
(i) Write the equation for this reaction.
N2(g) + O2(g) → 2NO(g).
Do you know?
CO2(g) + H2O(l) ⇌ H2CO3(aq).
However, if there are additional SO2 and NO2 in the air, the acidity of rainwater would increase because of the formation of “extra” acids:
SO2(g) + H2O(l) ⇌ H2SO3(aq);
and
4NO2(g) + O2(g) + 2H2O(l) → 4HNO3(aq).
This unusally acidic rainwater or other forms of precipitation, such as snow, is known as acid rain. It can have harmful effects on plants, aquatic life, and infrastructure:
• It reacts with metals and carbonates, hence damaging infrastructure.
• It lowers the pH of water bodies, hence affecting aquatic life.
• It reacts with important nutrients from the soil, such as phosphates, hence affecting plant growth.
(ii) Draw the dot-and-cross diagram of nitrogen monoxide.

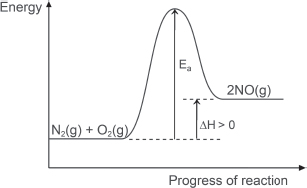
(iii) Explain why a high temperature is needed for the reaction.
A high temperature is needed to provide energy to overcome the strong N≡N triple bond.
| Q | So, does this mean that the activation energy for the above reaction is very high due to the strong N≡N triple bond? |
A: You are right!
Do you know?
N2(g) + O2(g) → 2NO(g).
This nitrogen monoxide can be further oxidized to form the brown nitrogen dioxide:
2NO(g) + O2(g) → 2NO2(g).
Nitrogen dioxide is also formed when the heat energy from lightning causes the nitrogen and oxygen to react during a thunderstorm. The smell of nitrogen dioxide can be obvious after the storm. NO2 dissolves in water to give acid rain and is an irritant to the eyes and lungs.
(iv) Given that the ΔH for the formation of nitrogen monoxide is positive, draw an energy profile diagram for the reaction of nitrogen with oxygen to form nitrogen monoxide.
SO2(g) + CaCO3(s) → CaSO3(s) + CO2(g).
(i) What is the oxidation state of the sulfur atom in sulfur dioxide?
Let the oxidation state of sulfur atom be x.
Therefore, x + 2(−2) = 0 {As SO2 is electrically neutral.} ⇒ x = +4.
(ii) Given that sulfur dioxide is an acidic gas, what is the nature of the above reaction?
The above reaction is an acid–base reaction since sulfur dioxide is acidic while CaCO3 is basic in nature.
| Q | Why is CO32− basic in nature? |
A: The CO32−ion comes from the dissociation of the weak carbonic acid, H2CO3:

When an acid dissociates, it would form the conjugate base ion. As the CO32− ion comes from a weak acid, when it is in water, it undergoes the following basic hydrolysis, resulting in the formation of a basic solution:
CO32–(aq) + H2O(l) ⇌ HCO3−(aq) + OH−(aq).
(iii) Suggest why the calcium carbonate is powdered.
Powdered calcium carbonate provides a greater surface area of contact for the SO2 gas to react. This would minimize the escape of the SO2 into the atmosphere.
(iv) If nitrogen dioxide behaves similarly to sulfur dioxide, write an equation to show how nitrogen dioxide reacts with calcium carbonate.
3NO2(g) + CaCO3(s) → Ca(NO3)2(s) + CO2(g) + NO(g).
(v) State a source of calcium carbonate.
Calcium carbonate comes from limestone.
(vi) Given that nitrogen dioxide forms a dimer easily, draw the dot-andcross diagram of nitrogen dioxide and use it to explain the ease of dimerization.

Nitrogen dioxide forms a dimer easily because in the dimer, all the atoms would achieve the octet configuration as shown below:

| Q | Other than using the octet rule, what other explanation can be used to explain why nitrogen dioxide dimerizes? |
A: When nitrogen dioxide dimerizes, the energy level of the dimer is lower than that of the monomer because when a bond is formed, energy is evolved. The evolution of energy lowers the energy level of the products. This is the driving force behind the dimerization process.
(vii) What is the color of nitrogen dioxide?
The colour of nitrogen dioxide is brown.
(viii) Calculate the mass of calcium carbonate needed to react with 900 kg of sulfur dioxide.
SO2(g) + CaCO3(s) → CaSO3(s) + CO2(g).
Molar mass of SO2 = 32.1 + 2(16.0) = 64.1 g mol−1.
Amount of SO2 in 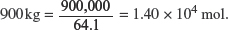
Amount of CaCO3 needed = Amount of SO2 in 900 kg = 1.40 × 104 mol.
Molar mass of CaCO3 = 40.1 + 12.0 + 3(16.0) = 100.1 g mol−1.
Mass of calcium carbonate needed = 1.40 × 104 × 100.1 = 1.40 × 106 g.
(c) In the air, sulfur dioxide reacts with nitrogen dioxide forming sulfur trioxide (SO3). The reactions that take place are shown in the equations:
SO2 + NO2 → SO3 + NO
2NO + O2 → 2NO2.
(i) State the oxidation state of the sulfur atom in sulfur trioxide.
Let the oxidation state of sulfur atom be x.
Therefore, x + 3(−2) = 0 {As SO3 is electrically neutral.} ⇒ x = +6.
(ii) What is the role of nitrogen dioxide in the above reactions? Explain your answer.
The nitrogen dioxide is acting as a catalyst because it remains unchanged at the end of the reaction.
(iii) Sulfur dioxide is used in the Contact Process to make sulfuric acid. Describe the conditions and name the catalyst in the Contact Process.
The temperature used in the Contact Process is about 400°C, with a pressure of about 1 atm, and a ratio of SO2 to O2 of 1:1 by volume. The catalyst used is vanadium(V) oxide (V2O5).
(i) Explain the term global warming.
Global warming refers to the additional trapping of heat energy in the Earth’s atmosphere brought about by the additional greenhouse gases such as carbon dioxide, methane, and nitrous oxide (N2O) that are released into the atmosphere due to rampant human activity.
Do you know?
— During the day, the Earth’s surface absorbs heat energy emitted from the Sun. And in the night, the Earth cools down by losing this absorbed heat energy. But before all this radiation can escape into outer space, greenhouse gases such as carbon dioxide, water vapor, and others in the atmosphere absorb some of it, which makes the atmosphere warmer. As the atmosphere gets warmer, it makes the Earth’s surface warmer too. If not for the greenhouse gases that trap the heat in the atmosphere, the Earth would be a very cold place. This process of keeping the Earth warm is known as the greenhouse effect. Hence, natural greenhouse effect is important to sustain life on Earth.
(ii) Describe two consequences of global warming.
Global warning raises the Earth’s temperature, causes polar ice caps to melt, raises the sea level, and eventually submerges cities. In addition, global warming also results in extreme droughts and rainfall around the world, disrupting the growth of plants.
(iii) Explain why carbon dioxide that is produced naturally does not lead to a serious greenhouse effect.
The carbon dioxide that is produced naturally is assimilated by green plants during photosynthesis. Thus, this does not lead to a serious greenhouse effect as long as the carbon cycle is balanced. The following shows the processes in the carbon cycle:

(a) Briefly explain how oxides of nitrogen and carbon monoxide are produced in vehicles.
When car engines use air to burn fuel, oxides of nitrogen are produced due to the reaction of nitrogen from the air with oxygen in the hightemperature car engines.
Carbon monoxide is produced due to the incomplete combustion of the carbon-containing compounds in car engines.
(b) Suggest how oxides of nitrogen and carbon monoxide can be removed from the exhaust gas.
Pollutants that are emitted from the combustion of fuel in car engines can be minimized by passing the exhaust gas through the catalytic converters. These pollutants are converted into less harmful products such as CO2, N2, and H2O.
Do you know?
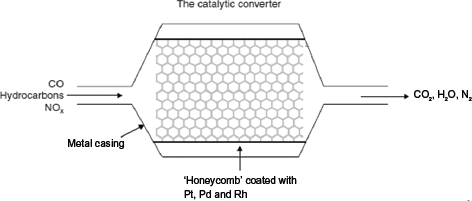
The chemical reactions that happen in the catalytic converter are as follows:
• As the gases enter, the oxides of nitrogen (NO and NO2) are reduced to N2 by the excess CO present, with rhodium acting as the catalyst:
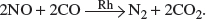
• The CO is also oxidized to CO2 with platinum and palladium as the catalysts:

• Non-combustible hydrocarbons are oxidized to CO2 and H2O with the help of platinum and palladium.
The catalytic activity of the catalytic converters would be destroyed in the presence of lead. This is because lead is preferentially adsorbed onto the active sites, making it unavailable for the reactants to adsorb on it. Hence, cars that are fitted with catalytic converters must be run on unleaded petrol.
(c) Give chemical equations to show the removal of oxides of nitrogen and carbon monoxide from the exhaust gas.
2NO + 2CO → N2 + 2CO2;
and
O2 + 2CO → 2CO2.
(d) Explain briefly why carbon monoxide is poisonous.
Carbon monoxide reacts with the hemoglobin in the blood to form carboxyhemoglobin, which prevents red blood cells from taking up oxygen for transport to the rest of the body for respiration.
(e) Why are oxides of nitrogen polluting?
The oxides of nitrogen can dissolve in rainwater and thus increase the acidity of the water. It can also have harmful effects on plants, aquatic life, and infrastructure.
In addition, nitrogen dioxide can also accelerate the break down of ozone. With a thin layer of ozone in the atmosphere, more harmful ultraviolet radiation would reach the Earth’s surface. Too much ultraviolet light can damage the skin and cause cancer.
(f) Explain why, in terms of collisions between particles, the rate of production of nitrogen oxide increases as the concentration of oxygen increases.
As the concentration of oxygen increases, the frequency of collisions between the nitrogen and oxygen particles increases. This would lead to a higher frequency of effective collisions; hence, a higher rate of production of nitrogen oxide.
(g) Explain why the rate of the reaction in part (f ) increases as the engine temperature increases.
As temperature increases, there are more particles possessing kinetic energies that are sufficient to overcome the activation energy. This thus leads to a higher frequency of effective collisions. In addition, with higher kinetic energies, the particles move faster. Hence, there is a higher frequency of collisions between the particles, which leads to a higher frequency of effective collisions. Thus, the rate of reaction increases.
(a) Briefly describe the sources of methane and ozone in the lower atmosphere.
Methane comes mainly from landfills and decaying organisms. Methane is a potent greenhouse gas.
Ozone in the lower atmosphere is formed by the chemical reactions between oxides of nitrogen and incompletely combusted hydrocarbons. Too much ozone in the lower atmosphere leads to the formation of photochemical smog or haze.
(b) Explain why methane is a pollutant.
Methane is a greenhouse gas that is capable of trapping heat energy within the atmosphere. It is even more potent than carbon dioxide!
(c) Ozone is important to help block out the dangerous ultraviolet radiation in the stratosphere. Explain why ozone is a pollutant in the lower atmosphere.
Ozone is a strong oxidizing agent and an irritant of the respiratory system. Thus, in the lower atmosphere, a high level of ozone becomes more of a pollutant.
(d) Describe briefly how ozone is being produced naturally.
Stratospheric ozone is formed naturally when the ultraviolet radiation from the Sun causes the oxygen molecules to react:
O2(g) + UV light → 2O(g);
O(g) + O2(g) → O3(g).
Thus, the ozone formation process is in fact an ultraviolet absorption process.
(e) State the cause of ozone depletion.
It has been found that chlorofluorocarbon (CFC) products, which are widely used as aerosol propellant or coolants in refrigerators, catalyze the breaking down of the ozone. This means that the formation of ozone is actually slower than the rate of it being broken down. Hence, there would be a depletion of ozone.
(f) Briefly explain how the compound mentioned in part (e) destroys ozone molecules.
When the CFC molecules reach the stratosphere, it breaks up to give chlorine atoms which accelerate the decomposition of ozone. As a result, there is an ozone “hole” in the atmosphere, which allows too much ultraviolet light to reach the Earth’s surface.
(g) Draw the dot-and-cross diagrams of both ozone and methane.

2O3(g) → 3O2(g).
(i) In terms of the energy changes that take place during bond-breaking and bond-making, explain why this reaction is exothermic.
2O−O=O → 3O=O.
The reaction is exothermic because the energy that is released during the bond-making process is more than the energy that is absorbed during the bond-breaking process.
| Q | The question did not provide us with the bond energies for the O−O and O=O bonds. So, how are we supposed to know that the breaking of two O−O bonds would absorb less energy than the amount of energy that is released during the formation of a O=O bond? |
A: Indeed, the question did not tell contain all those information. But the question did state that the decomposition reaction is exothermic in nature. So, what you are supposed to do is based on the fact that since the reaction is exothermic in nature, it must mean that the bond-making process releases more energy than the energy that is absorbed during the bond-breaking process.
(ii) Draw an energy level diagram for the above reaction.

(iii) Explain how you can tell from the energy level diagram that the reaction is an exothermic one.
Since the energy level of the product is lower than that of the reactant, it is an exothermic reaction. This is also indicated by a downward arrow.
(iv) Explain why the rate of this decomposition increases as the temperature increases.
As temperature increases, there are more particles possessing kinetic energies that are sufficient to overcome the activation energy. This thus leads to a higher frequency of effective collisions. In addition, with higher kinetic energies, the particles move faster. Hence, there is a higher frequency of collisions between the particles, which leads to a higher frequency of effective collisions. Thus, the rate of decomposition increases.
(v) Calculate the energy released when 16 g of ozone is decomposed.
Amount of O3 in 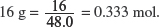
One mole of ozone will release 143 kJ when it is fully decomposed, thus the amount of energy that is released by 0.333 mole of ozone = 0.333 × 143 = 47.7 kJ.
(vi) The decomposition of ozone can be accelerated by the presence of a catalyst, the chlorine atom. Explain how a catalyst speeds up the rate of the reaction.
A catalyst lowers the activation energy of the reaction. As a result of the lowered activation energy, there would be more particles possessing kinetic energies that are greater than this lowered activation energy. Hence, the frequency of effective collisions increases, leading to an increase in the rate of the reaction.