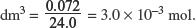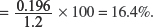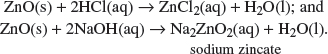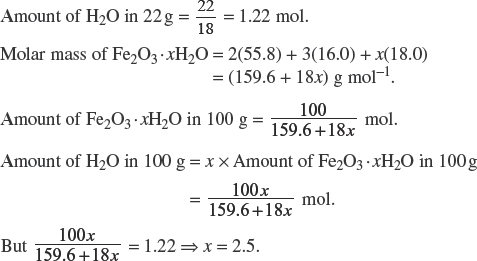CHAPTER 13
METALS AND EXTRACTION
Do you know?
— Most metals are found in the ground as compounds called minerals. Impure minerals are called ores. An
ore is a naturally occurring source of a metal, from which the metal is extracted via economical means. The ore of a metal is usually in the form of the oxides, sulfides, chlorides, or carbonates.

In Chapter 10, we learned that for metals that are above carbon in the reactivity series (K, Na, Ca, Mg, and Al), their oxides cannot be reduced by carbon back to their metallic form. Therefore, to extract the metal, electrolysis has to be employed. This thus makes the metals expensive. Oxides of Zn, Fe, Pb, and Cu can be reduced by carbon through heating as these metals are below carbon in the reactivity series. The prices of the metals that are extracted through such a method are relatively inexpensive.
(1) Iron is extracted form hematite, which contains iron(III) oxide, using the blast furnace.
(a) Explain why carbon can be used to extract iron from its oxide ores.
Explanation:
Iron is below carbon in the reactivity series. This makes carbon more likely to undergo oxidation as compared to iron. Hence, carbon can be used to reduce iron(III) oxide into the iron metal.
| Q | If the metal is below carbon in the reactivity series, it means that carbon is more likely to undergo oxidation than the metal. So how does this actually enable carbon to reduce the metal oxide? |
A: If you put the metal oxide and carbon together, since carbon is above the metal in the reactivity series, then CO2 is preferred to the metal oxide. The carbon will “extract” the oxygen from the oxide to form CO2, leaving the reduced metal oxide, which is the metal, behind. Get it? May be the following would be helpful:
Metal oxide + Carbon → Metal + Carbon dioxide.
Carbon dioxide is preferred over the metal oxide.
The metal is preferred over carbon.
Therefore, the right-hand side of the equation is preferred!
Do you know?
— Iron is extracted from its ore, hematite, using the blast furnace. A mixture of the iron ore, limestone (CaCO
3), and coke (carbon) is fed into the blast furnace from the top:

Hot air that has been preheated by the hot waste gases is introduced at the bottom of the furnace. In the blast furnace, there are a few important reactions taking place:
1. Formation of carbon dioxide
The burning of coke is highly exothermic, which provides the energy that is needed for the reduction:
C(s) + O2(g) → CO2(g).
The decomposition of limestone, CaCO3, also produces CO2:
CaCO3(s) → CaO(s) + CO2(g).
The purpose of the calcium(II) oxide is to help to remove impurities such as sand and clay in the ore, through the following reaction:
CaO(s) + SiO2(s) → CaSiO3(l).
The molten slag, CaSiO3(l), which is lighter than molten iron, can be drained off easily from the bottom of the furnace.
2. Formation of carbon monoxide
The carbon dioxide formed from the burning of coke would be reduced to carbon monoxide when it encounters more coke:
CO2(g) + C(s) → 2CO(g).
3. Reduction of iron(III) oxide in hematite
It is the carbon monoxide that is responsible for reducing the iron(III) oxide to iron:
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g).
Molten iron is known as pig iron or cast iron.
The waste gases, which include carbon dioxide, carbon monoxide, and others, are released into the atmosphere. This is environmentally unfriendly!
(b) Name and explain the type of chemical reaction involved when iron ore is converted to iron.
Explanation:
When Fe2O3 is converted to iron, the oxidation state of Fe3+ decreases from +3 to 0. This is a reduction process. Thus, there must be a corresponding oxidation reaction happening at the same time, which is the oxidation of the CO to CO2. Therefore, the overall reaction is redox in nature.
| Q | So, the oxidation state of carbon in CO increases from +2 to +4 in CO2? |
A: You are right!
(c) Write a balanced chemical equation for the reaction between iron(III) oxide and carbon monoxide.
Explanation:
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g).
(d) Draw the dot-and-cross diagram of iron(III) oxide.
Explanation:
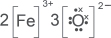
(e) Draw the dot-and-cross diagram of carbon monoxide if both atoms have achieved the stable octet configuration.
Explanation:
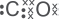
(f) What is the use of limestone in the blast furnace?
Explanation:
The decomposition of limestone, CaCO3, produces CaO and CO2:
CaCO3(s) → CaO(s) + CO2(g).
The purpose of the calcium(II) oxide is to help remove impurities such as sand and clay in the ore, through the following reaction:
CaO(s) + SiO2(s) → CaSiO3(l).
The CO2 is used to oxidize carbon to CO:
CO2(g) + C(s) → 2CO(g).
(g) The waste gases consist of carbon dioxide, carbon monoxide, and nitrogen. Explain the presence of each of these gases.
Explanation:
Carbon dioxide is produced from the oxidation of carbon by oxygen and also from the decomposition of the CaCO3.
Carbon monoxide is produced from the oxidation of carbon by carbon dioxide.
Nitrogen is from the air that is pumped into the furnace in which the oxygen is used to oxidize the carbon while the nitrogen remains unreacted.
(h) Given that 28 tons (1 ton = 1000 kg) of hematite are loaded into the furnace, calculate
(i) the minimum mass of coke required for complete extraction of the iron.
Explanation:
Molar mass of Fe2O3 = 2(55.8) + 3(16.0) = 159.6 g mol−1.
Amount of Fe2O3 in 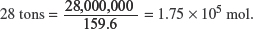
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g).
One mole of Fe2O3 reacts with three moles of CO. Assuming that all the CO that is needed come from coke (C), then 1.75 × 105 mol of Fe2O3 needs = 1.75 × 105 × 3 = 5.25 × 105 mol of CO.
Therefore, the minimum mass of coke that is required for the complete extraction of the iron = 5.25 × 105 × 12.0 = 6.30 × 106 g = 6.30 × 103 tons.
| Q | Why do you need to assume that all the CO that is needed comes from coke? |
A: In addition to the CO2 from the burning of the coke, the CO2 that is produced from the decomposition of CaCO3 can also help to convert the carbon to carbon monoxide as follows:
CO2(g) + C(s) → 2CO(g).
(ii) the maximum possible mass of iron extracted.
Explanation:
Amount of Fe extracted = 2 × Amount of Fe2O3 in 28 tons = 3.50 × 105 mol. Therefore, the maximum possible mass of iron extracted = 3.50 × 105 × 55.8 = 1.95 × 107 g.
(i) Given that only 18 tons of iron were retrieved from part (h), calculate the percentage purity of the hematite.
Explanation:
Amount of Fe in 18 tons 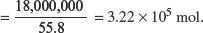
Amount of 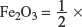 Amount of Fe in 28 tons = 1.61 × 105 mol.
Amount of Fe in 28 tons = 1.61 × 105 mol.
Mass of Fe2O3 = 1.61 × 105 × 159.6 = 2.57 × 107 g = 25.7 tons.
Percentage purity of hematite 
(j) Iron is often alloyed to reduce rusting.
(i) Explain the term alloy.
Explanation:
A pure metal by itself is soft, thus they are malleable and ductile. But if we introduce another metal atom of a different size into the regular lattice structure of a metal, we can increase the strength of the metal. Such a solid solution of metals is known as an alloy. Sometimes, instead of introducing another metal atom, carbon atoms are used instead.
| Q | Why is the strength of an alloy much stronger than that of a pure metal? |
A: As the size of the atom of the impurity is different from that of the metal, it disrupts the regular arrangement of the atoms in the pure metal. Thus, when a force is applied, the atom of the impurity, which is bigger in size, would prevent the layers of atoms from sliding over one another. This makes the metal harder!
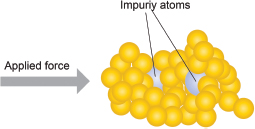
A: An alloy is usually made by melting different metal components together and then solidifying the mixture. As such, the metal components of an alloy must be soluble in one another when in the molten state, and they should not separate into distinct layers when solidified.
Do you know?
— Properties that an alloy has but are absent in a pure metal:
• An alloy is usually harder. For instance, brass, which is a composite of zinc and copper, is harder than its constituent metals.
• An alloy can be more resistant to corrosion. For instance, stainless steel, which is iron doped with other atoms such as carbon, chromium, manganese, etc., is more resistant to corrosion than pure iron itself.
• An alloy can have a lower melting point than the pure metal. For instance, solder, which is an alloy of tin and lead, has a lower melting point than its constituent metals.
• An alloy can have a better appearance than its constituent metals. For instance, pewter is an alloy of tin, copper, and antimony. It is aesthetically more appealing than the dull gray tin.
(ii) Name one element besides carbon that is used for this purpose.
Explanation:
Manganese or chromium.
(iii) Explain why an alloy can be more resistant to corrosion than a pure metal.
Explanation:
The metal that is used to dope the pure metal may be more likely to undergo corrosion than the pure metal itself. This thus protects the pure metal from undergoing corrosion.
Do you know?
— Other than using alloying to minimize corrosion, the common methods for rust prevention are:
Painting
Coating iron with a layer of paint excludes water and oxygen from coming in contact with the iron; this helps to prevent corrosion.
Galvanization
By coating a layer of zinc, which corrodes preferentially to the iron due to its higher reactivity (refer to Chapter 10 on Electric Cells and the Reactivity Series), the iron would be protected.
Cathodic protection
A piece of more reactive metal, such as magnesium, is connected to the iron. As the magnesium preferentially undergoes oxidation, it is acting as an anode. The electrons from this oxidation process would flow to the iron. This makes the iron electron-rich, in which only reduction can happen there. Hence, the iron is acting as a cathode where it cannot undergo oxidation. As time passes, the magnesium rod disappears and is replaced with a new one. The magnesium metal is known as the sacrificial metal. This method is commonly used to protect iron pipes.
(iv) Iron is malleable. Describe how this property can be explained in terms of its structure.
Explanation:
The atoms in iron are held together by metallic bonds which are strong and non-directional. The metallic bonds are the electrostatic attractive forces between the positive ions and the delocalized electrons. Thus, when a force is applied to a piece of iron, the atoms simply move over one another without breaking of the metallic bonds. This thus accounts for the malleability and ductility of metals.

(v) Explain why the strength of an alloy is different from that of a pure metal.
Explanation:
As the size of the atom of the impurity is different from that of the metal, it disrupts the regular arrangement of the atoms of the pure metal. Thus, when a force is applied, the impurity atom which is bigger in size, would prevent the layers of atoms from sliding over one another. This makes the metal harder.
(vi) Explain why iron is a good conductor of heat and electricity.
Explanation:
The solid lattice structure of iron has a sea of delocalized electrons which can act as mobile charge carriers under the influence of an applied electrical potential. This accounts for the good electrical conductivity of the metal.
In addition, because the delocalized electrons are small and light, they can transfer heat energy across a piece of metal more efficiently through collisions among themselves. This accounts for the good thermal conductivity of the metal.
(vii) Explain why hot water tanks used in industries are made of copper and not steel, which is an alloy containing iron.
Explanation:
Copper does not corrode under hot water as easily as steel does. In addition, copper is a better conductor of heat energy than steel, thus a hot water tank that is made of copper can heat up the water more uniformly.
| Q | Wait a minute, if we use stainless steel instead, would it be better as corrosion is minimized? |
A: Stainless steel does not corrode as easily as steel, but copper is still more resistant to corrosion in the long run.
| Q | Why is copper a better conductor of heat than steel? |
A: This is because the copper solid lattice has more delocalized electrons than steel. Thus, more heat energy can be transferred faster across a piece of copper of the same dimensions than a piece of steel. This also accounts for the higher electrical conductivity of copper compared to steel.
| Q | If copper is better at conducting heat, won’t it lose heat more easily than steel? |
A: Good thinking! This problem can be solved by using insulation. Thus, overall, it is still better to use copper to construct a hot water tank.
(k) State two other metals that can be extracted using carbon. Hence, explain why aluminum cannot be extracted from aluminum oxide using the method mentioned.
Explanation:
The two metals are tin and zinc.
Since alumimum is above carbon in the reactivity series, this implies that aluminum metal is more likely to undergo oxidation than carbon. This would mean that carbon cannot be used to reduce aluminum oxide.
(l) What technological breakthrough was required before the possible extraction of metallic aluminum?
Explanation:
The melting point of aluminum oxide is simply too high to melt and extract the aluminum economically. With the introduction of cryolite, Na3AlF6, the melting point is lowered drastically. This thus allows the aluminum to be extracted from the aluminum oxide through electrolytic means.
Do you know?
— The reactions that are thought to occur at the respective electrodes during the electrolysis of molten Al
2O
3 are as follows:
Anode: 2O2− → O2 + 4e−.
Cathode: Al3+ + 3e− → Al.
Overall ionic equation: 4Al3+(l) + 6O2−(l) → 4Al(l) + 3O2(g).
(m) A student suspected that a dilute solution of hydrochloric acid could speed up the rusting of iron. To test his hunch, the student immersed a piece of iron nail in a test tube half-filled with tap water. He then added a few drops of the acid.
(i) Can the student use boiled water in place of the tap water? Explain.
Explanation:
The student cannot use boiled water in place of tap water as boiled water lacks oxygen. This is because the rusting of iron needs both water and oxygen to be present simultaneously.
Do you know?
— The brown rust that is formed is actually a form of hydrated iron(III) oxide:
4Fe(s) + 3O2(g) + 2xH2O(l) → 2Fe2O3 · xH2O(s).
| Q | Do all metals corrode when expose to air? |
A: Both aluminum and chromium are protected from corrosion because of the formation of an impervious layer of oxide. That is why you need to know of the anodization of aluminum and chromium plating. These can help prevent corrosion.
(ii) What would happen when a layer of oil is spread on the surface of the water in the test tube? Explain.
Explanation:
The layer of oil would exclude oxygen gas from dissolving in the water. Thus, rusting would stop after some time when the oxygen that has already been dissolved in the water is used up.
(iii) In order to test the reliability of his hunch, a control experiment needed to be set up. Why was this important?
Explanation:
If there is no control experiment, he would not be able to compare the rate of rusting as there would be nothing to compare against.
(iv) Describe an appropriate set-up for the control experiment.
Explanation:
Since the presence of the acid is the subject of study, an appropriate set-up for the control experiment would be to immerse a piece of iron nail in a testtube that is half-filled with tap water, but without the addition of the acid. The two set-ups must be placed side-by-side under the same temperature.
(v) Other than excluding the factors that would accelerate rusting, suggest other methods that may be helpful to prevent corrosion.
Explanation:
To prevent corrosion, we can (i) electroplate the metal with chromium; (ii) paint the metal with a layer of paint; (iii) coat a layer of zinc that preferably undergoes corrosion (galvanization); and (iv) connect a piece of more reactive metal, such as magnesium, to the iron (cathodic prevention).
(n) A sample of a compound of iron is analyzed. The sample contains 0.547 g of potassium, 0.195 g of iron, 0.252 g of carbon, and 0.294 g of nitrogen. Calculate the empirical formula of this compound.
Explanation:
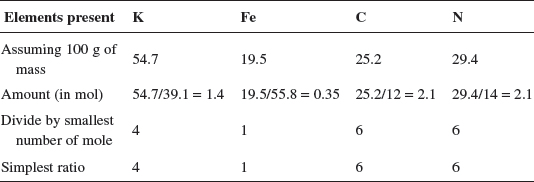
The empirical formula of the unknown compound is K4FeC6N6.
2. The metal antimony, Sb, has a proton number of 51. It can be extracted from its oxide via a reaction with carbon in a furnace. The equation for the reaction is:
Sb2O3(s) + 3C(s) → 2Sb(s) + 3CO(g).
(a) What is the relationship between the reactivity of the metals and their method of extraction?
Explanation:
Metals that are above carbon in the reactivity series are more likely to undergo oxidation than carbon. Hence, these metals cannot be extracted from their ores by reduction using carbon. So, to extract these metals, electrolysis has to be employed. As for the metals below carbon in the reactivity series, they can be extracted simply through reduction with carbon.
(b) From the method of extraction of antimony, suggest where the metal might be placed in the reactivity series.
Explanation:
Antimony is below carbon in the reactivity series.
(c) State the oxidation number for the atoms involved in the above chemical reaction. Hence, state the nature of the above reaction.
Explanation:
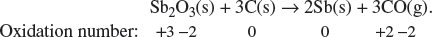
Sb undergoes reduction as its oxidation state decreases from +3 to 0. Carbon undergoes oxidation as its oxidation state increases from 0 to +2.
Hence, the above reaction is a redox reaction.
(d) What is the maximum amount of antimony, in moles, that can be produced from the reaction of antimony oxide with one mole of carbon?
Explanation:
One mole of carbon would give 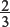 moles of antimony.
moles of antimony.
(e) What is the maximum mass of antimony, in tons, that can be formed from 25 tons of its oxide?
Explanation:
Molar mass of Sb2O3 = 2(121.8) + 3(16.0) = 291.6 g mol−1.
Amount of 
Amount of Sb = 2 × Amount of Sb2O3 = 1.71 × 105 mol.
Mass of Sb = 1.71 × 105 × 121.8 = 2.09 × 107 g = 20.9 tons.
(f) Based on the position of antimony in the Periodic Table, suggest the physical properties of pure antimony.
Explanation:
Antimony is positioned after tin and before iodine in the same period of the Periodic Table. Since tin is a metal while iodine is a non-metal, we would expect antimony to have the characteristics of a metalloid.
(g) State the other possible oxidation state for antimony.
Explanation:
Antimony belongs to Group 15, possessing five valence electrons. Hence, the two possible oxidation states for antimony are +3 and +5. Thus, the other possible oxidation state is +5.
(h) Explain your choice of the oxidation state in part (g).
Explanation:
The maximum oxidation state of an element corresponds to the number of valence electrons it has. This explains the choice of the oxidation state.
(i) How would you expect the reducing power of antimony to be as compared to phosphorous? Explain.
Explanation:
The reducing power of antimony is greater than that of phosphorous. The valence electrons of the elements that are lower down a group are farther away from the nucleus. Hence, the valence electrons are more easily removed. This would imply that elements that are lower down in a group are easily oxidized, thus having a greater reducing power.
| Q | If the reducing power increases down a group, does that mean that the oxidizing power would decrease down the group? |
A: Brilliant! An oxidizing agent undergoes reduction, i.e., takes in electrons. Down a group, since the valence shell is farther away from the nucleus, the extra electrons that an oxidizing agent takes in would be less strongly attracted. Hence, the oxidizing power would decrease down the group!
(j) How would you expect the oxidizing power of antimony to be as compared to tin? Explain.
Explanation:
An oxidizing agent takes in electrons and thus undergoes reduction. Antimony and tin belong to the same period. Antimony is positioned after tin in the same period. Since the electrostatic attractive force acting on the valence electrons increases across a period, we would expect the extra electrons that antimony takes in to be more strongly attracted than those for tin. Thus, antimony would have a greater oxidizing power than tin.
| Q | If the oxidizing power increases across a period, does that mean the reducing power would decrease across the period? |
A: Yes, you are right! A reducing agent undergoes oxidation, i.e., loses electrons. It is thus more difficult to lose electrons across a period.
Do you know?
— To understand whether it is easier to lose electrons across a period of elements, let us look at the electronic configurations of some Period 3 elements:
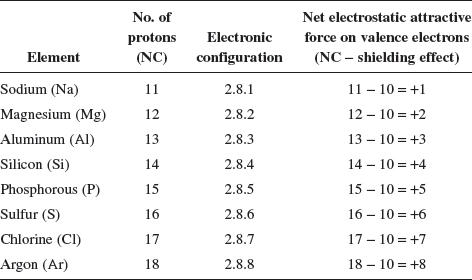
Do you see that the net electrostatic attractive force acting on the valence electrons has increased across the elements in Period 3? This trend is true even with Periods 2 or 4. So, with an increase in the net electrostatic attractive force acting on the valence electrons, we would expect increasing difficulty to remove valence electrons across the period. Relatively, it would mean that there is an increasing affinity for the elements to gain electrons across the period!
In a nutshell, remember:
• Elements across a period possess increasing ease in gaining electrons BUT decreasing ease in losing electrons.
• Elements down a group possess decreasing ease in gaining electrons BUT increasing ease in losing electron.
3. In the Stone Age, the only metals available were gold and silver. Later, the Bronze Age came about. Bronze is made by mixing copper and tin.
(a) What is the name given to such a mixture of metals from different elements?
Explanation:
A mixture of metals from different elements is known as an alloy.
(b) Why is bronze used instead of pure copper or tin?
Explanation:
Bronze is probably harder and stronger than pure copper or tin.
| Q | What is that greenish compound that is coated on a piece of old bronze artifact? |
A: It is probably copper(II) carbonate that was formed from the reaction between copper(II) oxide and carbon dioxide in the air:
CuO(s) + CO2(g) → CuCO3(s).
(c) Tin was extracted from its ore
cassiterite, SnO
2, through the process called smelting. In smelting, the ore is heated with carbon.
(i) What type of compound, ionic or covalent, is SnO2?
Explanation:
SnO2 is a covalent compound.
| Q | Why is SnO2 not an ionic compound? |
A: This is another example in which metal and non-metal does not react to give an ionic compound. If SnO2 is an ionic compound, then it would consist of Sn4+ and O2− ions. This would mean that a Sn atom has to lose four electrons. It is simply too energetically demanding to do that. Even if it does, the charge density of Sn4+ is too great. Its polarizing power would be very strong. This would then make SnO2 a covalent compound. Hence, when Sn and O2 react, SnO2 has to be a covalent compound.
(ii) Draw the dot-and-cross diagram of SnO2.
Explanation:

(iii) Write the chemical equation for the above extraction reaction.
Explanation:
C(s) + SnO2(s) → Sn(s) + CO2(g).
(iv) In view of the extraction reaction, where would the position of carbon be in the metal reactivity series relative to tin?
Explanation:
Carbon would be above tin in the reactivity series. This is because metals that are below carbon in the reactivity series are less likely to undergo oxidation than carbon. Hence, these metals can be extracted from their ores by reduction using carbon.
(d) Brass is another alloy consisting of zinc and copper.
(i) Describe, with the aid of a labeled diagram, the structure of a metal such as copper.
Explanation:
The copper metal can be viewed as a rigid lattice of positive ions surrounded by a sea of delocalized electrons. What holds the lattice together is the strong metallic bonding — the electrostatic attraction between the positive ions and the delocalized valence electrons.

(ii) Explain, in terms of their structures, why both zinc and copper are good conductors of electricity.
Explanation:
Both zinc and copper are good conductors of electricity because of the presence of the sea of delocalized electrons which can act as mobile charge carriers when an electrical potential is applied across a piece of the metal.
(iii) A 1.2 g sample of powdered brass was analyzed by reaction with excess dilute sulfuric acid. The zinc reacts as shown in the equation to form 0.072 dm
3 of hydrogen measured at room temperature and pressure:
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g).
(I) Give a name for the above reaction.
Explanation:
The reaction is an acid–metal reaction or a redox reaction.
| Q | Why does the copper not react with the H+? |
A: Copper is below hydrogen gas in the reactivity series. This means that hydrogen would prefer to be oxidized than copper. Thus, when you mix copper and acid together, the copper cannot reduce the H+ back to H2.
| Q | So, does it mean that we can use hydrogen gas to reduce copper oxide back to copper? |
A: Certainly. The concept is similar to the fact that you can use carbon to reduce any metal oxide only if the metals are below carbon in the reactivity series.
| Q | If copper does not react with acids, why does a piece of copper dumped into nitric acid produce brown fumes? |
A: Copper does not react with the H+ from HNO3 but it does react with the NO3− ion. This accounts for the formation of brown fumes which is actually NO2 gas. If you dump a piece of copper into HCl or H2SO4, there is no acid–metal reaction at all!
(II) What is the nature of the above reaction?
Explanation:
The reaction is in fact redox in nature. This is because Zn undergoes oxidation as its oxidation state increases from 0 to +2, while H+ undergoes reduction as its oxidation state decreases from +1 to 0.
(III) Suggest why brass was used in a powdered form rather than in a lump.
Explanation:
The powdered form ensures a greater rate of reaction between the metal and acid due to the greater surface area of contact between the acid and metal.
(IV) Calculate the mass of zinc in the sample of brass.
Explanation:
At room temperature and pressure, 1 mole of gas has a volume of 24.0 dm3.
Amount of H in 0.072 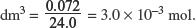
Amount of Zn = Amount of H2 in 0.072 dm3 = 3.0 × 10−3 mol.
Therefore, mass of Zn = 3.0 × 10–3 × 65.4 = 0.196 g.
(V) Calculate the percentage of zinc in the sample of brass.
Explanation:
Percentage of zinc in the sample of brass 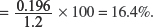
(VI) Describe how aqueous ammonia can be used to show that only the zinc in the sample reacted with the acid.
Explanation:
If aqueous ammonia is added to the solution slowly till in excess, a white precipitate of Zn(OH)2 would form. In excess aqueous ammonia, the white precipitate would dissolve to give a colorless solution.
Do you know?
— Zinc oxide is an amphoteric oxide. It can react with both an acid and base:
(VII) If you are to study how the rate of the reaction is dependent on the concentration of the acid, with the aid of a labeled diagram, briefly describe how you would do it.
Explanation:
The volume of hydrogen gas can be collected as shown below:

The volume of the hydrogen that is collected can be monitored with respect to time. This would give us the rate of the reaction for different concentrations of the acid used.
4. In the early 19th century, cans made of tin-plated iron were first used to preserve food. Tin has several advantages, including: it corrodes much less readily than iron and it forms a protective oxide layer over the iron. When the tin coating is scratched, however, the iron rusts faster when it is in contact with tin. Fortunately, neither Fe
2+ nor Sn
2+ ions are toxic.
(a) State two substances which are necessary for iron to rust and from which iron is protected by the tin layer.
Explanation:
Essentially, iron needs oxygen and water in order to rust. The tin layer would exclude the iron from coming in contact with these two elements for rusting.
(b) Write a balanced equation for the reaction where the presence of tin ions encourages the iron to corrode.
Explanation:
Sn2+ + Fe → Fe2+ + Sn.
| Q | Why does the Sn2+ ion cause the Fe to corrode? |
A: From the reactivity series, iron is above tin. This means that iron would prefer to undergo oxidation than tin. Hence, when you have Fe and Sn2+ together, the Fe would prefer to become Fe2+, while Sn2+ prefers to be formed back to Sn.
(c) Rust is often given the formula Fe2O3 · xH2O, where x has a variable non-integral value. Calculate the value of x for a sample of rust which lost 22% mass (as steam) when heated to constant mass.
Explanation:
Assuming 100 g of Fe2O3 · xH2O, there are 22 g of H2O.
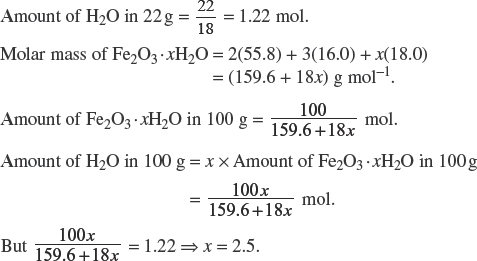
(d) An underground iron pipe is less likely to corrode if bonded at intervals to magnesium stakes. Give a reason for this. Explain why aluminum would be a poor substitute for magnesium.
Explanation:
Magnesium is above iron in the reactivity series. This means that magnesium would prefer to undergo oxidation than iron. When magnesium is oxidized, the electrons would flow to the iron. This would make the iron less likely to be oxidized.
Although aluminum is also above iron in the reactivity series, the oxidation of aluminum would create a layer of impervious aluminum oxide. As a result, oxygen cannot penetrate this layer of oxide layer. Hence, the aluminum cannot protect the iron from undergoing further oxidation.



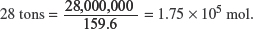
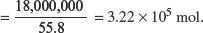
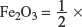 Amount of Fe in 28 tons = 1.61 × 105 mol.
Amount of Fe in 28 tons = 1.61 × 105 mol.



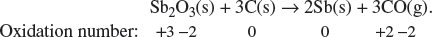
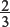 moles of antimony.
moles of antimony.