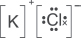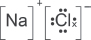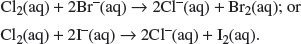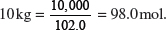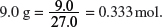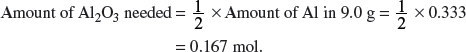CHAPTER 11
ELECTROLYSIS
Do you know?
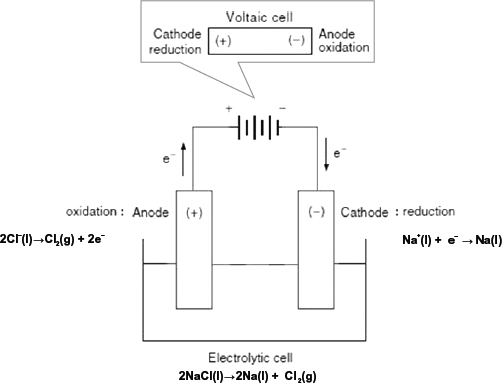
— In an electric cell, chemical energy is converted into electrical energy by directing the electron transfer during a redox reaction, through an external circuit. But in the electrolytic cell, an electric current is passed through an electrolyte to force an otherwise non-spontaneous redox reaction to occur. This process is known as electrolysis and the set-up is termed the electrolytic cell.
— In the electrolytic cell, we have:
• The electrolyte, which is a compound in solution or a molten compound that is able to conduct electricity because there are mobile charge carriers in the form of ions. The electrolyte is decomposed in the process.
• An electric cell or power source, which is used to drive the flow of electrons in the electrolytic set-up. It acts like a “pump” to push electrons in a single direction.
• The electrodes, which are metal conductors or graphite, by which an electric current enters or leaves the electrolyte.
• The cathode, which is the reduction electrode and it is negatively charged because it is connected to the negative terminal of the electric cell. Take note that the polarity or sign of the cathode for an electrolytic cell is opposite of that for an electric cell.
• The anode, which is the oxidation electrode and it is positively charged because it is connected to the positive terminal of the electric cell. Take note that the polarity or sign of the anode for an electrolytic cell is opposite of that for an electric cell.
1. Electrolysis involves the decomposition of a compound by the passage of an electric current.
(a) Complete the table, which relates to the electrolysis of different solutions using inert electrodes.
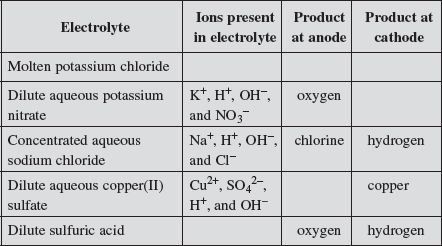
Explanation:
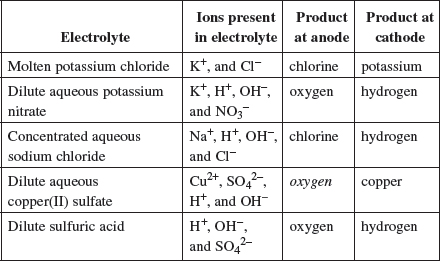
| Q | Why are there OH− ions present in dilute sulfuric acid? |
A: Water can undergo auto-ionization:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH−(aq).
Thus, both H+(aq) and OH−(aq) ions are present in the aqueous solution irrespective of whether it is an acidic or alkaline solution. In an acidic solution, [H+(aq)] is greater than [OH−(aq)]; vice versa in an alkaline solution.
Do you know?
— In Chapter 10, we learned about the reactivity series, which tells us that different metals have different potentials to undergo oxidation. At the same time, we also learned that the greater the ease of a metal in undergoing oxidation, its correspondng metal cation would be less likely to be reduced. With this, we have the following diagram to show us the relative ease of discharge of the various cations and anions:
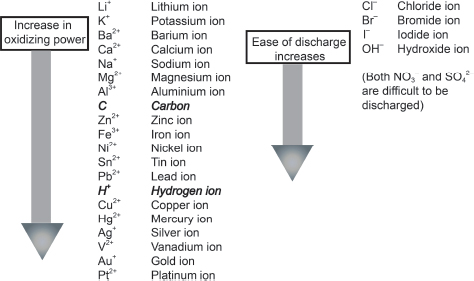
This diagram would help us decide which anion would undergo discharge during electrolysis. Students need to remember that both SO42− and NO3− would have difficulty undergoing discharge in an aqueous solution. Instead, the predominant discharge to replace the discharge of both the SO42− and NO3− would be the hydroxide ion that is present:
4OH−(aq) → 2H2O(l) + O2(g) + 4e−.
(i) Explain the terms inert electrode, anode, and cathode.
Explanation:
An inert electrode is an electrode that does not react with the components inside the electrolytic cell. An anode is the electrode in which oxidation takes place while the cathode is where reduction occurs.
| Q | Why is graphite preferred to platinum as an inert electrode? |
A: Well, because it is cheap!
(ii) Explain the source of the H+ and OH– ions in the electrolyte.
Explanation:
The H+ and OH– ions in the electrolyte come from the auto-ionization of water:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH−(aq).
If acid molecules are dissolved in water, the additional source of H3O+ ions come from the dissociated acid molecules. If a base is dissolved instead, then additional OH− ions come from the alkali.
(iii) Explain why the electrolysis of concentrated aqueous sodium chloride liberates chlorine rather than oxygen at the anode.
Explanation:
At the anode, although OH– is preferentially discharged, it is unlikely to happen as the concentration of OH– is extremely low. Thus, it is the high concentration of Cl– ions that undergoes discharge.
| Q | So, does this mean that the concentrations of the species would affect which species is discharged at the electrode? |
A: Yes, you are right! In a nutshell, the concentration of a species in electrolysis does affect whether it gets discharged or not!
Do you know?
— Brine, which is simply concentrated sodium chloride solution, is an important starting material for the production of
hydrogen,
chlorine gases, and
sodium hydroxide. The reaction of chlorine gas and sodium hydroxide is important for the production of sodium chlorate(I) (NaClO), a powerful oxidizing agent, which acts as the active ingredient in
bleaching agent:
2NaOH(aq) + Cl2(aq) → NaOCl(aq) + NaCl(aq) + H2O(l).
The ions that are present during electrolysis would be similar to those for the dilute solution!
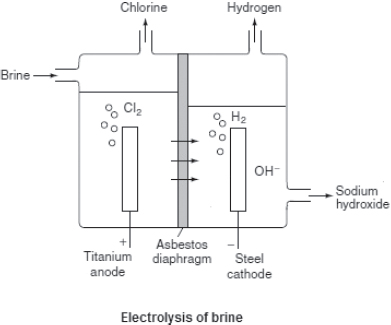
At the positively charged anode:
2Cl−(aq) → Cl2(g) + 2e− Oxidation.
At the negatively charged cathode:
2H+(aq) + 2e− → H2(g) Reduction.
Overall ionic equation:
2Cl−(aq) + 2H+(aq) → Cl2(g) + H2(g).
| Q | Is the above process used to manufacture chlorine gas? |
A: Yup! The chlorine gas that is produced can be used to disinfect water.
(iv) Explain why the electrolysis of concentrated aqueous sodium chloride liberates hydrogen rather than sodium at the cathode.
Explanation:
It is more difficult to discharge Na+ than H+ because from the reactivity series, sodium metal is above hydrogen. This means that sodium is more likely to undergo oxidation than hydrogen. Hence, Na+ is less likely to be reduced than H+.
| Q | But even if the Na+ is reduced to Na(s), would Na(s) still be able to react with the water that is present in the electrolyte? |
A: Yes! Na metal would react with the water to give hydrogen gas. So, it is back to square one.
(v) Explain why the concentration of sodium hydroxide in the electrolyte increases during the electrolysis of concentrated aqueous sodium chloride.
Explanation:
Both H+ and OH− ions come from the auto-ionization of water. Thus, as H+ is reduced to H2, the concentration of OH− ions would increase. In addition, since Cl− ions are oxidized to Cl2 while leaving Na+ ions behind, the concentration of sodium hydroxide in the electrolyte increases during the electrolysis.
(vi) Explain why the electrolysis of dilute aqueous copper(II) sulfate liberates copper metal rather than hydrogen at the cathode.
Explanation:
It is more difficult to discharge H+ than Cu2+ because from the reactivity series, copper metal is below hydrogen. This means that hydrogen is more likely to undergo oxidation than copper. Hence, H+ is less likely to be reduced than Cu2+.
Do you know?
— During the electrolysis of aqueous CuSO
4 using inert electrodes, the ions that are present in the solution are: Cu
2+, SO
42−, H
+, and OH
−. Thus,
At the positively charged anode:
4OH−(aq) → 2H2O(l) + O2(g) + 4e− Oxidation.
At the negatively charged cathode:
Cu2+(aq) + 2e− → Cu(s) Reduction.
Overall ionic equation:
4OH−(aq) + 2Cu2+(aq) → 2H2O(l) + O2(g) + 2Cu(s).
The OH− and Cu2+ ions selectively get discharged at the anode and cathode, respectively!
— If aqueous CuSO
4 is electrolyzed using copper electrodes instead, the ions present are still: Cu
2+, SO
42−, H
+, and OH
−. But,
At the positively charged anode:
Cu(s) → Cu2+(aq) + 2e− Oxidation.
At the negatively charged cathode:
Cu2+(aq) + 2e− → Cu(s) Reduction.
Overall ionic equation:
Cu2+(aq) + Cu(s) → Cu(s) + Cu2+(aq).
At the anode, it is the copper metal that undergoes oxidation and not OH−(aq). This is the basis for the purification of copper:
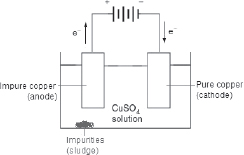
The impure copper is made the anode while a piece of pure copper acts as the cathode. As time passes, the anode dissolves, leaving the impurities behind while the pure copper grows in size.
In a nutshell, the types of electrode used in electrolysis can affect the outcome of the process!
| Q | Why is it important to purify copper? |
A: Pure copper has lower resistance; this would help to minimize the loss of electrical energy through heat.
| Q | If we compare electroplating to the purification of copper, these two electrolytic processes are very similar. Am I right? |
A: It is great that you managed to identify the similarities between the two processes. Electroplating is primarily used to coat a thin layer of material (of desirable properties) onto another material (which lacks the desired property). For instance, a popular use is in the electroplating of jewellery. Inexpensive jewellery is often coated with a thin layer of a precious metal such as silver or gold.
The piece of metal that is to be coated with silver, is made the cathode, which is very similar to the making of a piece of pure copper as the cathode during the purification process. This cathode is than placed into an electrolytic solution that contains the ions of the coating material, i.e., Ag+ ions.
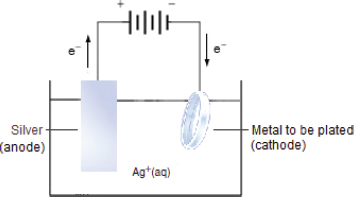
The coating material, i.e., Ag(s) is made the anode, which is very similar to the making of a piece of impure copper as the anode during the purification process.
When an electric current is passed, the Ag+ ions in the solution will migrate to the cathode and undergo reduction to form Ag(s) on the surface of the cathode.
At the positively charged anode:
Ag(s) → Ag+(aq) + e− Oxidation.
At the negatively charged cathode:
Ag+(aq) + e− → Ag(s) Reduction.
Overall ionic equation:
Ag+(aq) + Ag(s) → Ag(s) + Ag+(aq).
(vii) During the electrolysis of dilute aqueous copper(II) sulfate using inert electrodes, the blue coloration of the aqueous copper(II) sulfate fades and the pH of the solution decreases after some time. Explain why these changes take place.
Explanation:
The blue coloration of the aqueous copper(II) sulfate fades because the Cu2+(aq) ions, which give rise to the blue coloration, have been reduced to copper metal at the cathode.
The pH of the solution decreases after some time is due to the oxidation of OH−(aq) at the anode, as follows:
4OH−(aq) → 2H2O(l) + O2(g) + 4e−.
As both OH−(aq) and H+(aq) ions come from the auto-ionization of water, when the concentration of OH−(aq) decreases, the concentration of H+(aq) increases relatively. Thus, the pH of the solution thus decreases.
(viii) State some possible uses for the hydrogen gas produced.
Explanation:
Hydrogen can be used (i) as a fuel in the fuel cell; (ii) to hydrogenate fats using nickel catalyst; and (iii) in the synthesis of ammonia from nitrogen in the Haber Process.
(ix) Give the dot-and-cross diagram of potassium chloride.
Explanation:
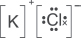
(x) State the types of bonding in molten potassium chloride.
Explanation:
In molten potassium chloride, the oppositely charged ions are attracted to each other by ionic bonds.
| Q | So, we did not actually break the ionic bonds in an ionic compound when we melted it? |
A: Nope! You just weakened them.
(b) Aqueous copper(II) sulfate was electrolyzed using copper electrodes. The copper anode lost mass as copper(II) ions were formed, and the copper cathode gained mass as copper atoms were formed.
(i) State one industrial application of this electrolysis.
Explanation:
The impure copper can be made the anode while the pure copper can be made the cathode. In such a way, we can get purified copper. Thus, this method is used to purify copper in the industry.
(ii) Explain the likely factors that would affect the rate of the deposition of copper at the cathode.
Explanation:
How fast the copper can be deposited would depend on the quantity of electrons that flow through the system per unit time. This is determined by the magnitude of the current.
In addition, the concentration of the copper(II) sulfate solution would also affect the rate of deposition. The higher the concentration of the solution, the higher the electrical conductivity; hence, the faster the rate of deposition of the copper.
The temperature would also affect the rate of deposition. The higher the temperature, the higher would be the rate of collision of the particles with the surface of the electrode; hence, the faster the rate of deposition.
(iii) Explain why copper is such a good conductor of electricity.
Explanation:
Copper has delocalized valence electrons which can act as mobile charge carriers when a potential difference is applied across the metal. This accounts for its good electrical conductivity.
| Q | Since a metal and an electrolyte can conduct electricity, are there any differences between them? |
A: The charge carriers in a metal are delocalized electrons while those in an electrolyte are free ions. In addition, a piece of metal remains unchanged after electrical conduction but for an electrolyte, it undergoes discharged or it “decomposes.”
2. An aqueous solution of barium hydroxide is electrolyzed between carbon electrodes.
(a) What gas would you expect to be produced at the anode? Give the species that produces the gas.
Explanation:
The species that are present in aqueous barium hydroxide are: Ba2+, H+, OH−, and H2O. At the positively charged anode, oxidation takes place. Thus, the species that would likely be attracted to the anode is the OH− ion and it will be oxidized to form oxygen gas:
4OH−(aq) → 2H2O(l) + O2(g) + 4e−.
(b) How would you test for the gas evolved at the anode?
Explanation:
Oxygen gas can be tested by a glowing splint. The gas would relight a glowing splint.
(c) Give the half-equations for the reaction at the anode and cathode.
Explanation:
Anode (oxidation): 4OH−(aq) → 2H2O(l) + O2(g) + 4e−.
At the cathode, reduction can only occur for H+, forming hydrogen gas.
Cathode (reduction): 2H+(aq) + 2e− → H2(g).
(d) Give the molar ratio of the products that are formed at the anode to the cathode.
Explanation:
A balanced redox equation is:
4OH−(aq) + 4H+(aq) → 2H2O(l) + O2(g) + 2H2(g).
Hence, the molar ratio of O2: H2 is 1:2.
| Q | So, based on the above equation, would the pH of the solution change during electrolysis? |
A: Since equal amounts of OH−(aq) and H+(aq) are “consumed” during the electrolytic process, the pH is not going to change!
| Q | So, the electrolysis of aqueous barium hydroxide is essentially the electrolysis of water? |
A: You are right! Essentially, it is just this reaction: 2H2O(l) → 2H2(g) + O2(g).
| Q | So, why don’t we just electrolyze plain water instead? |
A: It is difficult to electrolyze plain water to get hydrogen and oxygen as the electrical conductivity of plain water is very low. This is due to the minute amount of ions that is generated from the auto-ionization of water. You can simply treat the presence of barium hydroxide as just to increase the electrical conductivity of the solution!
| Q | So, what would we get when we electrolyze aqueous sulfuric acid solution? |
A: Simple! The ions that are present in aqueous sulfuric acid are: SO
42−, H
+, and OH
−. Since SO
42− ions are difficult to be discharged, we have the following reactions occurring at the electrodes:
At the positively charged anode:
4OH−(aq) → 2H2O(l) + O2(g) + 4e− Oxidation.
At the negatively charged cathode:
2H+(aq) + 2e− → H2(g) Reduction
Overall ionic equation:
4OH−(aq) + 4H+→ 2H2O(l) + O2(g) + 2H2(g).
Thus, the electrolytic process is simply the electrolysis of plain water!
Do you know?
— The electrolysis of dilute aqueous sodium chloride solution is also equivalent to the electrolysis of plain water. The ions present are: Na+, Cl−, H+, and OH−. Both Na+ and Cl− ions would not undergo discharge as they are less likely to do so. Thus, when we electrolyze this solution using an inert electrode of platinum or carbon, we would have the following reactions happening at the cathode and anode:
At the positively charged anode:
4OH−(aq) → 2H2O(l) + O2(g) + 4e− Oxidation.
At the negatively charged cathode:
2H+(aq) + 2e− → H2(g) Reduction.
Overall ionic equation:
4OH−(aq) + 4H+→ 2H2O(l) + O2(g) + 2H2(g).
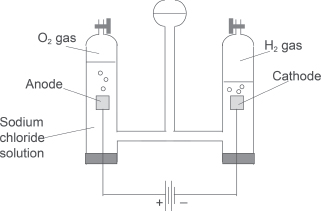
(e) It is observed that, in fact, during the electrolysis, the mass of the anode decreases and a white precipitate forms around it.
(i) Suggest an explanation for these observations.
Explanation:
As oxygen gas is evolved at the anode, it can react with the carbon electrode to give carbon monoxide and carbon dioxide gases. This accounts for the decreases in the mass of the anode. As the carbon dioxide dissolves in the alkaline solution, the CO2 undergoes the following reaction:
CO2(g) + 2OH−(aq) → CO32−(aq) + H2O(l).
The CO32−(aq) ions form the insoluble BaCO3 precipitate, which is white in color.
(ii) Give an equation that can be used to explain the decrease in mass of the anode.
Explanation:
C(s) + O2(g) → CO2(g).
(iii) Identify the white precipitate and give the equation for its formation.
Explanation:
The white precipitate is BaCO3.
(iv) Explain why the white precipitate has a high melting point.
Explanation:
Barium carbonate is an ionic compound with strong ionic bonds between the oppositely charged ions. This accounts for its high melting point.
| Q | If barium carbonate has a high melting point due to the strong ionic bonds, then why does it decompose upon heating? |
A: The melting and decomposition processes are two separate issues. Barium carbonate decomposes to barium oxide (BaO) and carbon dioxide due to the high charge density of the Ba2+ ion. The high charge density “pulls” the electron cloud of the CO32− ion toward itself. This in turn weakens the intramolecular covalent bonds within the CO32− ion, causing it to be easily broken up upon heating. Thus, when barium carbonate decomposes, it has not melted!
(3) Sodium chloride has a melting point of 801°C. Electrolysis of molten sodium chloride was carried out under an inert atmosphere of argon gas with platinum as the electrodes.
(a) Explain why sodium chloride has such a high melting point.
Explanation:
Sodium chloride is an ionic compound with strong ionic bonds between the oppositely charged ions. This accounts for its high melting point.
(b) Explain why molten sodium chloride and not solid sodium chloride was used for electrolysis.
Explanation:
In solid sodium chloride, the ions are rigidly held in the solid lattice structure. Hence, they cannot act as mobile charge carriers. In contrast, in the molten state, the ions are mobile and thus can act as charge carriers.
(c) Give the dot-and-cross diagram of sodium chloride.
Explanation:
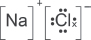
(d) Give the half-equations for the reactions that occurred at the anode and cathode. Hence, give the overall reaction equation with state symbols.
Explanation:
At the positively charged anode:
2Cl− → Cl2 + 2e− Oxidation.
At the negatively charged cathode:
Na+ + e− → Na Reduction.
Overall reaction equation:
2Cl−(l) + 2Na+(l)→ Cl2(g) + 2Na(l).
| Q | Why is the state symbol for the sodium “(l)”? Shouldn’t it be “(s)”? |
A: Under the high temperature, the sodium would have already melted.
| Q | What happened to the Cl− ions that were originally present near the cathode (negative terminal) during the electrolysis of molten sodium chloride? |
A: The Cl− near the negative terminal would be repelled by the negatively charged electrode, and likewise for the Na+ ions that were originally near the positive terminal (anode). So, the movement of charges in the electrolyte constitutes an electrical current. In addition, such a movement of charges also ensures that there is electrical neutrality in the electrolyte.
| Q | Does electrolysis help us verify the existence of ions? |
A: Certainly! For instance, the ions in a solid ionic compound are not mobile but when an electrical potential is applied across molten ionic compound, it can actually conduct electricity. This is a demonstration of the presence of ions!
(e) Describe a test you would carry out to identify the product obtained at the anode. Give the reaction equation for the positive test.
Explanation:
A piece of filter paper strip soaked with starch and potassium iodide solutions can be used to test for the presence of chlorine. If the starch paper turns blue-black, then the gas is chlorine.
Cl2(g) + 2I−(aq) → 2Cl−(aq) + I2(aq).
The chlorine oxidizes the iodide ions to form iodine, which forms a blueblack coloration with the starch.
Do you know?
— Group 17 elements are strong oxidizing agents. As a result of their high electronegativity, they tend to accept electrons readily from other substances and become reduced. The product is the halide ions, which carry a charge of –1 and has a stable octet configuration in the valence shell.
X2 + 2e− → 2X−, X = F, Cl, Br, or I.
Since electronegativity decreases down the group, it is expected that the ease in accepting electrons also decreases. In other words, the oxidizing power of the Group 17 elements decreases down the group with fluorine being the strongest and iodine the weakest oxidizing agent in the group.
— One important reaction to show the decrease in oxidizing power down Group 17 is through the displacement reaction. In a displacement reaction, the halogen which is a stronger oxidizing agent will be able to oxidize the halide ion of the weaker oxidizing agent that is below itself in the group. The halide ion is said to be displaced from the solution.
— We can make use of this type of reaction to identify the presence of Br
− and I
− ions in a solution. Simply add aqueous Cl
2 followed by an organic solvent. Upon shaking the mixture, the presence of Br
− or I
− can be confirmed as Br
2 and I
2 provides distinct coloration in the organic layer.
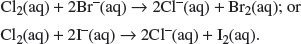
If we try to react I2 with Cl−, no reaction will occur. I− and Cl2 are not produced!

(f) State one problem you would expect when carrying out this experiment in the laboratory.
Explanation:
Chlorine is a very pungent gas that would irritate the eyes.
(g) Give the name of the reaction when the product at the cathode reacts with water. Hence, give an overall equation for this reaction.
Explanation:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g).
This is a redox reaction.
| Q | Can we call it an acid–metal reaction? |
A: It is in fact an acid–metal reaction as the water is behaving as an acid, but the expected answer is redox reaction. So, it is better not to.
(h) Give the electronic configuration of the atom of the product produced at the cathode.
Explanation:
The electronic configuration of Na is 2.8.1.
(i) Explain why the product at the cathode is highly reactive.
Explanation:
By losing an electron, the Na atom becomes the Na+ ion, which has an electronic configuration of 2.8. It thus has a stable octet configuration. This explains why the sodium is highly reactive.
| Q | But you have said before that the octet rule is not the rationale why a sodium atom would prefer to lose an electron. So, why did you use it as an explanation here? |
A: Well, what should you do? This is the requirement for the examination, you still need to answer according to what the examiner expects from you. As long as you know the actual reasoning, it is great!
(j) Identify the polarity of these two electrodes.
Explanation:
Since Na+ is attracted to the cathode to undergo reduction, the cathode is negatively charged. As the Cl− is attracted to the anode to undergo oxidation, the anode is positively charged.
(k) Explain why platinum is used as the electrodes.
Explanation:
Platinum is an inert metal that does not react with the electrolytic products.
(l) Why was the electrolysis carried out under an inert atmosphere of argon.
Explanation:
Argon is an inert gas that does not react with the electrolytic products.
(m) What would be the result you would obtain if concentrated aqueous sodium chloride was used instead of molten sodium chloride?
Explanation:
If concentrated aqueous sodium chloride was used instead of molten sodium chloride, then the following reactions would take place at the anode and cathode:
At the positively charged anode:
2Cl−(aq) → Cl2(g) + 2e− Oxidation.
At the negatively charged cathode:
2H+(aq) + 2e− → H2(g) Reduction.
Overall ionic equation:
2Cl−(aq) + 2H+→ Cl2(g) + H2(g).
(n) What would be the result you would obtain if dilute aqueous sodium chloride was used instead of molten sodium chloride?
Explanation:
If dilute aqueous sodium chloride was used instead of molten sodium chloride, then the following reactions would take place at the anode and cathode:
At the positively charged anode:
4OH−(aq) → 2H2O(l) + O2(g) + 4e− Oxidation.
At the negatively charged cathode:
2H+(aq) + 2e− → H2(g) Reduction.
Overall ionic equation:
4OH−(aq) + 4H+(aq) → 2H2O(l) + O2(g) + 2H2(g).
(4) The extraction of aluminum from its ore is done by electrolyzing a mixture of aluminum oxide and cryolite (NaAlF
4) at 800°C using carbon electrodes.
(a) Give the dot-and-cross diagram of aluminum oxide.
Explanation:

Do you know?
— As aluminium is above carbon in the reactivity series, it is extracted through electrolysis. There are two important stages involved in the extraction process of pure aluminum:
Stage 1: Concentrating the ore
The mineral must first be separated from most of the worthless compounds in the bauxite ore, usually by physical methods. But for aluminum, bauxite is reacted with hot concentrated sodium hydroxide to isolate the aluminum oxide:
Al2O3 + 2NaOH + 3H2O → 2NaAl(OH)4.
The hot solution is then cooled to crystallize out the aluminum(III) hydroxide:
NaAl(OH)4 → Al(OH)3 + NaOH.
Then, the aluminum(III) oxide is obtained by dehydrating the aluminum(III) hydroxide:
2Al(OH)3 → Al2O3 + 3H2O.
Stage 2: Extracting the crude metal from the ore
The aluminum oxide is electrolyzed by dissolving it in molten cryolite, Na3AlF6, at about 1000° C. This helps to save electrical energy as aluminum oxide melts at about 2000° C. The following show the electrolytic set-up:
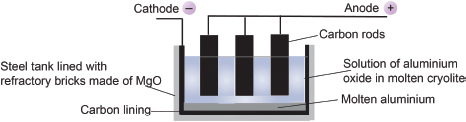
Simplistically, the following reactions are thought to happen:
Anode: 2O2− → O2 + 4e−
Cathode: Al3+ + 3e− → Al
Overall ionic equation: 4Al3+(l) + 6O2−(l) → 4Al(l) + 3O2(g).
As the temperature of the electrolytic bath is high, the oxygen reacts with the carbon electrodes to form carbon monoxide and carbon dioxide gases. As a result, the electrodes need constant replacement. The molten aluminum that is formed at the anode can simply be drained off from the bottom of the bath.
(b) Why is a mixture of aluminum oxide and cryolite used in the above instead of pure aluminum oxide?
Explanation:
Aluminum oxide melts at about 2000°C. With the addition of cryolite, the melting point is lowered to about 1000°C. This helps to save electrical energy.
(c) Write the ionic equations for the reactions that occurred at the anode and cathode.
Explanation:
Anode: 2O2− → O2 + 4e−.
Cathode: Al3+ + 3e− → Al.
(d) After some time, the carbon electrode decreased in mass and was needed to be replaced. Explain.
Explanation:
As the temperature of the electrolytic bath is high, the oxygen reacts with the carbon electrodes to form carbon monoxide and carbon dioxide. As a result, the electrodes need constant replacement.
(e) Write an equation to help explain the decrease in mass of the anode.
Explanation:
C(s) + O2(g) → CO2(g).
(f) Given the trouble of regular replacement of a graphite anode, would you suggest replacing the graphite anode with a steel anode? Explain your reason.
Explanation:
I would not suggest replacing the graphite anode with a steel anode. This is because although the melting point of steel is higher than the operating temperature of the electrolytic process, the structure of steel may actually weaken during the high operating temperature.
| Q | Can I give “yes” as an answer instead? |
A: Well, you can. Then all you need to do is to give the reason that steel has a high melting point, thus there is no need for regular replacement. But if steel can really replace carbon as the anode electrode, then why did your teacher explain to you that carbon electrodes are still used in the electrolysis of aluminum oxide?
(g) Aluminum is commonly used to make saucepans although steel is stronger and cheaper. Give a reason.
Explanation:
Aluminum is relatively more resistant to corrosion than steel because the layer of aluminum oxide coated on the surface is impervious to further chemical attack by air and water. In addition, aluminum is lighter than steel. This makes the saucepans lighter, and hence, easier to handle.
Do you know?
— Although the layer of aluminum oxide can be formed naturally in air, the layer is not thick enough. The layer of aluminum oxide on the surface of the saucepan can be increased via anodization, i.e., by making the aluminum objects as the anode during the electrolytic process. The layer of aluminum oxide is of substantial commercial and technological importance for the prevention of corrosion for automobile and aerospace structures and also for electrical insulation.
— Anodization of aluminum is usually carried out in a sulfuric acid electrolyte. During the electrolytic process of anodization, oxygen gas is discharged at the anode, which would react with the unoxidized aluminum metal to form a thick aluminum(III) oxide layer. The freshly formed film can then further be dyed to give color-anodized aluminum. The cathode can be graphite, stainless steel, or other electrical conductors that are inert in the anodizing bath.
At the positively charged anode:
4OH−(aq) → 2H2O(l) + O2(g) + 4e−
4Al(s) + 3O2(g) → 2Al2O3(s).
At the negatively charged cathode:
2H+(aq) + 2e– → H2(g).
| Q | If the electrolyte is sulfuric acid, wouldn’t it react with the amphoteric Al2O3? |
A: It would not react because the H+ would be repelled from the anode, which is positively charged!
(h) Calculate the maximum possible mass of aluminum that can be extracted from 10 kg of aluminum oxide.
Explanation:
Molar mass of Al2O3 = 2(27.0) + 3(16.0) = 102.0 g mol−1.
Amount of Al2O3 in 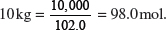
Amount of Al that can be extracted = 2 × 98.0 = 196.0 mol.
Maximum possible mass of aluminum that can be extracted = 196.0 × 27.0 = 5294 g.
(i) Aluminum is extracted from aluminum oxide. A major use of aluminum is in the manufacture of drink cans. A typical drink can contains 9.0 g of aluminum. Calculate the mass of aluminum oxide required to make a drink can.
Explanation:
Amount of Al in 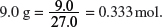
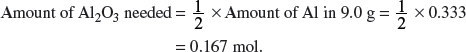
Mass of Al2O3 needed = 0.167 × 102.0 = 17.0 g.
(j) Suggest why manufacturers of drink cans are always trying to find ways of reducing the mass of aluminum required for a typical drink can.
Explanation:
By reducing the mass of aluminum that is required for a typical drink can, less aluminum oxide is needed. More money can be saved as smaller amount of energy would be used to extract a smaller amount of aluminum from the oxide.
(k) Explain why the recycling of aluminum is economically viable.
Explanation:
The costs involved in the extraction, especially the amount of electrical current needed, are high. Usually for metals that are obtained through electrolysis, it is not cheap. Thus, recycling of metals obtained via electrolytic means is viable.