(a) Barium nitrate solution reacts with sodium sulfate solution to form an insoluble salt and sodium nitrate solution.
Ba2+(aq) + SO42−(aq) → BaSO4(s).
| Q | Why are some ionic compounds soluble in water while others are not? |
A: For ionic compounds that are soluble in water, we can say that in general, it is because the attractive forces that are formed between the ions and water molecules are able to provide a sufficient amount of energy to break up the ionic lattice. For those that are insoluble, it is probably due to the insufficient amount of energy supplied to overcome the greater strength of ionic bonds. Thus, we can classify salts into soluble salts and insoluble salts:
| Soluble salts | Insoluble salts |
|
• Salts containing Na+, K+, and NH4+ as cation are soluble. • Salts containing NO3− as the anion are soluble. |
|
• Salts containing Cl−, Br−, and I− as the anion are soluble EXCEPT • Salts containing SO42+ as the anion are soluble EXCEPT • Na2CO3, K2CO3, (NH4)2CO3. |
• PbCl2, PbBr2, PbI2 • AgCl, AgBr, AgI • PbSO4, BaSO4 and CaSO4 (sparingly soluble) • Salts containing CO32− as the anion are insoluble EXCEPT |
Do you know?
Step 1: |
Mix two solutions together. |
|
Ba(NO3)2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaNO3(aq). |
Step 2: |
Filter the mixture and collect the residue. |
Step 3: |
Wash the residue with deionized water. |
Step 4: |
Dry it and you get the salt crystals! |
(b) Magnesium reacts with dilute hydrochloric acid to form a soluble salt and hydrogen.
Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g).
Do you know?
• An acid reacts with a metal to give off hydrogen gas.
• An acid reacts with a carbonate/hydrogencarbonate to give off carbon dioxide gas.
• An acid reacts with base to give a salt and water.
— But not all acids would display all the above three characteristics simultaneously. Some acids may just display one out of the three. For example, water reacts with sodium metal to give off hydrogen gas but does not react with carbonates or with a base.
| Q | How do you prepare a soluble salt? |
• Acid reacting with excess metal:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) + unreacted metal.
• Acid reacting with excess insoluble base:
Zn(OH)2(s) + 2HCl(aq) → ZnCl2(aq) + 2H2O(l) + unreacted base.
• Acid reacting with excess insoluble carbonate:
ZnCO3(s) + 2HCl(aq) → ZnCl2(aq) + CO2(g) + H2O(l) + unreacted carbonate.
Step 2: Filter the mixture and collect the filtrate.
Step 3: Concentrate the filtrate through evaporation.
Step 4: Crystallize out the salt by cooling the hot solution.
Step 5: Filter and you get the salt crystals!
| Q | Why is excess metal, excess base, excess carbonate used when preparing the salt? |
A: As the metal, base, and carbonate used are insoluble, when we use these substances in excess in the preparation of the salt, we ensure that all the acid is completely reacted. Otherwise later during the crystallization of the salt, the salt might be contaminated with the leftover acid.
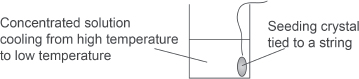
| Q | How would you crystallize the salt out? |
Step 1: Dissolve the mixture with excess water in a beaker.
Step 2: Filter the mixture with a filter funnel and collect the filtrate.
Step 3: Concentrate the filtrate by evaporating most of the water off.
Step 4: Let the saturated solution cool down slowly.
Step 5: Put in a small crystal of the pure salt as a “seed.” As the temperature decreases, the solubility of the salt also decreases. The salt would then deposit on the seed of crystal.

(c) Zinc reacts with aqueous copper(II) sulfate to form aqueous zinc(II) sulfate and copper metal.
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s).
Do you know?
— The above reaction is a displacement reaction. In fact, it is a redox (reduction−oxidation) reaction. The Zn metal becomes Zn2+ by losing electrons, while the Cu2+ becomes Cu by gaining electrons. The Zn metal is said to have undergone oxidation (i.e., being oxidized), while the Cu2+ undergoes reduction (i.e., being reduced). There is a transfer of electrons from the Zn atom to the Cu2+ ion!
(d) Calcium carbonate reacts with sulfuric acid to form carbon dioxide, salt, and water.
CaCO3(s) + 2H+(aq) + SO42−(aq) → CaSO4(s) + CO2(g) + H2O(l).
(e) Calcium carbonate reacts with nitric acid to form carbon dioxide, salt, and water.
CaCO3(s) + 2H+(aq) → Ca2+(aq) + CO2(g) + H2O(l).
Do you know?
— CO2 is not very soluble in water and at the same time, if there is a large evolution of the gas, the gas escapes from a liquid, causing a bubbling effect. This bubbling effect is known as effervescence. Do not mistake effervescence for the bubbling that you see during boiling. The bubbles that you see during boiling are actually bubbles of water vapor.
(f) Aluminum oxide reacts with sulfuric acid to form salt and water.
Al2O3(s) + 6H+(aq) → 2Al3+(aq) + 3H2O(l).
(a) Write the balanced chemical equation for the reaction.
SiO2(s) + MgO(s) → MgSiO3(s).
Do you know?
— Magnesium silicate is a major component of talcum powder, which is widely used in the cosmetic industry, and also in baby powder. It is likewise often used in basketball or weightlifting to keep the player’s hands dry.
(b) Explain why these two oxides can react together.
MgO is a basic oxide while SiO2 is acidic in nature. Thus, the reaction between the two oxides is in fact an acid–base reaction.
| Q | MgO consists of Mg2+ and O2− ions; how does the O2− ion get “incorporated” into SiO2? |
A: The SiO2 has a giant covalent/macromolecular lattice structure in which each Si atom is covalently bonded to four other O atoms, and each O atom is in turn bonded to two Si atoms.
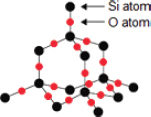
Since the Si atom is bonded to four highly electronegative O atoms, the Si atom is highly electron-deficient as the shared covalent electrons are increasingly attracted toward the O atoms. As a result, the highly electron-rich O2− ion can easily be attracted to the electron-deficient Si atom. It is simply another example of electrostatic attraction between oppositely charged particles.
Do you know?
• Basic oxides are formed from the reaction of metals with oxygen, for example, Na2O, MgO, and CaO. Basic oxides can react with an acid or even with an acidic oxide:
Na2O(s) + CO2(g) → Na2CO3(s).
• Acidic oxides result from the reaction of non-metals, such as CO2 and SO2, with oxygen. It reacts with a base to give a salt and water:
CO2(g) + Ca(OH)2(aq) → CaCO3(s) + H2O(l).
• Neutral oxides neither react with acids nor bases. An example is CO.
• Amphoteric oxides react with both acids and alkalis. It is formed from the reaction of a metal with oxygen. Examples are Al2O3, ZnO, and PbO. The oxide ion component, O2−, enables the amphoteric oxide to react with an acid. Due to the high charge density of the cation, the metal cation component reacts with a base as follows:
| Q | Why does the high charge density of the cation enable it to react with the base? |
A: If the charge density of the positive cation is very high, it would be strong enough to attract the base, OH−, which has an opposite charge as compared to the cation. And if the attractive force is so strong, then the two oppositely charged particles can form another particle, such as AlO2− and ZnO22−.
(a) Explain the meaning of the terms weak and monobasic.
A weak acid undergoes partial dissociation in aqueous solution.
The basicity of an acid refers to the number of moles of hydroxide ions that is needed to completely neutralize one mole of the acid molecule.
Do you know?
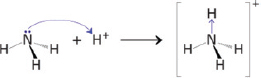
— According to Brønsted–Lowry definition, an acid is a proton donor while a base is a proton acceptor. This would mean that in order for an acid–base reaction to occur, both the acid and base must coexist to facilitate proton transfer. But what happens to the proton after it is accepted by the base? The proton forms a dative covalent bond/- coordinate bond with the base:
— A strong acid completely dissociates in aqueous solution. That is, one mole of HCl will provide one mole of H3O+and one mole of Cl– upon dissociation in water, i.e., [HCl] = [H3O+]. We use a single arrow “→” for the dissociation equation:
HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq).
— As a weak acid does not fully dissociate in water, one mole of CH3COOH will provide less than one mole of H3O+ and CH3COO– each, upon partial dissociation in water, i.e., [CH3COOH]initial > [H3O+]equilibrium. We use a double arrow “⇌” for the dissociation equation:
CH3COOH(aq) + H2O(l) ⇌ CH3COO–(aq) + H3O+(aq).
A weak acid dissociates to a lesser extent than a strong acid because the dissociation of a weak acid is energetically less favorable than that for a strong acid. A weak acid needs to absorb more energy in order to fully dissociate!
| Q | Can we simply write the equation as HCl(aq) → Cl–(aq) + H+(aq) to indicate that HCl is acidic? |
A: For the purpose of simplicity, yes. But it is more meaningful to write the equation as: HCl(aq) + H2O(l) → Cl–(aq) + H3O+(aq). This is because the H–Cl bond does not automatically break up when a HCl molecule “plunges” into the water. The “loss” of a proton from a H−Cl molecule is mediated by the lone pair of electrons from a water molecule. One can actually visualize the lone pair of electrons “extracting” the proton. In addition, hydrogen ions do not exist in solution. A H+ ion has such a high charge density that it is actually bonded to at least one water molecule when in aqueous solution, i.e., H+ binds with H2O to form H3O+ (hydronium ion).
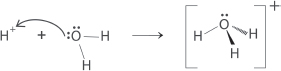
Without the water molecule, the HCl cannot function as an acid! It is common to find the symbol “H+(aq)” used in many texts for simplicity’s sake, including this one. It is okay to use it but we must bear in mind that when we write “H+(aq),” we are actually referring to “H3O+(aq).”
| Q | What is a lone pair of electrons? |
A: A lone pair of electrons is a pair of electrons that is in the valence shell that is not involved in covalent bonding.
Do you know?
— Acids are classified as monoprotic, diprotic, or triprotic, depending on the number of H atoms in a molecule that are able to ionize to form H+ ions. A monoprotic acid is a monobasic acid because one mole of the acid molecules needs one mole of hydroxide ions (OH−) to fully neutralize it, and so on and so forth for dibasic and tribasic acids. Thus, the basicity of an acid refers to the number of moles of hydroxide ions that is needed to completely neutralize one mole of the acid molecule. It should not be confused with the term “basicity” alone, which refers to how alkaline the solution is!
— But not all hydrogen atoms in a molecule will ionize to form H+ ions. For example, in ethanoic acid, only one hydrogen atom out of the four will ionize:
CH3COOH ⇌ H+ + CH3COO–.
— The basicity of an acid should not be confused with the strength of an acid. The number of hydrogen ions liberated per molecule of acid does not determine its strength. A dibasic or tribasic acid need not necessarily be a stronger acid than a monobasic acid. A good example is carbonic acid, H2CO3, which is a weak dibasic acid. On the other hand, both nitric acid and hydrochloric acid are strong monobasic acids.
(b) Calculate the concentration of hydrogen ions present in the above acid solution.
The pH of a solution is defined as negative log10 of the concentration of hydrogen ion in mol dm–3
pH = –log10 [H+(aq)].
Thus, given pH = 2.43, the [H+(aq)] = 10−2.43 = 3.72 × 10−3 mol dm−3.
Do you know?
• has no units;
• ranges from 0 to 14; and
• the greater the [H+], the smaller the pH value.
By knowing the [H+], we can calculate the pH of an acid, and vice versa for an alkali!
| Q | What if we are asked to calculate the pH of NaOH(aq)? NaOH(aq) is a base and it doesn’t produce H+ ions. So, how is it possible to assign a pH value to an aqueous solution of NaOH(aq) when we do not have a value for [H+]? |
A: Well, it turns out that for all aqueous solutions, be it acidic, neutral, or basic, there are both H+ and OH– ions present. In pure water, the water molecules actually undergo auto-ionization:
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH−(aq),
[H3O+(aq)] = [ OH−(aq)] = 10−7 mol dm−3 at 25ºC, i.e., the reason why the pH of pure water is 7! [H3O+(aq)] varies depending on whether an acid or base is added to the pure water.
Depending on the types of solution, the concentrations of these two ions differ:
• for neutral solutions, [H3O+(aq)] = [OH−(aq)];
• for acidic solutions, [H3O+(aq)] > [OH−(aq)]; and
• for basic solutions, [H3O+(aq)] < [OH−(aq)].
The product of [H3O+(aq)][OH−(aq)] = 10−14 mol2 dm−6, hence we can calculate the [H3O+(aq)] simply by using [H3O+(aq)] = 10−14/ [OH−(aq)].
Many students assume that there is no OH− ions in an acidic solution and vice versa. This is incorrect! Both the H3O+(aq) and OH−(aq) ions are always present simultaneously in a solution!
(c) With reference to your answer in part (b), explain whether lactic acid is a strong or weak acid.
Lactic acid is a weak acid since the [H+(aq)] = 3.72 × 10−3 mol dm−3 ≠ 0.10 mol dm−3. This shows that not all lactic acid molecules have completely dissociated.
(d) Calculate the volume of sodium hydroxide with a concentration of 0.10 mol dm−3 that is needed to neutralize 30 cm3 of the lactic acid solution.
Amount of lactic acid 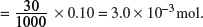
Since lactic acid is monobasic, let it be HA. Thus, the ionic equation for the acid–base reaction is
HA(aq) + OH−(aq) → A−(aq) + H2O(l).
Amount of lactic acid = Amount of NaOH = 3.0 × 10−3 mol.
Volume of NaOH 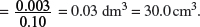
Do you know?
— Although a weak acid does not fully dissociate in water, the amount of a strong base, such as NaOH, required for complete neutralization is still the same as compared to one that is needed to neutralize a strong acid of the same concentration, same volume and same basicity.
| Q | Wait a minute, you say that the amount of a strong base, such as NaOH, required to neutralize a weak acid and a strong acid of the same concentration, same volume and same basicity, are the same. Is it because NaOH is too strong that it is not able to differentiate the strengths of the two acids? |
A: Now, an acid such as CH3COOH, is weak as compared to HCl when we use H2O as a base to differentiate their relative strengths. But if a stronger base is used, such as NaOH, it cannot differentiate between the strengths of the two acids; the NaOH would fully react with these two acids. So, you are right! Hence, it is important to take note that “strong” or “weak” is a relative concept. As the saying goes, “one base’s strong acid is another base’s weak acid.”
| Q | What is basicity of an acid? |
A: Basicity of an acid refers to the number of moles of hydroxide ions that is needed to completely neutralize one mole of the acid molecules. For examples, HCl is a monobasic acid while H2SO4 is a dibasic acid. Similarly, we say that NaOH is a monoacidic base because one mole of NaOH needs one mole of H+ ions for complete neutralization while Ca(OH)2 is a diacidic base.
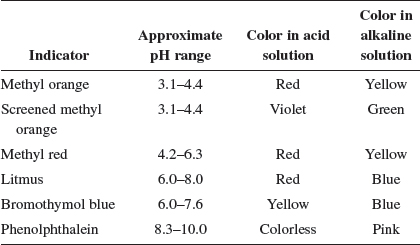
(e) Suggest a suitable indicator for the above titration.
Since this is a weak acid−strong base titration, a suitable indicator would be phenolphthalein or bromothymol blue.
| Q | What is the reason behind the choice of indicator for a weak acid−strong base titration? |
Now, the endpoint pH of a strong base−weak acid titration is not at the neutral pH of 7, but it is in the alkaline range. The pH is greater than 7 because of basic hydrolysis that is brought about by the conjugate base (A–) of the weak acid (HA):
A–(aq) + H2O(l) ⇌ HA(aq) + OH−(aq).
Both phenolphthalein and bromothymol blue change color in the alkaline pH range. Hence, they are suitable indicators for the weak acid−strong base titration.
| Q | So, does this mean that the endpoint pH of a weak base−strong acid titration would have a pH < 7? |
A: Yes, indeed. The endpoint pH of a weak base−strong acid titration would have a pH < 7 because of acidic hydrolysis brought about by the conjugate acid (e.g., NH4+) of the weak base (e.g., NH3), i.e., NH4+(aq) + H2O(l) ⇌ NH3(aq) + H3O+(aq). Hence, the choice of indicator for different types of titration is important! The following shows three possible titration curves:
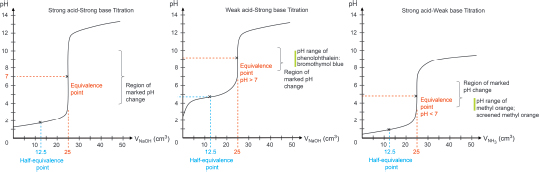
Different indicators change colour at different pH ranges; this is known as the working range of the indicator.
| Q | So, does that mean that if we use methyl orange as the indicator for a weak acid−strong base titration, the moment the methyl orange changes color (at working range of pH 3−4), it would mean that we have added a smaller amount of the titrant as compared to if we use phenolphthalein as the indicator? |
A: Absolutely brilliant! If we use phenolphthalein as the indicator for the weak base−strong acid titration, we would have “over-added” the titrant as compared to the use of methyl orange. The following tables show the appropriate indicators for different types of titration and their color changes in solutions of different pH values.
| Type of titration | Marked pH change | Suitable indicator |
| Strong acid–strong base | 4–10 | Phenolphthalein, methyl orange; bromothymol blue; screened methyl orange |
| Strong acid–weak base | 3.5–6.5 | Methyl orange; screened methyl orange |
| Weak acid–strong base | 7.5–10.5 | Phenolphthalein; bromothymol blue |
| Weak acid–weak base | No marked change | No suitable indicator |
Calculate the pOH of a solution of sodium hydroxide of concentration 0.10 mol dm−3.
Since NaOH is a strong base, [OH−] = [NaOH] = 0.10 mol dm−3.
Therefore, pOH = −log 0.10 = 1.
A monoacidic base means one mole of the base would react with one mole of H+ ions.
Amount of HCl used 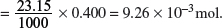
Amount of compound A in 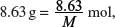 where M = molar mass of compound A.
where M = molar mass of compound A.
Amount of HCl used in 23.15 cm3 = Amount of compound A in 25.0 cm3.
Amount of compound A in 250 cm3
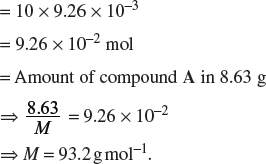
Do you know?
— A base is the chemical opposite of an acid. Why? An acid increases the concentration of H+ but a base decreases it. A reaction between an acid and a base is termed neutralization, producing a salt and water. Thus, metal oxides and hydroxides are considered as bases because of the reaction of the O2− ion (of the oxide) and the OH− (of the hydroxide) with the H+ from the acid.
— A base can be soluble or insoluble; soluble bases are called alkalis and they dissolve in water to produce the hydroxide ion, OH− ion:
NaOH(s) → Na+(aq) + OH−(aq).
— Aqueous ammonia is a weak base because of the partial hydrolysis of ammonia in water as shown:
NH3(g) + H2O(l) ⇌ OH–(aq) + NH4+(aq).
— Calcium(II) hydroxide (Ca(OH)2) or slaked lime is a weak alkali in water. Due to the sparing solubility of calcium(II) hydroxide in water, it forms a dilute solution called limewater. Limewater is thus Ca(OH)2(aq). This is an important example to show that the solubility of an oxide or hydroxide in water would affect its basicity!
| Q | In the previous parts, we learned that the conjugate base (CH3COO–) of a weak acid (CH3COOH) is basic because of its high affinity for a H+. So, does it mean that the conjugate acid (NH4+) of a weak base (NH3) is acidic because the conjugate acid has low affinity for a H+? |
A: You are absolutely right again here! A weak base is weak because the base has low affinity to accept a H+ as compared to a strong base. This would mean that even after the weak base is protonated, it would not “hold on” to the proton long enough before it lose it again. This thus accounts for the reversible reaction!
(i) Calculate the concentration of the acid in mol dm−3.
H2AO4(aq) + 2OH−(aq) → AO42−(aq) + 2H2O(l).
Amount of NaOH used 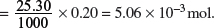
Amount of H2AO4 used 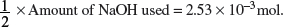
Concentration of 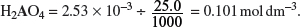
(ii) Calculate the relative molecular mass of the acid.
Let the molar mass of the acid be M g mol−1.
Concentration of H2AO4 = 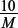 = 0.101 mol dm−3.
= 0.101 mol dm−3.
Therefore, 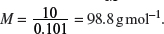
Hence, the relative molecular mass of the acid is 98.8.
Do you know?
— You can make use of a simple titration experiment to determine the molar mass or relative molecular mass of an acidic or basic compound.
(iii) Suggest an identity for the element A.
Let the molar mass of A be x g mol−1.
Molar mass of H2AO4 = 2(1.0) + x + 4(16.0) = 98.8 g mol−1.
Therefore, x = 32.8 g mol−1.
From the Periodic Table, A is probably sulfur (S).
(iv) Give the electronic configuration of element A.
Sulfur atom has 16 electrons; the electronic configuration is 2.8.6.
(v) Draw the dot-and-cross diagram for H2AO4.
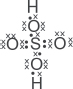
| Q | Why are there 12 electrons around the sulfur atom in the above dot-and-cross diagram? Why does the sulfur atom not obey the octet rule? |
A: Sulfur and other Period 3 elements and beyond can have more than eight electrons in their valence electronic shell. This is because the valence shell of Period 3 elements is the n = 3 electronic shell, which can hold a maximum of 18 electrons (2n2 = 2(3)2 = 18). Thus, when Period 3 elements form compounds, it does not necessary need to fulfill the octet configuration. Take note that the octet rule is not a “dictating rule” that all elements must follow!
(c) A solution is made by dissolving 7.5 g of sodium hydroxide, containing an inert impurity, in water and making up to 250 cm3 of solution. If 20 cm3 of this solution is exactly neutralized by 13 cm3 of 1.0 mol dm−3 HNO3, calculate the percentage purity of the sodium hydroxide.
HNO3(aq) + NaOH(aq) → NaNO3(aq) + H2O(l).
Amount of HNO3 used 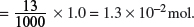
Amount of NaOH in 20 cm3 = Amount of HNO3 used = 1.3 × 10−2 mol.
Amount of NaOH in 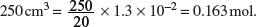
Mass of NaOH in 7.5 g of impure NaOH = 0.163 × (23.0 + 16.0 + 1.0) = 6.5 g.
Percentage purity of the sodium hydroxide 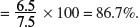
Do you know?
— You can make use of a simple titration experiment to determine the purity of an acidic or basic compound.
(d) Limestone is actually made up of calcium carbonate which is insoluble in water. 2.0 g of the impure calcium carbonate was added to 50.0 cm3 hydrochloric acid solution with a concentration of 1.0 mol dm−3. The mixture was then transferred to a 250 cm3 volumetric flask and topped up to the mark. 25.0 cm3 of this solution was found to require 20.0 cm3 of sodium hydroxide of concentration 0.20 mol dm−3 for complete neutralization. Determine the percentage purity of the calcium carbonate.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l).
Amount of NaOH used 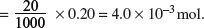
Amount of HCl in 25.0 cm3 = Amount of NaOH used = 4.0 × 10−3 mol.
Amount of leftover HCl in 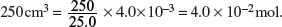
Initial amount of HCl used in 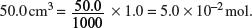
Amount of HCl used to react with calcium carbonate = 5.0 × 10−2 − 4.0 × 10−2 = 1.0 × 10−2 mol.
2HCl(aq) + CaCO3(s) → CaCl2(aq) + CO2(g) + H2O(l).
Amount of CaCO3 present = 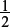 × Amount of HCl used to react with calcium carbonate =
× Amount of HCl used to react with calcium carbonate = 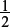 × 1.0 × 10−2 = 5.0 × 10−3 mol.
× 1.0 × 10−2 = 5.0 × 10−3 mol.
Mass of CaCO3 in 2.0 g of impure CaCO3 = 5.0 × 10−3 × {40.1 + 12.0 + 3(16.0)} = 0.50 g.
Percentage purity of the 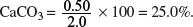
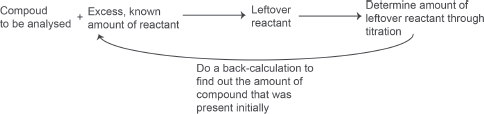
Do you know?
— The above titration is known as a back-titration experiment. Essentially, a compound is added to an excess but known amount of reactant. The leftover reactant is then determined through titration to find out how much is left. The actual amount of reactant that has reacted with the compound can then be back calculated.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l).
(a) Explain the terms strong acid and strong base.
A strong acid or a strong base fully dissociates in water. An example of a strong acid and a strong base are given below:
HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq); and
NaOH(s) + aq → Na+(aq) + OH–(aq).
(b) Calculate the pH of the acid solution before the titration.
Since HCl is a strong acid, [HCl] = [H3O+] = 0.100 mol dm−3.
pH of the acid solution before the titration = −log [H3O+] = −log 0.100 = 1.0.
(i) the number of moles of H+ remaining in the conical flask;
HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq).
Amount of NaOH added 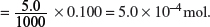
Amount of HCl reacted with the NaOH = Amount of NaOH added = 5.0 × 10−4 mol.
Amount of HCl present initially 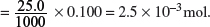
Amount of H+ remaining in the conical flask = 2.5 × 10−3 − 5.0 × 10−4 = 2.0 × 10−3 mol.
(ii) the new concentration of H+ in the conical flask; and
New volume of solution = 25.0 + 5.0 = 30.0 cm3.
New concentration of H+ in the conical flask = 2.0 × 10−3 ÷ 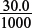 = 6.67 × 10−2 mol dm−3.
= 6.67 × 10−2 mol dm−3.
(iii) the new pH of the mixture in the conical flask.
The new pH of the mixture in the conical flask = −log[H3O+] = −log 6.67 × 10−2 = 1.18.
(d) Calculate the pH of the mixture after 24.0 cm3 of 0.100 mol dm−3 sodium hydroxide was added.
Amount of NaOH added =  × 0.100 = 2.4 × 10−3 mol.
× 0.100 = 2.4 × 10−3 mol.
Amount of HCl reacted with the NaOH = Amount of NaOH added = 2.4 × 10−3 mol.
Amount of HCl present initially = 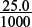 × 0.100 = 2.5 × 10−3 mol.
× 0.100 = 2.5 × 10−3 mol.
Amount of H+ remaining in the conical flask = 2.5 × 10−3 − 2.4 × 10−3 = 1.0 × 10−4 mol.
The new concentration of H+ in the conical flask = 1.0 × 10−4 ÷ 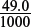 = 2.04 × 10−3 mol dm−3.
= 2.04 × 10−3 mol dm−3.
The new pH of the mixture in the conical flask = −log[H3O+] = −log 2.04 × 10−3 = 2.69.
(e) Would the pH of the mixture in the conical flask after 26.0 cm3 of sodium hydroxide has been added be closer to pH 7 or 11? Explain your choice.
Amount of NaOH added = 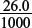 × 0.100 = 2.6 × 10−3 mol.
× 0.100 = 2.6 × 10−3 mol.
Amount of HCl reacted with the NaOH = Amount of NaOH added = 2.5 × 10−3 mol.
Amount of NaOH left = 2.6 × 10−3 − 2.5 × 10−3 = 1.0 × 10−4 mol.
Since there is an excess NaOH that is unreacted, the pH of the solution would be closer to 11 and not neutral at pH 7.
| Q | How do you calculate the pH of the above alkaline solution? |
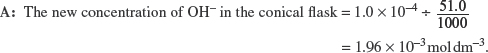
pOH = −log [OH–] = −log 1.96 × 10−3 = 2.71.
Since pH + pOH = 14, therefore pH = 14 − 2.71 = 11.29.
(f) Outline the most appropriate method to prepare a pure dry sample of sodium chloride starting with dilute hydrochloric acid.
To prepare a pure dry sample of sodium chloride starting with dilute hydrochloric acid:
Step 1: To get the salt solution, react an equimolar amount of HCl(aq) and NaOH(aq).
Step 2: Concentrate the solution through evaporation.
Step 3: Crystallize out the salt through cooling the hot solution.
Step 4: Filter and you get the salt crystals!
(a) Explain why calcium dihydrogen phosphate is both an acid and a salt.
Calcium dihydrogen phosphate is considered a salt as it is an ionic compound that is formed when phosphoric(V) acid (H3PO4) reacts with a base or a metal. For example,
Ca(OH)2 + 2H3PO4 → Ca(H2PO4)2 + 2H2O; and
Ca + 2H3PO4 → Ca(H2PO4)2 + H2.
Calcium dihydrogen phosphate is considered an acid as it can dissolve in water to give an acidic solution due to the following dissociation:
H2PO4−(aq) + H2O(l) ⇌ HPO42+(aq) + H3O+(aq).
| Q | So, does that mean that phosphoric(V) acid can in fact undergo the following three dissociations? H3PO4(aq) + H2O(l) → H2PO4−(aq) + H3O+(aq) H2PO4−(aq) + H2O(l) → HPO42−(aq) + H3O+(aq) HPO42−(aq) + H2O(l) → PO43−(aq) + H3O+(aq). Does each of the above three dissociations occur to the same extent? |
A: Phosphoric(V) acid is a tribasic acid which can dissociate in three stages as shown above. But the amount of dissociation for each stage is not to the same extent. It is logical to understand why: imagine after the first dissociation, for example H3PO4 → H+ + H2PO4–, you have the negatively charged H2PO4– ion formed. Do you think it is as easy to remove another H+ ion from a negatively charged species as compared to from a neutral H3PO4? Of course not! Hence, the second or third dissociation usually occurs to a lesser extent than the previous dissociation.
(i) Write an equation for the reaction of calcium hydroxide with phosphoric acid.
Ca(OH)2 + 2H3PO4 → Ca(H2PO4)2 + 2H2O.
(ii) Give the formulae of two other calcium salts formed from calcium hydroxide and phosphoric(V) acid.
Ca(OH)2 + H3PO4 → CaHPO4 + 2H2O; and
3Ca(OH)2 + 2H3PO4 → Ca3(PO4)2 + 6H2O.
(i) Explain which acid, calcium dihydrogen phosphate or ethanoic acid, is a weaker acid.
A lower pH value would mean a higher concentration of H+ ions.
If the calcium dihydrogen phosphate in the 0.25 mol dm−3 solution has fully dissociated, then [H+] = 0.50 mol dm−3. Hence, the pH should be 0.30.
Similarly, if the ethanoic acid in the 0.50 mol dm−3 solution has fully dissociated, then [H+] = 0.50 mol dm−3. Hence, the pH should be 0.30.
But since the pH value of ethanoic acid (3.8) is lower than that of the calcium dihydrogen phosphate (4.4), we could probably say that calcium dihydrogen phosphate is a weaker acid.
| Q | What do you mean when you say that “we could probably say that calcium dihydrogen phosphate is a weaker acid”? |
A: We can only use pH value to gauge the strength of an acid provided that you are comparing two acids of the same concentrations. Only then would it be fair to conclude that the stronger acid would be the one that dissociates more and thus gives a higher [H3O+].
| Q | But how does the concentration of the weak acid affects the dissociation and hence the concentration of the H+ ions? |
A: Assuming that we have the following weak acid, HA, dissociating in water:
HA(aq) + H2O(l) ⇌ H3O+(aq) + A−(aq).
The amount of the weak acid dissociated depends very much on its concentration. How? According to Le Chatelier’s Principle, a higher concentration of a weak acid would cause it to dissociate to a larger extent, hence the amount of dissociation increases, which leads to an increase in [H3O+] and decrease in pH. But there is a limit to the increase in the amount of a weak acid that has dissociated and a decrease in pH! This is because as a larger amount of a weak acid dissociates, more free ions are formed. It would also mean that the backward reaction is also more likely to occur. From the above equation, we can see very clearly that in order for HA to function as an acid, we need a base (H2O) to be present. Imagine if there are too many molecules of a weak acid but not enough H2O, can the weak acid dissociate? So, we can only use pH to compare the strengths of two weak acids provided they are of equal concentrations. Secondly, both must be of a similar type; for example, both must be monobasic, i.e., one mole of the acid reacts with one mole of OH−.
| Q | A higher concentration of a weak acid causes more weak acid to dissociate and at the same time, causes the rate of the backward reaction to increase. Then, at a lower concentration value, when the weak acid dissociates, there is less chance for the backward reaction to occur because the volume of the system is larger. So, would this cause more H3O+(aq) to form and hence lower the pH value? |
A: There is a fallacy here! Yes, at a lower concentration of the weak acid, you can perceive that dissociation somehow increases because the rate of the backward reaction decreases, so the number of moles of H3O+(aq) increases. But do not forget that the volume of the system also increases because of dilution. Thus, when you calculate the pH, which is pH = −log[H3O+] = 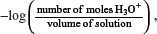 the increase in the volume is much more signification than the number of moles. Hence, overall, the pH increases. Just imagine, if the solution of the weak acid is greatly diluted, just like in pure water, would the pH just be about 7 instead?
the increase in the volume is much more signification than the number of moles. Hence, overall, the pH increases. Just imagine, if the solution of the weak acid is greatly diluted, just like in pure water, would the pH just be about 7 instead?
(ii) The pH of 1.0 mol dm−3 solution of sulfuric acid is 0.30. Rank in order of increasing acidity for sulfuric acid, calcium dihydrogen phosphate, and ethanoic acid.
A lower pH value would mean a higher concentration of H+ ions.
If sulfuric acid in the 1.0 mol dm−3 solution has fully dissociated, then [H+] = 2.0 mol dm−3. Hence, the pH should be 0.30, which coincides with the measurement. Thus, sulfuric acid is a strong acid!
Therefore, based on the given information, in order of increasing acidity: calcium dihydrogen phosphate < ethanoic acid < sulfuric acid.
(iii) Describe an experiment, other than measuring pH, which you would carry out to show that sulfuric acid is a strong acid but ethanoic acid is a weak acid. Show what measurements you would make and what results you would expect.
The idea that we are using to determine the strength of the acid is simple: the stronger the acid, the greater the amount of dissociated H+. Thus, if we put in a fixed amount of solid CaCO3 into same volume of each of the two acids, we can measure the volume of CO2 gas that is collected with respect to time. The stronger the acid, the faster the rate of production of the CO2.
But, we have to take note that sulfuric acid is a dibasic acid while ethanoic acid is a monobasic acid. Hence, the concentration of sulfuric acid must be half that of ethanoic acid.
| Q | Other than the above method, is there any other way to differentiate the strengths of the two acids? |
A: Well, you can add a known volume of sodium hydroxide solution into the same volume of each of the two acids. Then, measure the temperature change for the acid−base reaction. Since the strong acid has fully dissociated in water, the energy change for the acid−base reaction would be more exothermic than that between the weaker acid and the base. This is because since the weak acid has not fully dissociated in water, part of the heat energy that is released during the acid−base reaction would be diverted to “help” dissociate the undissociated weak acid molecules. Thus, the temperature change for the acid−base reaction between the weaker acid and the base would be smaller than that between the strong acid and the base.
(a) Anhydrous iron(III) chloride, FeCl3, by direct reaction of the elements.
Dry chlorine gas can be passed over heated iron.
2Fe(s) + 3Cl2(g) → 2FeCl3(s).
The mixture is then dissolved in water to get rid of the unreacted iron metal. The solution is filtered, then concentrated through evaporation. Next, crystallize out the salt through cooling the hot solution. Filter and you get the salt crystals!
(b) Sodium nitrate, NaNO3, by reacting an acid with an alkali.
Step 1: To get the salt solution, react an equimolar amount of HNO3(aq) and NaOH(aq).
HNO3(aq) + NaOH(aq) → NaNO3(aq) + H2O(l).
Step 2: Concentrate the solution through evaporation.
Step 3: Crystallize out the salt through cooling the hot solution.
Step 4: Filter and you get the salt crystals!
(c) Copper(II) sulfate, CuSO4 · 5H2O, by reacting an acid with a metal oxide.
Step 1: To get the salt solution, react excess CuO(s) with sulfuric acid.
CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l) + unreacted oxide.
Step 2: Filter the mixture and collect the filtrate.
Step 3: Concentrate the filtrate through evaporation.
Step 4: Crystallize out the salt through cooling the hot solution.
Step 5: Filter and you get the salt crystals!
| Q | Why is excess metal oxide used when preparing the salt? |
A: As the metal oxide used is insoluble, when we used an excess of these substances in the preparation of the salt, we ensure that all the acid used is completely reacted. If not, during the crystallization of the salt, it might be contaminated by the leftover acid.
Do you know?
— When there are water molecules embedded within the giant ionic lattice structure of a salt, we have the formation of a hydrated salt. These water molecules are known as the water of crystallization. The water molecules are attracted to the ions of the ionic lattice structure via ion−dipole interactions as the water molecules have permanent dipole (refer to Chapter 3 on Chemical Bonding). But nevertheless, the water of crystallization can be easily removed via heating. For example:

— The desiccant, silica gel, has cobalt(II) chloride embedded within the giant covalent lattice structure of the SiO2. The hydrated form of cobalt(II) chloride is CoCl2 · 6H2O which is pink in color. When CoCl2 · 6H2O loses its water of crystallization, it reverts back to the blue anhydrous CoCl2. Thus, the color of the cobalt(II) chloride serves as an indicator as to when the drying agent needs to be changed.