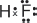
H2(g) + F2(g) → 2HF(g) + aq → 2HF(aq).
(a) Give the oxidation states of hydrogen and fluorine in hydrogen fluoride.
The oxidation states of hydrogen and fluorine in hydrogen fluoride are +1 and −1, respectively.
| Q | What is an oxidation state? |
A: An oxidation state indicates the ease of the atom in a species (which can be an atom, ion, or molecule) to undergo oxidation. A positive oxidation state indicates that the atom would be oxidized, whereas a negative oxidation state indicates reduction. The oxidation state is indicated by the oxidation number.
| Q | So, if an atom has an oxidation number of +1, does it mean that it has lost an electron? |
So, remember that the oxidation number indicates the ease of the atom to lose electrons, but it does not necessarily mean that the atom has already lost electrons. Thus, the oxidation number may not be equivalent to the formal charge that an atom carries.
| Q | So, how does one determine the oxidation number of an atom in a species? |
If the atom is covalently bonded to other atoms, one needs to analyze each of the covalent bonds in turn, and determine which of the two bonding atoms has a higher electronegativity value. The one that is more electronegative would polarize the shared electron cloud more toward itself. Hence, this atom would have a negative oxidation number. This would mean that it has a potential to undergo reduction as compared to the other less electronegative atom. The oxidation number would then be determined by the number of electrons contributed by this atom in the bond-sharing process.
Do you know?
(1) The oxidation state of atoms in the elemental form is being assigned a zero value. E.g., Ca, F atoms in Ca(s), and F2(g).
(2) In all compounds, fluorine has the oxidation state of –1.
(3) In all compounds, hydrogen has the oxidation state of +1. An exception will be in the case of metal hydrides such as NaH, where the oxidation state of H is –1.
(4) In all compounds, oxygen has the oxidation state of –2. An exception will be in the case of peroxides such as H2O2, where the oxidation state of O is –1 and in OF2 where it is +2.
(5) For a pair of covalently bonded atoms with a single bond, the more electronegative atom is assigned an oxidation state of –1 and the less electronegative atom has an oxidation state of +1.
(6) The sum of the oxidation states of all atoms in a neutral compound (e.g., KCl and CO2) is zero.
(7) For a monoatomic ion, its oxidation state corresponds to the net charge on the ion.
(8) For a polyatomic ion, i.e., an ion with more than three atoms, the sum of the oxidation states of all atoms corresponds to the net charge on the ion.
Recall that the electronegativity of elements
• increases across the period, and
• decreases down the group.
| Q | So, if two dissimilar atoms form a double bond, the more electronegative atom would have an oxidation state of −2? |
A: You are right! The less electronegative one would have an oxidation number of +2. Likewise, for a triple bond, the more electronegative atom would have an oxidation number of −3.
(b) Identify both the oxidizing agent and the reducing agent.
An oxidizing agent itself undergoes reduction, while a reducing agent itself undergoes oxidation. Now, since hydrogen undergoes oxidation, it is acting as a reducing agent. Likewise, fluorine is the oxidizing agent in the above reaction.
Do you know?
• The species that loses electron(s) is said to be oxidized, i.e., it has undergone oxidation. This species is known as the reducing agent or a reductant.
• The species that accepts the electron(s) is said to be reduced, i.e., it has undergone reduction. This species is known as the oxidizing agent or oxidant.
— In a nutshell, an oxidizing agent will itself undergo reduction. A reducing agent will itself be oxidized. And in a redox reaction, both the reducing agent and oxidizing agent must be present together! Otherwise who would provide the electrons and who would take up the electrons? So, do you see that this is very similar to an acid–base reaction? An acid must be present in order for the base to behave as a base, and vice versa!
(c) Explain, in terms of electron gain and loss, why this is a redox reaction.
The oxidation number of hydrogen increases from 0 to +1, which indicates that hydrogen has “lost” an electron. In contrast, the oxidation number of fluorine has decreased from 0 to −1, an indication of a “gain” of an electron. Thus, the above reaction is a redox reaction.
(d) Give the dot-and-cross diagram for hydrogen fluoride.
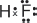
(e) Explain why hydrogen fluoride is a liquid at room temperature.
Hydrogen fluoride is a liquid at room temperature because of the strong hydrogen bonds between the HF molecules.
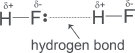
| Covalent bond | Bond energy in kJ mol−1 |
| H−H | 436 |
| F−F | 151 |
| H−F | 299 |
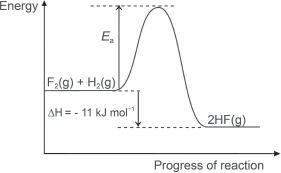
calculate ΔH for the reaction between hydrogen and fluorine to give gaseous hydrogen fluoride.
H–H + F–F → 2H–F.
Total energy absorbed during bond breaking = BE(H–H) + BE(F–F) = 436 + 151 = 587 kJ mol−1.
Total energy released during bond forming = 2 × BE(H–F) = 598 kJ mol−1.
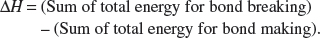
Hence, ΔH = 587 – 598 = –11 kJ mol−1.
(g) Draw an energy profile diagram for the formation of hydrogen fluoride gas. Indicate on the diagram the activation energy and the ΔH for the reaction.
(a) The ionic equation for the redox reaction between acidified MnO4– and Fe2+ is given below:
MnO4–(aq) + 8H+(aq) + 5Fe2+(aq)
→ Mn2+(aq) + 4H2O(l) + 5Fe3+(aq).
Explain, in terms of changes in oxidation number, why this reaction involves both oxidation and reduction. Hence, identify both the oxidizing agent and reducing agent.
The oxidation number of Mn in MnO4−(aq) decreases from a +7 to +2 in Mn2+. This is a reduction process; hence, MnO4−(aq) is an oxidizing agent.
The oxidation number of Fe2+(aq) increases from a +2 to +3 in Fe3+. This is an oxidation process; hence, Fe2+(aq) is a reducing agent.
Do you know?
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s).
To represent the oxidation of Zn, we have the oxidation half-equation:
Zn(s) → Zn2+(aq) + 2e−,
where e− represents a negatively charged electron. Do you see that in an oxidation half-equation, the electrons are on the right-hand side of the equation? This is an indication of the release of electrons during an oxidation reaction.
To represent the reduction of Cu2+, we have the reduction half-equation:
Cu2+(aq) + 2e− → Cu(s).
Do you see that in a reduction half-equation, the electrons are on the left-hand side of the equation? This is an indication of the “consumption” of electrons during a reduction reaction. If we combine these two half-equations, we get the overall redox equation! Take note that in a balanced redox equation, the amount of electrons released during oxidation MUST be all taken in for reduction. There cannot be “unconsumed” electrons floating around!
(i) Give the color change for MnO4–(aq) and explain why an indicator is not needed for the above titration.
In an acidic medium, the manganate(VII) ion is reduced as follows:
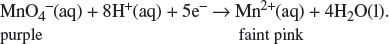
Since KMnO4(aq) is purple in color while its reduced product, Mn2+(aq), in an acidified medium is essentially colorless, an external indicator is not required to be added in the course of the titration. The endpoint is taken when the first permanent pink coloration is formed because of an excess drop of potassium manganate(VII) is added.
| Q | In the above equation, Mn2+(aq) is said to have a faint pink coloration. So, shouldn’t the formation of the Mn2+(aq) be used as an indication for the endpoint of titration? |
A: In reality, the faint pink coloration of Mn2+(aq) is virtually undetectable.
(ii) Calculate the amount of MnO4– used in the titration.
Amount of MnO4– used in the titration 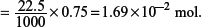
(iii) Hence, calculate the mass of iron present in the 9.50 g sample of moss killer.
MnO4–(aq) + 8H+(aq) + 5Fe2+(aq) → Mn2+(aq) + 4H2O(l) + 5Fe3+(aq).
Amount of Fe2+ present = 5 × Amount of MnO4– used in the titration = 5 × 1.69 × 10−2 = 8.45 × 10−2 mol.
Mass of iron present in the 9.50 g sample of moss killer = 8.45 × 10−2 × 55.8 = 4.72 g.
(iv) Determine the percentage by mass of iron in the moss killer.
Percentage by mass of iron in the moss killer 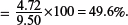
6Fe2+(aq) + Cr2O72−(aq) + 14H+(aq) → 6Fe3+(aq) + 2Cr3+(aq) + 7H2O(l),
determine the volume of ammonium dichromate(VI) of concentration 0.750 mol dm–3 that is required to completely oxidize all the iron(II) sulfate.
Amount of Fe2+ present = 8.45 × 10−2 mol.
Amount of Cr2O72− used = 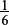 × Amount of Fe2+ present =
× Amount of Fe2+ present = 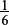 × 8.45 × 10−2 = 1.41 × 10−2 mol.
× 8.45 × 10−2 = 1.41 × 10−2 mol.
Volume of ammonium dichromate(VI) needed = 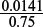 = 0.0188 dm3 = 18.8 cm3.
= 0.0188 dm3 = 18.8 cm3.
(NH4)2Cr2O7(s) → Cr2O3(s) + 4H2O(l) + N2(g).
Use changes in oxidation state to explain why this is a redox reaction.
The oxidation number of N in NH4+ increases from −3 to 0 in N2. This is an oxidation process. Hence, NH4+ is a reducing agent.
The oxidation number of Cr in Cr2O72− decreases from a +6 to +3 in Cr2O3. This is a reduction process. Hence, Cr2O72− is an oxidizing agent.
Therefore, the above reaction is a redox reaction.
| Q | Can the above reaction be termed a “disproportionation” reaction? |
A: A disproportionation reaction is a redox reaction in which a single substance is simultaneously being oxidized and reduced. Since the above redox reaction does not involve the simultaneous oxidation (NH4+) and reduction (Cr2O72−) of the same species, it is not a disproportionation reaction.
(vii) Give the electronic configurations for Fe2+, Fe3+, and Mn2+.
The electronic configurations of: Fe2+ is 2.8.14; Fe3+ is 2.8.13; and Mn2+ is 2.8.13.
Do you know?
— When the particles have the same number of electrons, they are termed “isoelectronic.” The unique feature of isoelectronic particles is that they have the same amount of inter-electronic repulsion.
(viii) Usually, for such titrations, the solution needs to be acidified by an acid. Explain why sulfuric(VI) acid is commonly used for acidification.
Sulfuric(VI) acid itself will not act as an oxidizing or reducing agent during the titration process.
| Q | Can we use other acids such as HCl(aq) or HNO3(aq)? |
A: No! MnO4− is strong enough to oxidize the Cl− ion to the chlorine gas (Cl2). So, if you acidify it using HCl(aq), the oxidizing agent would not be effective anymore. This would also mean that we can use acidified potassium manganate(VII) to test for HCl gas! As for HNO3, it can also act as an oxidizing agent due to the presence of the NO3− ion.
2Fe3+(aq) + Sn2+(aq) → 2Fe2+(aq) + Sn4+(aq).
(a) Give both the oxidation and reduction half-equations.
Oxidation half-equation: Sn2+(aq) → Sn4+(aq) + 2e−.
Reduction half-equation: Fe3+(aq) + e− → Fe2+(aq).
| Q | How do we derive the half-equations for a more complex redox equation? |
MnO4–(aq) + 8H+(aq) + 5Fe2+(aq) → Mn2+(aq) + 4H2O(l) + 5Fe3+(aq),
we can derive the half-equations as follows:

(i) Explain the formation of the iodine.
The iodine was formed from the oxidation of the iodide by the Fe3+ ion.
(ii) Give both the oxidation and reduction half-equations.
Oxidation half-equation: 2I−(aq) → I2(aq) + 2e−.
Reduction half-equation: Fe3+(aq) + e− → Fe2+(aq).
(iii) Hence, give the overall ionic equation.
Overall ionic equation: 2I−(aq) + 2Fe3+(aq) → I2(aq) + 2Fe2+(aq).
| Q | So, does that mean we cannot synthesize FeI3 compound? |
A: You are right! You can make FeCl3 and FeBr3 but you just can’t make FeI3, because the moment you put I− and Fe3+ together, they undergo a redox reaction!
(iv) Draw the dot-and-cross diagrams for iodine and potassium iodide.
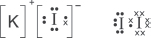
(v) Explain why iodine and potassium iodide have such a great difference in melting points.
Iodine is a non-polar molecule with weak intermolecular forces of the instantaneous dipole–induced dipole (id–id) type. For potassium iodide, it is an ionic compound with strong ionic bonding. Hence, potassium iodide has a much higher melting point than iodine.
(vi) Explain why solid potassium iodide is an electrical insulator but molten potassium iodide is not.
In the solid state, the ions in potassium iodide are rigidly held in fixed positions in the solid lattice structure. Hence, these ions are not mobile and cannot act as charge carriers. But in the molten state, the ions are mobile enough to act as charge carriers.
3SO2(g) + Cr2O72−(aq) + 2H+(aq)
→ 3SO42−(aq) + 2Cr3+(aq) + H2O(l).
(i) What would be the expected observation for a positive test?
If the orange Cr2O72– solution turns green, it indicates that Cr2O72– has been reduced to Cr3+. This means that the unknown substance has caused the reduction of the Cr2O72– ion, and hence contains a reducing agent.
| Q | Is acidified potassium dichromate(VI) used in the breathalyzer? |
A: Yes! Ethanol can be oxidized by potassium dichromate(VI) and the amount of Cr3+ formed, which is indicated by the intensity of the green coloration, tells us the amount of ethanol in the exhaled air.
(ii) What reaction has occurred to give the observation in part (a)?
A redox reaction has occurred to give the observation in part (a). The Cr2O72−(aq) undergoes reduction while the SO2 undergoes oxidation.
(iii) A 3 dm3 sample of polluted air contains 5.2% sulfur dioxide by volume (measured at s.t.p.). Calculate the volume of 0.20 mol dm–3 aqueous dichromate(VI), Cr2O72−, required to react with all the sulfur dioxide in the sample.
At s.t.p. (0°C and 1 bar), 1 mole of gas occupies a volume of 22.7 dm3.
Thus, 3 dm3 of gas would contain 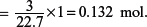
Amount of SO2 in 3 dm3 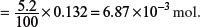
Three moles of SO2 react with one mole of Cr2O72−.
Amount of Cr2O72− needed = 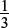 × 6.87 × 10−3 = 2.29 × 10−3 mol.
× 6.87 × 10−3 = 2.29 × 10−3 mol.
Volume of 0.20 mol dm–3 aqueous dichromate(VI), Cr2O72−, required = 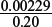 = 0.0115 dm3.
= 0.0115 dm3.
(i) Describe the bonding present in solid calcium and explain why calcium can conduct electricity.
Calcium atoms are bonded together via metallic bonds. It is the electrostatic attractive force between the positive ions and the sea of delocalized valence electrons.
Calcium can conduct electricity because the delocalized valence electrons can act as charge carriers under the influence of an applied electrical potential difference.
(ii) Write an equation for the reaction of calcium in water. Identify the oxidation numbers of all the atoms involved by writing the oxidation numbers underneath each symbol in the equation.
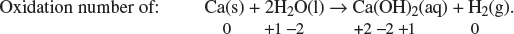
| Q | Is water acting as an acid in the above reaction? |
A: Yes, you are right! According to the properties of an acid, water is acting as an acid because it reacts with a metal to give off hydrogen gas. But take note that water is not a very strong acid that would react with all metals. Water is a weak acid that only reacts with very reactive metals.
| Q | So why does pure water at 25°C not have a pH < 7? In fact, why is it pH = 7? |
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH−(aq).
[H3O+(aq)] = [OH−(aq)] = 10−7 mol dm−3 at 25°C, i.e., the rationale why the pH of pure water is 7! [H3O+(aq)] varies depending on whether an acid or base is added to the pure water.
(iii) State which substance in part (b)(ii) has been oxidized and write a half-equation to show the oxidation process.
The oxidation state of calcium increases from 0 to +2; thus, calcium metal has been oxidized.
Oxidation half-equation: Ca → Ca2+ + 2e−.
(iv) What is the common name of the solution formed by the reaction of calcium with water?
The common name of the solution formed by the reaction of calcium with water is called “limewater.”
| Q | Isn’t calcium hydroxide partially soluble in water? |
A: Yes, calcium hydroxide is not very soluble in water but we can still dissolve some to get a decent amount of calcium hydroxide solution that can be used to test for CO2 gas.
(v) What is this solution commonly used for in the laboratory? Give a chemical equation for this reaction of the solution.
Limewater is commonly used to test for the presence of CO2 gas. A white precipitate of insoluble calcium carbonate will form when you bubble CO2 through limewater:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l).
| Q | Will we get a white precipitate if we bubble CO2 into aqueous sodium hydroxide solution? |
2OH−(aq) + CO2(g) → CO32−(aq) + H2O(l).
This is an acid-base reaction as the CO2 is an acidic gas. Thus, insoluble CaCO3 can be obtained because the CaCO3 is insoluble! If CO2 is bubbled into NaOH(aq), the Na2CO3 that is formed is soluble in water. Hence, you would not get a white precipitate! This would mean that if CO2 is bubbled into aqueous Ba(OH)2, you would get a white precipitate of BaCO3 as well.
| Q | But when too much CO2 is bubbled into aqueous Ca(OH)2 solution, no white precipitate is observed. Why? |
CO2(g) + H2O(l) → H2CO3(aq).
Thus, when too much CO2 is bubbled into aqueous Ca(OH)2 solution, both the CO32− and H2CO3 that are formed can react in an acid–base reaction:
CO32−(aq) + H2CO3(aq) → 2HCO3−(aq).
Hence, no white precipitate is formed because Ca(HCO3)2 is soluble in water.
(vi) Suggest the likely pH of the solution.
The likely pH of the calcium hydroxide solution would be about 12.
| Q | What is the best guess of the pH for such a solution? |
A: Aqueous ammonia has a pH of about 11. Since Ca(OH)2 is a stronger base than ammonia, the best guess would be a pH value that is more than 11 but less than 13.
AgCl(s) → Ag(s) + Cl(s).
Chlorine atoms immediately react with copper(I) chloride to make copper(II) chloride:
CuCl(s) + Cl(s) → CuCl2(s).
When the exposure to bright light is stopped, silver atoms reduce copper(II) chloride back into copper(I) chloride and silver chloride.
(i) Calculate the maximum mass of silver that can be formed when 0.305 g of silver chloride decomposes.
Molar mass of AgCl = 107.9 + 35.5 = 143.4 g mol−1.
Amount of AgCl in 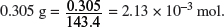
Amount of Ag formed = Amount of AgCl in 0.305 g = 2.13 × 10−3 mol.
Maximum mass of silver that can be formed = 2.13 × 10−3 × 107.9 = 0.229 g.
| Q | Why is the state symbol for the Cl atom given as (s)? |
A: Well, the state symbol (s) for the Cl atom indicates that it is an atom being trapped within the solid glass matrix.
(ii) Explain why the reaction between copper(I) chloride and chlorine involves both oxidation and reduction.
The oxidation state of Cu+ in CuCl increases from +1 to +2 in CuCl2. This is an oxidation reaction.
The oxidation state of the Cl atom decreases from 0 to –1 in CuCl2. This is a reduction reaction.
(iii) Give the oxidation and reduction half-equations for the reaction between silver and copper(II) chloride.
Reduction half-equation: Cu2+ + e− → Cu+.
Oxidation half-equation: Ag → Ag+ + e−.
(iii) Hence, give the overall ionic equation.
Overall ionic equation: Cu2+ + Ag → Cu+ + Ag+.
(iv) Construct the overall equation for the reaction between silver and copper(II) chloride.
When the exposure of light is stopped, we have redox reactions that are reversed of the following two reactions:
AgCl(s) → Ag(s) + Cl(s);
and
CuCl(s) + Cl(s) → CuCl2(s).
Thus, by reversing the given two equations, we have the following two reactions in sequence:
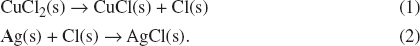
Adding equations (1) and (2), we have:
Ag(s) + Cl(s) + CuCl2(s) → AgCl(s) + CuCl(s) + Cl(s)
i.e.,
Ag(s) + CuCl2(s) → AgCl(s) + CuCl(s).
OR
We can simply add two Cl− ions to each sides of the ionic equation,
Cu2+ + Ag → Cu+ + Ag+,
to get an electrically neutral equation:
Ag(s) + CuCl2(s) → AgCl(s) + CuCl(s).
| Q | Wow! Why are you able to add the two chemical equations together like the simultaneous addition of two mathematical equations? What is the reasoning behind such an addition? |
A: Well, basically, reaction (1) takes place first. The product in reaction (1), i.e. Cl(s), then participates in reaction (2). So, the overall reaction equation, Ag(s) + CuCl2(s) → AgCl(s) + CuCl(s), seems to indicate that it is the actual reaction between Ag(s) and CuCl2(s), which is actually not the case. Therefore, you can add chemical equations like that if one product of an equation is also the reactant of another equation!
(v) Aqueous copper(II) chloride reacts with aqueous sodium hydroxide to form a precipitate. Write the ionic equation, including state symbols, for the precipitation reaction.
Cu2+(aq) + 2OH−(aq) → Cu(OH)2(s).
(vi) What is the name and color of the precipitate?
The blue precipitate is known as copper(II) hydroxide.
(vii) Give the dot-and-cross diagrams for the precipitate and copper(II) chloride.