Agency 2011), but a 2012 regulation banned the sale of all products in the EU containing phenylmercury compounds in more than trace amounts starting in 2017 (European Commission 2012). Phaseouts of phenylmercury in paints have been slow in other parts of the world as well.
Countries have also initiated several regulatory measures on other mercury-added product categories, including electronics. The EU member states, together with the European Parliament, took on a leadership role in regulating the content of hazardous substances in electronics both regionally and globally in the early 2000s (Selin and VanDeveer 2006). A 2002 directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment (RoHS), which was updated in 2011, limits the use of mercury in electrical and electronic equipment. The EU RoHS directive spurred similar regulatory measures in other countries including China, Japan, Taiwan, and South Korea. In the United States, California acted along the same lines (Wright and Elcock 2006). Following additional regulatory measures, a 2017 EU directive banned the manufacturing, export, and import of all mercury-added products covered by the Minamata Convention by either the end of 2018 or the end of 2020 (European Union 2017).
Actions taken by both industry and government influenced trends in mercury use in batteries, with the total amount of mercury used declining with time. Municipalities in Japan started battery recycling in the 1980s (Pollack 1984). US states around the Great Lakes and in the Northeast took action to restrict mercury use in batteries in the early 1990s (Cain et al. 2011). New Jersey restricted the sale and disposal of many mercury-containing batteries in 1992, and, along with Arkansas and Minnesota, banned mercuric-oxide batteries in 1993 (Sznopek and Goonan 2000). Federal legislation in the US phasing out mercury use in batteries through the Mercury-Containing and Rechargeable Battery Management Act followed in 1996. Much state and federal legislation exempted mercury use in small button-cell batteries because of a lack of alternatives, but mercury-added button-cell batteries were banned by some US states in the 2010s (Zero Mercury Working Group 2012). Major battery manufacturers advertise that all of their batteries are mercury free; Duracell, for example, has been making button-cell hearing aid batteries without mercury since 2011 (Panasonic 2018; Duracell 2019).
Countries in other regions implemented bans and restrictions on mercury in batteries later than in the United States. A 2006 EU directive prohibited batteries with mercury content greater than 0.0005 percent by weight, but exempted button-cell batteries with mercury content less than 2 percent (similar to earlier US legislation). Another 2015 directive updated the prohibition to include mercury-containing button-cell batteries. China banned mercuric oxide batteries in 1999 and set regulatory limits on mercury content for other types of batteries starting in the early 2000s (Cheng and Hu 2011). Statistics on trends in mercury use for battery production in China are conflicting for the late 1990s and early 2000s, but overall use was more than an order of magnitude greater than in the United States (Feng 2005). Mercury use in Chinese batteries was reported to drop after the year 2000 (China Council for International Cooperation on Environment and Development 2011). However, in China, which is the main producer of alkaline button cells (used, for example, in toys and remote controls), only 10 percent of battery production was reported to be mercury free in 2012 (Zero Mercury Working Group 2012).
Actions on mercury-added measuring devices involved interplay between governments and industrial innovation, including the introduction of digital thermometers. US states took the lead in controlling the sale of mercury-added measuring devices in the 2000s. New Hampshire passed a law in 2000 that prohibited the sale of mercury thermometers and other measuring devices (Health Care Without Harm 2018). This was followed by similar legislative measures in 13 other states over the following three years, shrinking the US market for such products. The US EPA largely relied on such state action to encourage firms to voluntarily cease production and sale (US Environmental Protection Agency 2014). In addition, the US National Institute of Standards and Technology in collaboration with the EPA in 2011 declared that it would no longer provide calibration services for producers and users of mercury thermometers, something it had been doing since 1901. This put further pressure on the private sector to end the production of mercury thermometers. By the early 2010s, there was only one manufacturer of mercury thermometers left in the United States (Roylance 2011).
Other jurisdictions have also acted on restricting the availability of mercury-added measuring devices. The EU in a 2007 directive banned the sale of mercury-added measuring devices including thermometers and barometers starting in 2009, although some member states had acted earlier. For example, Sweden banned the manufacturing and sale of medical mercury thermometers in 1998, and subsequently implemented additional controls on other measuring devices (Swedish Chemicals Inspectorate 2004). Other countries began to introduce controls on mercury-added measuring devices in the early 2010s, spurred on by the negotiations of the Minamata Convention. The Canadian federal government adopted a ban on the manufacturing and import of mercury-added measuring devices in 2014 (Government of Canada 2014). Other countries have also taken legislative steps, but with extended time lags before the controls enter into force. China, a main producer of mercury-added measuring devices, announced in 2017 that it would prohibit the production and export of mercury thermometers as of 2026 (Chemical Watch 2017).
Governmental efforts to address mercury in lamps and light bulbs have focused on a combination of bans and controls on maximum allowable mercury content, creating incentives for innovation and product development. For example, the US banned mercury vapor lamps as of 2008 (Energy Policy Act 2005). In contrast, most controls on CFLs have focused on limiting their mercury content rather than on phasing them out. Early traditional fluorescent lamps, containing mercury, were more efficient and provided more light per unit of energy than the older incandescent lamps (Bright and Maclaurin 1943), and these tubular-shaped lamps were adopted for certain lighting applications. CFLs using mercury were invented in 1976, but were not marketed widely until the 1990s, when they were hailed as an environmentally friendly substitute for the more energy-inefficient light bulb–shaped incandescents (Smithsonian National Museum of American History 2017). Although CFLs are still widely sold all over the world, governments have taken steps to phase out the early versions, which have relatively high mercury content, while still allowing (and in many cases encouraging) the continued use of those that contain less mercury.
Some government policies in other areas have, at least in the short-term, expanded markets for CFLs. The US Energy Independence and Security Act of 2007 set energy efficiency standards requiring that new light bulbs after 2012 use at least 28 percent less energy than the incandescent light bulbs. Several different kinds of light bulbs on the market met this requirement, including CFLs that use 75 percent less energy than a comparable incandescent light bulb (Natural Resources Defense Council n.d.). This may appear to be an increase in mercury use with no mercury-related benefits, but the situation is complex when considering both mercury use and discharges. The reduction of energy use can prevent a larger quantity of mercury from entering the environment than results from its use in the bulb itself, depending on recycling rates and levels of mercury emissions from the energy sector. In countries with high levels of mercury emissions from coal-fired power generation, switching to a CFL from an incandescent bulb results in reduced mercury emissions (Eckelman et al. 2008). Light-emitting diode (LED) bulbs, an even more efficient alternative, contain no mercury. In late 2019, however, the Trump administration blocked further implementation of the phaseout of incandescent bulbs.
Government interventions to control mercury use in products have influenced, as well as been influenced by, international actions. The 1998 heavy metals protocol under the Convention on Long-Range Transboundary Air Pollution (CLRTAP) limits the use of mercury in batteries, and urges parties to manage the risks of mercury for other uses. The Minamata Convention controls mercury uses in nine product categories and requires parties to discourage the manufacture and distribution of new mercury-added products. The nine categories are: pesticides, batteries, switches and relays, CFLs and linear fluorescent lights, high-pressure mercury vapor lamps, cold cathode fluorescent lamps and external electrode fluorescent lamps (CCFL and EEFL) for electronic display, cosmetics, and several kinds of measuring devices (including thermometers, barometers, and sphygmomanometers). The deadline for actions on these products is 2020, but parties can apply for up to two five-year extensions. For some products such as pesticides and non-electronic thermometers and barometers, parties must phase out all uses. For other product categories, the Minamata Convention merely sets maximum mercury amounts allowed. For example, it allows mercury in certain button-cell batteries up to 2 percent, consistent with earlier legislation in the United States and the EU.
Some Minamata Convention controls on mercury-added products are more difficult to enforce than others, especially if they concern mercury use in relatively low-tech and easily produced products. For example, mercury-containing skin-lightening soaps, creams, and powders are in continuing production and use, mainly in Africa and Asia, but also in some immigrant communities outside of these regions, including the United States (Zero Mercury Working Group 2010; World Health Organization 2011a; Minnesota Department of Health 2019). The sale of these products, which often takes place in market stalls and small shops, is difficult to address because the deep cultural roots of their application are associated with a desire for lighter skin, especially by women. The Minamata Convention also does not address mercury-containing products used in traditional and religious practices. China and Sri Lanka argued for exempting traditional medicines during the treaty negotiations (Earth Negotiations Bulletin 2012). Similarly, Nepal called for excluding religious uses of mercury from the Minamata Convention (Earth Negotiations Bulletin 2013a). In addition, the Minamata Convention explicitly exempts all military uses of mercury—the global scope of such uses is unknown.
Similar to their actions on products, industries and experts also designed mercury-free production processes (box 2-2). Private sector technological and economic considerations drove a gradual phaseout of mercury use in many sectors. This included the switch to mercury-free alternatives in acetaldehyde and VCM production in Europe and North America. The choice between mercury-based and mercury-free manufacturing techniques depended in large part on whether coal or oil was used as a raw material. Acetaldehyde can be produced from acetylene from coal in the presence of a mercury catalyst, or from oil-derived ethylene, a process that does not require mercury (Othmer et al. 1956). The coal-based acetylene process was the common technique in many regions for more than the first half of the twentieth century, but industries in most countries switched to the ethylene-based Hoechst-Wacker process in the late 1950s because the oil-based feedstock was cheaper to produce and easier to handle (Trotuş et al. 2013). A similar choice between coal and oil affected choices in VCM production. The dominant technology for VCM production today relies on a cheaper and more efficient mercury-free process that begins with ethylene, which is produced from oil. It is also possible to make VCM from acetylene produced from coal; this is the reason that countries with large coal reserves, such as China, continue to use the mercury-based process (UNEP 2017).
National and local governments in some instances also played a substantial role in controlling mercury use in production processes by adopting laws and regulations (box 1-2). Government actions often occurred in tandem with technological improvements spurred by the private sector. In some cases, government signals that an area of mercury use would become subject to legislative action pushed industry to look for alternatives. Refinement of the membrane-cell technology in chlor-alkali production, for example, was prompted in part because of growing environmental concerns about mercury in the 1960s that foreshadowed potential controls (O’Brien et al. 2005). In some of these instances, government action pushed industry to switch to a new production method that they later abandoned, despite having made large investments in it. For example, Japan was the first country to phase out the mercury-cell manufacturing process for chlor-alkali production in the early 1970s, in response to concerns about mercury that stemmed from the Minamata disease experience (Ministry of the Environment Japan 2013). This caused Japanese firms to invest in a less optimal technology, the diaphragm-cell process, which resulted in future inefficiencies when those plants were prematurely retired in favor of an improved ion-exchange process that was also mercury-free (Yarime 2007).
Governmental efforts to reduce mercury use in production processes sometimes had unintended effects. For example, the Chinese government attempted to reduce mercury use in the VCM sector in the early 2010s (J. Liu, pers. comm. February 28, 2019). From 2011 to 2015, plants producing VCM were required to switch from using a high-mercury catalyst (10.5–12.5 percent mercury content) to a low-mercury catalyst (4–6.5 percent mercury content), aiming for a 50 percent reduction in mercury use. Some plants failed to make necessary technical changes to adjust to the lower-mercury catalyst, and needed to use more catalyst per production unit or to increase manufacturing capacity to maintain production levels. This offset some of the intended reductions, and also increased levels of catalyst waste. International cooperation has supported the development of mercury-free technologies for coal-based VCM production in China (Zhang et al. 2011). Developing and implementing such technologies will be key, as China plans to continue to use coal as a feedstock in the domestic production of VCM.
The Minamata Convention bans or restricts all major remaining mercury uses in the chemicals industry. Parties were required to stop using mercury in acetaldehyde production in 2018 and to phase out mercury use in chlor-alkali production no later than 2025. Although there was no evidence of any remaining mercury-based acetaldehyde production anywhere in the world when the Minamata Convention negotiations began in 2010, the ban was included in the treaty at the request of Japan for symbolic reasons because of its ties to the Minamata pollution tragedy. Parties can apply for up to two five-year extensions to the phaseout date for chlor-alkali production. The Minamata Convention imposes restrictions on mercury use in three other processes: VCM production (reduce mercury use per unit of production by 50 percent between 2010 and 2020); sodium or potassium methylate or ethylate production (phase out mercury use as fast as possible, within 10 years of entry into force, and reduce emissions per unit of production by 50 percent between 2010 and 2020); and polyurethane production (phase out mercury use as fast as possible, within 10 years of entry into force).
Substitution may not always be unequivocally positive; alternative technologies used to replace mercury in production processes may also be harmful to human well-being. For example, in the chlor-alkali industry, modern plants use membrane technology that consists of per- and polyfluoroalkyl substances (PFAS). PFAS are highly toxic substances that are sometimes referred to as “forever chemicals” because of their inability to break down in the environment (Johnson 2018). The uses for two of the most well-known examples of this larger class of substances—perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA)—are regulated by several countries and controlled under the Stockholm Convention (Wang et al. 2017). Yet, the use of PFAS membranes for chlor-alkali production is common across the world where chlor-alkali production does not rely on mercury and/or asbestos. PFAS can be released during the production processes involving these membranes (Strynar et al. 2015). The human health impacts of the use of PFAS in chemicals production, however, remain unknown (Cousins et al. 2019). Newer membrane-free technologies are being developed as further alternatives (Hou et al. 2018).
The existence of transnational institutions, such as the Global Mercury Partnership, has driven much of the more recent international technical work to identify and diffuse substitutes for mercury in products and processes. These partnerships link stakeholders and their societal interactions to new technologies. In doing so, they enhance capacity transnationally to develop further technical know-how by raising awareness and building knowledge and support. There are specific partnership areas on mercury reduction in products and in the chlor-alkali sector. Stakeholders involved include different government ministries, private sector actors (producers, importers, and sellers), and civil society organizations. The United Nations Environment Programme (UNEP) and other international organizations, including through their involvement in the partnerships that predated the Minamata Convention, have helped raise awareness, mainly in developing countries, since the early 2000s. Identifying substitutes for mercury uses can be complex, but some actors have argued for broader visions for phaseouts of mercury. The EU has established a goal of a mercury-free economy, arguing that the dangers and costs of mercury to the environment and human health are too extreme to allow any ongoing uses (European Commission 2017a; European Commission 2018).
Preventing Environmental Releases and Promoting Cleanup
Many national and local governments have restricted and banned discharges of mercury from point sources (box 2-3). Such controls on mercury discharges from industrial sources date back to the 1970s. The US EPA set mercury emission limits on mercury-cell chlor-alkali plants in 1973 (see chapter 5). Regulations under the Convention for the Prevention of Marine Pollution from Land-Based Sources, which set mercury limit values for releases from chlor-alkali production in the early 1980s, encouraged European producers to increase production efficiency (Yarime 2007). The share of chlorine production in Europe based on mercury-cell technology fell from 63 percent to 26 percent between 1997 and 2012 due to a combination of plant retirements and growing environmental concerns about mercury discharges (European Commission 2014). Reduced demand for chlorine because of regulations on ozone-depleting substances and other chlorine-containing products influenced shutdowns of facilities that used mercury-based production techniques in the United States (Snyder et al. 2003). Canadian laws on maximum allowable releases of mercury from chlor-alkali plants led most to convert their underlying technology to mercury-free alternatives (Commission for Environmental Cooperation 1997).
Another area of government controls involves removing mercury from waste streams, where leakages from mercury-added products in landfills can cause water contamination and other problems. A 2002 EU directive on waste electrical and electronic equipment (WEEE) targeted mercury together with other hazardous substances (Selin and VanDeveer 2006). This directive, which was revised in 2012, is designed to operate alongside the RoHS directive on hazardous substances in products that was initially adopted around the same time. The WEEE directive regulates 10 common categories of electrical and electronic equipment that have often contained mercury, including household appliances, information technology and telecommunications equipment, lighting equipment, electrical and electronic tools, medical devices, and monitoring and control instruments. Under the directive, producers take on a greater responsibility for recycling, reprocessing, and safe disposal. This form of extended producer responsibility for dealing with waste is also intended to provide incentives for industry to design more environmentally friendly products that are easier and cheaper to manage safely once they have been returned.
National and local government interventions driven by concerns about environmental discharges have implemented standards for managing mercury in stockpiles and landfills (box 2-2). Sometimes this has involved collaboration with international bodies, which have developed guidelines for managing such stockpiles and landfills (box 2-2). These guidelines aim for the environmentally sound storage of mercury. International bodies have also developed guidelines for managing mercury wastes (box 2-3). National and local governments in turn implement standards for waste management (box 2-3). Mercury has posed storage and waste disposal problems for centuries, but it is only during the last 50 years or so that countries have developed and implemented legislation around the management of hazardous wastes. One Global Mercury Partnership area focuses on mercury supply and storage and another centers on waste management. The increasing phaseout of mercury uses in products and processes creates needs for the institutionalization of environmentally safe storage and disposal mechanisms. If mercury-use bans are combined with export bans, such mechanisms must be set up domestically. It is estimated that globally somewhere between 30,000 and 50,000 tonnes of excess mercury will become available by 2050 (UNEP 2015).
The Minamata Convention mandates that parties shall store and dispose of discarded mercury and mercury waste in an environmentally sound manner. In this area, the Minamata Convention connects with other international agreements including the 1989 Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal (Selin 2010). The Minamata Convention, consistent with the Basel Convention, identifies three categories of mercury wastes: (1) waste mercury or mercury compounds; (2) wastes that contain mercury or mercury compounds (for example, thermometers and CFLs); and (3) wastes that are contaminated with mercury or mercury compounds (for example, residues from mining and industrial processes). Significant variations exist across countries when it comes to developing national inventories of mercury and mercury wastes. To ensure sound management, it will be important to further establish effective mechanisms for both identifying and managing waste that contains or is contaminated with mercury. An important part of the work of the Minamata Convention Conference of the Parties (COP), in collaboration with the Basel Convention, is to further develop technical guidelines for environmentally sound storage and disposal.
High levels of excess mercury in society from phaseouts of commercial uses increase the demand for environmentally safe management of waste mercury. One study estimates that roughly 11,000 tonnes of metallic mercury from the chlor-alkali industry, non-ferrous metal production, and other processes need to be disposed of in the EU alone before midcentury (Hagemann et al. 2014). EU law mandates that liquid elemental mercury must undergo appropriate conversion before it is transferred to permanent storage because it is so hazardous (Science for Environment Policy 2017). Treatment options for liquid mercury include forming an amalgam with solid metals, such as zinc or copper, or converting it into mercuric sulfide. Long-term storage can be either above or below ground. Above-ground storage requires access to specialty warehouse-style storage facilities. Options for underground storage in Europe include depositing mercury in salt mines or hard rock formations (Science for Environment Policy 2017). The development and implementation of similar kinds of waste management strategies and policies are important to many countries, as waste mercury is a challenge worldwide.
National and local governments have cleaned up mercury-contaminated sites (box 3-3). Sites that are contaminated with mercury (sometimes together with other hazardous substances) pose significant management problems. Many of these sites are located close to factories that used mercury in products and manufacturing processes. The Minamata Convention requires that parties endeavor to develop strategies for identifying and assessing mercury-contaminated sites. One option to address contaminated terrestrial sites is the washing of mercury-contaminated soils, where mercury binds to other particles and is later separated for further processing. Phytoremediation and phytostabilization can use plants and plant roots to immobilize mercury and other contaminants from soil and water before these plants are recovered and stored (Science for Environment Policy 2017). In addition, bacterial remediation is used to remove mercury from water; it can also work for soil (Mahbub et al. 2017).
Addressing contaminated sites can be contentious. The clean-up of Minamata Bay involved a politically sensitive and extensive reclamation project. Sediments were dredged from Minamata Bay in the 1980s and were placed underneath the eco-park in Minamata. Some residents worry that untreated methylmercury in the sediments may leak back into the water, as Minamata is located in an earthquake prone region. Researchers believe this risk is minimal, however, arguing that the methylmercury has transformed into the more stable form of mercuric sulfide (Sokol 2017). More recently, local groups in Kodaikanal and authorities in the state of Tamil Nadu have been in protracted debates with the Indian subsidiary of Unilever about how to address soils contaminated with mercury from the thermometer factory that was forced to close in 2001 (see chapter 4). One area of disagreement has involved how much soil should be cleaned up: the factory has argued that only the most contaminated soil needs to be removed, while local activists believe it is necessary to treat a much larger quantity of soil that has lower mercury concentrations (Dev 2015).
Cleanup of mercury in contaminated sites represents a significant economic cost to society. Based on the well-established principle that a polluter should pay the costs of pollution, at least some of these costs should be borne by the polluting factory. Yet a large portion of the cleanup costs often falls on the public sector (and, by extension, taxpayers). In one of the most expensive mercury cleanups to date, the cost of removing mercury that was released from lithium enrichment for nuclear weapons production at Oak Ridge is estimated at USD 3 billion. During the period from 2012 to 2015, cleanup costs for reported mercury spills by the US EPA ranged from tens to hundreds of thousands of dollars per incident (Wozniak et al. 2017). While North America and Europe struggle to address old contaminated sites, the number and severity of mercury-contaminated sites continue to increase in Asia and other parts of the world (Li et al. 2009; Kocman et al. 2013). The global economic costs for dealing with all of these sites are unknown, but given the fact that many sites in North America and Europe remain untreated, and that the problem continues to increase in many other parts of the world, costs will only grow with time.
Insights
The story of the mercury-based thermometer at the beginning of this chapter shows that even a single product is part of a system in which mercury connects with issues of human well-being, prosperity, industrial production, technological change, and legacy contamination. In this section, we examine insights from the products and processes system for mercury. First, the use and presence of mercury in products and processes has varied over space and time, with different drivers and consequences. Second, mercury has both benefited and detracted from human well-being, and technological change as well as government intervention have driven gradual transitions toward mercury-free alternatives. Third, cross-scale actions by governments and the private sector occurred simultaneously, showing the complex nature of designing effective interventions.
Systems Analysis for Sustainability
The unique physical properties of mercury intersected with technology development, economic factors, and concerns about environmental and human health damages to drive changes in the use of mercury in products and processes. Mercury use at times decreased in some sectors while it increased in others. For example, mercury use was phased out in mirror and hat making around the same time it began and expanded in battery and light bulb production. The continuing availability of mercury mined from cinnabar facilitated its growing use in products and processes, and industrialization dramatically increased its use as a combined result of technological innovations and growing consumer demand for new mercury-containing products and goods made using mercury. Much of this mercury was eventually discharged into the environment, and the application of mercury-treated pesticides added to this environmental burden. Local and national governments increasingly adopted standards and laws controlling mercury uses based on growing concerns about the environmental and human health impacts of mercury in the late 1900s. Advances in scientific and technical knowledge made it possible to switch to mercury-free alternatives for major products and processes.
The products and processes system for mercury has been able to continue to provide societal benefits from goods and industrial manufacturing while gradually reducing its reliance on mercury-based technology. Development of new techniques allowed producers to adapt to mercury phaseouts, some of which were prompted by government mandates. Mercury-free mirrors, pesticides, thermometers, batteries, and light bulbs have continued to provide important societal benefits, and the chemicals industry has found ways to keep manufacturing high-volume chemicals by using mercury-free production processes. In contrast, many environmental components have much more limited capacity to change once mercury has entered ecosystems. Contaminated sites and landfills that contain high levels of mercury can continue to cause local harm to wildlife and people for decades to centuries. Effective cleanup of such sites is both very expensive and technically complicated. Some discharged mercury from products and processes adds to the global environmental cycling of mercury; this cycling continues for a long time and is difficult to alter.
Mercury has been ubiquitous in global commerce, and its use, environmental presence, and behavior in particular places have influenced its effects on ecosystems and human well-being. Relatively small amounts of discharges, compared to the global use of mercury, can have severe negative impacts on the environment and human health in particular places. Industrial discharges of mercury in Minamata, Niigata, and Grassy Narrows led to many human deaths, permanent neurological damages, and highly contaminated aquatic ecosystems. Accidental consumption of mercury-treated seeds in several countries resulted in additional human fatalities, and the application of mercury-containing pesticides caused harm to birds and other local animal populations. The long-range transport of some of the mercury that has been discharged into the environment from products and processes also transfers its environmental and human health damage far and wide, by adding to the amount of mercury globally that can be converted into methylmercury in faraway ecosystems. These distant effects are often not as visible as the more local consequences, but they can also be substantial and long lasting.
Sustainability Definitions and Transitions
Present-day efforts toward sustainability emphasize the reduction and ultimate elimination of mercury use in products and processes. Yet historically, mercury uses have both contributed to and detracted from human well-being in complex ways. Many known societal uses of mercury, going back to ancient China and the Roman Empire, were closely linked with prosperity and high social status; coveted items such as cosmetics, mirrors, and fur hats were made using mercury. Thus, mercury itself had a commercial value, both as an independent commodity and as a contributor to the value of manufactured goods. The positive value of mercury was not just economic. Stocks of mercury contributed to human well-being, for example, through their uses in developing scientific knowledge, in producing scientific instruments, and in allowing for the production of chlorine used in water disinfection. At the same time, much mercury has been dispersed into the environment, adding to contaminated sites and damaging human health. The gradual phaseouts of mercury in products and processes reduced risks of local contamination as well as the amount of mercury going into global environmental cycling.
Gradual transitions away from mercury use in products and processes, shaped by a combination of private-sector innovation and government action, dramatically reduced the global amount of mercury use. In some cases, mercury-specific interventions drove change. In others, the use of mercury played an incidental role in technological development and other environmental concerns. Economic drivers prompted some switches away from mercury-using technologies. One example is the replacement of mercury-using acetylene-based production processes with mercury-free ethylene-based production processes, a change driven largely by the advantages of using oil instead of coal as a raw material. Another example is light bulbs, which first involved increases and then decreases in mercury use, as the switch to compact fluorescent bulbs was driven by concerns about energy efficiency. These mercury-containing bulbs are now being replaced by newer non-mercury technology in the form of LEDs. Some changes in mercury use in products and processes took hold in specific places and then spread internationally. This was seen, for example, in the cases of batteries and chlor-alkali production processes.
Some transitions to mercury-free products or processes occurred relatively rapidly, but many were preceded by a longer history of related actions. It can be difficult to define how quickly change occurred without identifying a clear starting point. For example, is it when the use of mercury started, when such mercury use was discovered to be dangerous, or when the first mercury-free alternative became commercially available? Japan banned the use of mercury in chlor-alkali manufacturing leading to plant closings, a rapid and discontinuous change. In the use of mercury in mirror making, in contrast, slower dynamics of technology improvements occurred over time that ultimately led to silver nitrate mirrors outcompeting those made using mercury. In the case of batteries, innovation and subnational action emerged over time before national regulatory bans took effect. Some dynamics were slowed by the influence of powerful actors who resisted change, such as when chemical companies in Minamata and Niigata denied responsibility for mercury discharges, or when industry fought pesticide bans in US courts. In other cases, such as for batteries, industries accelerated action through technology innovation.
Sustainability Governance
The use of mercury in products and processes is governed by a combination of domestic controls in the context of global goals. Many countries developed their own policies and strategies for addressing mercury use over the past century, but the Minamata Convention now sets deadlines for phaseouts and limits on most of the remaining commercial uses of mercury in products and processes. The Minamata Convention introduces harmonized top-down controls for major current commercial mercury-added products and processes. Many of these controls are of particular importance to developing countries where relatively few regulations on mercury use in products and processes had previously been adopted. The existence of mercury-free alternatives for banned mercury-added products and processes, as well as products that meet the requirements for maximum allowable mercury content, greatly facilitates implementation of the Minamata Convention controls. In addition, domestic and international markets for mercury-added products had already shrunk significantly before the Minamata Convention was adopted. However, the Minamata Convention does not control military uses of mercury or mercury used in traditional medicines and religious practices.
Governance efforts to address mercury in products and processes have affected how economic benefits and mercury impacts have varied across space and time. For instance, the EU emission standard for chlor-alkali production reduced mercury use but delayed the phaseout of mercury to give the industry a longer time to reconfigure its production system. This benefited producers in the short term, but allowed more mercury to circulate in society and the environment in the long term. In addition, had mercury use in chlor-alkali production been phased out before the EU’s export ban, it is possible that this mercury might have been resold and potentially used in ASGM (see chapter 3). In contrast, the approach taken by Japan resulted in more near-term adjustment costs to the domestic chemicals industry, but an earlier stop to mercury-containing technology eliminated risks of mercury exposure to workers and also lessened the impact of environmental discharges of mercury to ecosystems and people, both locally and in faraway locations.
Efforts to manage the benefits and harms of mercury have varied in their effectiveness. Different interventions over time had differential impacts, and have sometimes involved trade-offs. Conflicts between public health interests and industry interests are well illustrated by the stories of Minamata, Niigata, Grassy Narrows, and Kodaikanal, which follow an all too common pattern of behavior of entrenched economic interests denying responsibility and fighting the local communities harmed by their activities. This type of clash often involves citizens banding together to take on powerful economic actors, including cases that involve contaminated sites and waters. Many of these are the result of industrial discharges and illegally stored wastes, which add large amounts of mercury to environmental components. This situation is echoed in other communities worldwide. Often, community members may perceive a trade-off between their economic security—where their jobs and livelihoods might be dependent on the polluting industry—and the health and environmental impacts of mercury.
Mercury, the god of commerce, has witnessed a significant change in societal perceptions of the benefits and liabilities of mercury uses, particularly over the past half century. Mercury used in products and manufacturing processes for millennia provided many benefits to society, but also caused a wide range of harms to human health and the environment. Many early substitutions within the private sector, which shifted toward mercury-free products and industrial manufacturing processes, occurred primarily for economic reasons, but more recent changes and government controls have been introduced because of environmental and human health concerns about mercury. Societies use less mercury now than five decades ago, as a result of bottom-up innovation, top-down regulation, and public pressures. Some manufacturers phased out mercury use relatively quickly, while others tried to reduce use over longer periods of time. Large-scale industrial uses of mercury are mostly in the past, but the negative environmental and human health impacts and the economic costs of mercury discharges from products and processes, and from contaminated sites, will persist long into the future.