
· · ·
The Great Lakes . . . darkly reflect how a global tradefest can turn into an ecological makeover. What was once a distinct North American body of water . . . is now little more than a degraded multicultural aquarium.
ANDREW NIKIFORUK, Pandemonium, 2006
THE GREAT LAKES HAVE ALWAYS been attractive to invaders and colonizers: they are relatively young and their waters support less biodiversity than older lakes. And wherever there is room in nature, something is bound to move in. Those organisms that arrived naturally, without any help from us—the Atlantic salmon or the herring gull, to name two—seem to have settled into the Great Lakes ecosystem without causing serious disruptions. Invasion isn’t always a destructive force; it is the way species expand and ecosystems mature and evolve.
Some organisms that have arrived in the Lakes region through human intervention, on the other hand, have caused tremendous changes in the makeup of the region’s biota, not all of them beneficial. Unfortunately, examples abound. Of the 278 species of plants found growing in 1980 on Toronto’s Leslie Street Spit—a 5-kilometer (3-mile) artificial arm protruding into Lake Ontario that has been allowed to grow wild—only 122 were native to southern Ontario. No one knows who brought dandelions over from Europe, or why, since there were two species of dandelions here already. Garlic mustard was sown as cattle feed with seeds imported from Europe in the nineteenth century, as was lamb’s-quarter, a wild variety of spinach. We had to introduce forage plants, such as alfalfa, to feed our domestic animals, because they too had come from Europe and were more accustomed to eating European crops. Dodder, a rootless, leafless, parasitic plant that squeezes nutrients out of host plants with its long, stringlike vines, made the crossing in sacks of alfalfa seeds, which dodder seeds strongly resemble.
So many invasive aquatic plant and animal species have moved into the Great Lakes ecoregion that a 1998 report by the Illinois Natural History Society’s Center for Aquatic Ecology stated that “some biologists argue that it is now a man-made aquaculture system.” Whereas before the settlement period there were 150 native fish species in the Great Lakes, nearly half of those have since declined or vanished, and 162 new, nonindigenous species have taken over their habitat. Most of these new species are here as a result of human action, by either accidental or deliberate introductions, and have thrived so well that the very nature of the Great Lakes aquatic ecosystem has been significantly altered. As Ian Duggan, a biologist with the Great Lakes Institute for Environmental Research, has remarked, “Nonindigenous species pose the leading threat to the biological integrity of the lakes, and may interact with other ecosystem stresses, including climate change and toxic contaminants, potentially compounding their effects.”

Dandelions, an introduced species, grace a meadow interspersed with Indian paintbrush, a colorful native species.
The massive and essentially unsolvable problem of invasive species in the Great Lakes was the focus of a congressional hearing held in Michigan’s Macomb County in the fall of 2005. At the hearing, Emily Fennel, an environmental analyst with Michigan’s Department of Natural Resources, noted that “the economic loss from invasive species in 2004 was $5 billion,” and that “42 percent of native species are threatened by invasive species.” Whereas half a century ago the Lakes provided relatively free food and water to the many communities built on its shores, today federal and local governments spend billions of dollars annually stocking and protecting native fish, controlling introduced species, and cleaning and clearing water intake valves clogged by invasive organisms—all of them introduced by us. If we have succeeded in turning the Great Lakes into a huge aquaculture experiment, it seems the experiment is running out of control.

The highly invasive garlic mustard has been in the Great Lakes basin since settlement days but has only recently begun to take over vast areas of forest meadowland.
A SURFEIT OF LAMPREYS
The first nonindigenous fish species recorded in the Great Lakes has arguably been the most devastating for native fish. The sea lamprey appeared in Lake Ontario in 1835, and its subsequent conquest of all five lakes has been called “a biological legend,” which puts it in the same category as the introduction of rabbits to Australia. The saga of the sea lamprey reads like a template for the many other pernicious species that have since invaded the system, from the alewife to zebra mussels.
Although it looks like an eel—and is sometimes erroneously called the lamprey eel—the sea lamprey differs from the American eel in several ways. Eels have spines, with up to 111 vertebrae; sea lampreys are cartilaginous, like sharks. The dorsal, anal, and caudal fins of eels form a continuous fringe that begins a third of the way along the body and goes completely around the tail and up the lower body almost to the midpoint, while lampreys have two small dorsal fins only. Eels are catadromous—they live in freshwater and spawn in saltwater— whereas lampreys swim up freshwater streams, like salmon, to spawn. And the American eel population has declined drastically in the Great Lakes and St. Lawrence River in the past few decades despite measures to save them, whereas sea lampreys are doing very well despite our best efforts to rid the system of them.

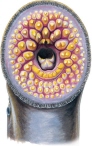
SEA LAMPREY, (above) AND MOUTH CLOSE-UP
The sea lamprey is the most primitive vertebrate known to science. Fossil evidence shows it to have been around for the past 400 million years. It is little more than a leathery, cartilaginous tube and a digestive tract, with a mouth at one end and an anus at the other; it has no jaws and therefore no teeth, but rather rows of sharp, disklike protuberances on its tongue and lips. It breathes through gill pouches arranged along the length of its body, which is covered by a layer of slime. The sea lamprey feeds by attaching its mouth to the side of a fish, lacerating the fish’s flank with its tongue, and then sucking the bodily fluids out of it. An anticoagulant in its saliva keeps the juices flowing freely. Fish have been found in the Great Lakes with eight or ten lamprey scars on their sides and three or four lampreys hanging off them, still sucking. A lake sturgeon was once caught in Lake Michigan with eighteen lampreys attached to it. Such fish are usually thrown back. “They cook dry,” one fisherman commented.
Lampreys live for only about eighteen months as adults, spending most of that time in the shallows of the open lakes. But like salmon they swim up natal streams to spawn. The female makes a shallow nest in the streambed with her mouth, then deposits 30,000 to 50,000 eggs in the depression. The male comes along and fertilizes them, whereupon both male and female adults die. Two weeks later the eggs hatch, and the larvae burrow into the sand or silt, where, as ammocoetes, they remain for the next three to eighteen years, depending on water temperature and food supply, feeding on organic detritus. When they have reached a certain sub-adult size (slightly under 38 centimeters or 15 inches in landlocked lakes, nearly twice that in anadromous populations), having accumulated enough body fat to sustain them until they become adults, they enter a metamorphosis, developing their toothlike oral disks and functional eyes and changing color. Once this phase is complete, they emerge from their natal streambeds and move downstream to open water, where they eventually become predators.
Some believe lampreys have always been in Lake Ontario, as well as in Lake Champlain and the Finger Lakes of upper New York State. Others think they originated in the Atlantic Ocean and entered Lake Ontario via the Erie Canal, which opened in 1825. In any case, the lamprey population remained stable and confined to Lake Ontario for almost a hundred years after 1825, even though the Welland Canal opened to shipping in 1829. They caused severe damage to several Lake Ontario fish populations, including ciscoes, lake trout, and walleye, but did not migrate further upstream into Lake Erie until 1921, two years after the Welland Canal was deepened to allow larger ships into the upper Lakes, and when the supply of sizable prey species in Lake Ontario had been reduced.
In 1936, they were reported in Lake Michigan; the following year they showed up in Lake Huron; and by 1946, they were in Lake Superior. The effect of their presence on large fish is reflected in commercial fishing statistics for lake trout, once the preeminent species in both the commercial and sport fisheries. The total lake trout catch in Lake Huron in 1937 was 1.5 million kilograms (3.4 million pounds); ten years later it was zero. In 1946, the lake trout catch in Lake Michigan was 2.5 million kilograms (5.5 million pounds); in 1953, it was 183 kilograms (402 pounds). By the mid-1950s, the lake trout population of Lakes Michigan-Huron was no longer commercially viable. At that time, the average lake trout catch in Lake Superior was still 2 million kilograms (4.5 million pounds); by 1961, the year the lamprey population peaked in Lake Superior, the total catch was 167 kilograms (368 pounds).

Sea lampreys clamped to the flank and gill of a lake trout. Since their introduction in the 1820s, lampreys have all but extirpated native salmon stocks.
Lake trout, ciscoes, and walleye were hit hard because lampreys feed only on large fish, adults greater than 43 centimeters (17 inches) long. Lake trout reach that size in about four years but do not become sexually mature until they are six or seven years old, which means most are either weakened or killed by lampreys before they are old enough to reproduce. As fish populations plummeted, lamprey numbers soared, increasing twenty- to fiftyfold per generation throughout the 1940s and ’50s. The population peaked in 1961, and the following year commercial fishing of lake trout in Lake Superior was prohibited. By then there were hardly any lake trout anyway, and even the lamprey had turned to other species. Similar declines started showing up in burbot, lake whitefish, suckers, lake herring, and even large chub.
Attempts to control lampreys began in Lake Superior in the 1960s, in order to try to preserve genetic pools of lake trout that could be hatchery-reared and used to reseed populations in the other Great Lakes. Various methods were tried, including electrocution— huge electrical probes inserted into the water at dam sites along freshwater streams known to be spawning beds for lampreys— and the release of neutered males. The most successful control was a chemical larvicide called 3-trifluormethyl-4-nitrophenol (TFM) for short, developed by Vernon Applegate, a U.S. Fish and Wildlife biologist at the Hammond Bay Biological Station in Michigan. Many fish exhibit toxic responses to TFM, as do many mollusk and amphibian species, but most fish have the ability to metabolize TFM in their livers. Lampreys do not. TFM was first sprayed on lamprey spawning streambeds in 1962, and by 1966 the lamprey population had been reduced to 5 percent of its former size.
The number of lampreys declined to such an extent that the Lakes were restocked with lake trout in the hope that they would reclaim their original dominance, and in some lakes the strategy seemed to be working. By the 1990s, the lake trout population was large enough that stocking was no longer necessary; in Lake Michigan, the trout numbers were thought to be exceeding historical levels. Then sea lamprey numbers began to climb again, probably as a delayed reaction to improvements made in the 1970s to the St. Marys River, between Lake Superior and Lake Huron, to make a more hospitable habitat for lake trout and other salmonids: it also made the river more hospitable to lampreys. Today the lamprey population in the upper Lakes is nearly as large as it was in the early 1960s, and there seems no effective way of controlling it. “They duck into little streams and freshets and avoid the chemical blocks,” says Jerome Keen, an analysis technician with the Great Lakes Fisheries Commission in Sault Ste. Marie, Ontario. “We hike up the smaller streams and spray, but it’s hard to get them all, and it only takes a few lampreys to make a lot.” Stocking lake trout in Lake Superior was halted in 1996 because it became simply an exercise in feeding lampreys: between 1995 and 1999, lampreys killed 500 tonnes (550 tons) of Lake Superior lake trout every year. In Lake Huron, only 17 percent of the lake trout population survive each year; 10 percent die naturally, 19 percent are caught by commercial and sport fisheries, and 54 percent are killed by sea lampreys. Apparently, like death and taxes, sea lampreys will always be with us.
SOME INTRODUCTIONS ARE NECESSARY
Smelts are slender, silvery fish that grow from 15 to about 30 centimeters (6 to 12 inches), depending on the species, and were once thought to be salmonids—Linnaeus named the European smelt Salmo eperlanus—but now have their own family, the Osmeridae. The largest, the rainbow smelt, is the anadromous Atlantic variety, introduced into Crystal Lake, near the east coast of Lake Michigan, in 1912 as food for Japanese salmon, which had been introduced earlier. The Japanese salmon disappeared, however, and the smelts escaped into Lake Michigan, where they flourished. For a while they were cursed as a nuisance, because they clogged up gill nets set for larger fish, but eventually they became an important commercial species in their own right, so much so that 1.8 million kilograms (4 million pounds) of them were taken from that lake in 1940. By that time smelts had spread into all five Great Lakes; they seem to have been introduced into Lake Ontario separately, from Cayuga Lake in northern New York.
Smelts spawn in massive numbers, surging like a wall of fish flesh up freshwater streams and into shallow, shoreline waters, much like their marine cousins on the Atlantic Coast, the similarly behaved (but not related) caplin and the (closely related) eulachon on the Pacific Coast. Like caplin, Great Lakes rainbow smelts were literally shoveled into garbage pails by the millions in the 1950s and ’60s. In 1964, the commercial catch of rainbow smelts from Lake Erie alone was 7.3 million kilograms (16 million pounds). But the population fell abruptly in the 1980s, probably because of predation by alewives, and it does not seem to have recovered its former abundance.

The rugged coastline of Lake Superior near Rossport, east of Thunder Bay, Ontario.
The increasing numbers of alewives and smelts in the Lakes led to the introduction of larger, predatorial fish to replace the Atlantic salmon and lake trout that had once kept prey species in check, but which had been nearly extirpated by the dual threats of sea lampreys and overfishing. There was also a perceived need to bolster the commercial and recreational fisheries. The first deliberate introductions were four species of Pacific salmon (of the genus Oncorhynchus, as distinct from the Atlantic salmon genus, Salmo). Although primarily a saltwater family, all salmonids (salmon, trout, char, grayling, and whitefishes) may have originated in freshwater, as suggested by their habit of returning to freshwater streams to spawn. There are no permanently saltwater salmonids, but there are several permanently freshwater species.
The kokanee, for example, introduced into Lake Michigan in 1950 with stock taken from Kootenay Lake, British Columbia, is a smaller, freshwater form of the sockeye salmon, rarely weighing more than 1.4 kilograms (3 pounds). They are now found in Lakes Huron, Michigan, and Superior, where they live on zooplankton and aquatic insect larvae such as those of the mayfly and caddisfly until the age of four. They then spawn, late in the season, in November and December, when ice is forming on the lake, after which, as with all Pacific salmon, the adults die. The young fry emerge from the gravel in April and move down into the “nursery” lake, where they feed on zooplankton and are fed on in turn by larger fish and diving birds.

RAINBOW SMELT

CHINOOK SALMON

COHO SALMON

BROWN TROUT
The sockeye was the first salmon to be introduced to the Lakes, in Lake Ontario in 1873. Chinook are the largest salmon, often weighing more than 13.6 kilograms (30 pounds) at which stage they are called tyee on the West Coast. Steelhead, a coastal strain of rainbow trout, were next, in 1900, and now range widely in Lakes Michigan and Huron and spawn in their tributaries, as well as migrating into tributaries of Lake Superior. In 1956, there was an accidental release of pink salmon into Lake Superior when a shipment of fertilized pink salmon eggs destined for an experimental introduction into Hudson Bay was stored temporarily at a hatchery on the Nipigon River. Somehow, 10,000 eggs were flushed down the hatchery sewer, and within a few years pink salmon were found spawning throughout Lakes Superior and Huron. A large, humpbacked salmon that feeds on alewives, the pink did very well and by 1979 had spread to all five lakes. And in 1966, the state of Michigan released 660,000 coho salmon into Lake Michigan and 192,000 into Lake Superior. Ontario planted 130,000 in Lake Ontario in 1969. Coho spawning runs now take place in many Great Lakes tributaries every eighteen months, when the adults are three or four years old.
The success of these introductions is still a matter of debate. For a long while it was not known whether these alien species were reproducing in Great Lakes waters, but it now seems fairly certain that they are, although stocking programs are still in place to keep population levels high enough to sustain a sport fishery. Since the 1960s, annual restockings have increased almost yearly. Between 1966 and 1998, 745 million hatchery-raised salmon were released into the Great Lakes. Ontario still releases 3 million exotic salmonids into Lake Ontario every year.
The brown trout is an Atlantic river species introduced into Michigan rivers in 1863 and Ontario in 1913, specifically for sport fishers. Anglers initially objected to these plantings because they thought the fish was too difficult to catch and because brown trout carry furunculosis, a disease of brook trout. But brown trout have endured in the Lakes and are now a popular species. They spawn in late autumn, using the same spawning headwaters as brook trout but later in the season. The female creates a shallow depression, or redd, in the streambed, deposits her eggs, then covers them with gravel. The adults grow to 18 kilograms (40 pounds) in their native marine environments, but only to about 6.4 kilograms (14 pounds) in freshwater lakes, down to 900 grams (2 pounds) in inland streams.
There is no doubt that such massive releases of Pacific salmonids and Atlantic trout compete with remnant populations of lake and brook trout for food and habitats, not to mention that Pacific salmonids take heavy tolls on the young of such native species as slimy sculpins, bloaters, and yellow perch. In salmon spawning streams, the larger and more aggressive Pacific species usually take over the most promising nest sites. They have been blamed for the failure of Atlantic salmon reintroduction programs in Lake Ontario, and for declines in the alewife population, the mainstay of the Lake Michigan fishery. But it is also true that their presence in the Lakes has been a boon to commercial fisheries as well as to sport fishing and tourism, upon which many formerly prosperous fishing communities have come to rely.
SOME INTRODUCTIONS ARE NOT
Other exotic species have no such benefits to recommend them. The carp was an early instance. Native to the temperate zones of Asia and Europe (the name comes from the Latin carpum, referring to a fish of the Danube), it was brought to England in the fifteenth century as an ornamental pond fish. It evidently found its way into the wild, since Izaak Walton, in The Compleat Angler (1653), refers to it as “the Queen of Rivers; a stately, good and very subtle fish.” It was brought to North America in the early 1800s by Captain Henry Robinson, of Newburgh, New York, who brought six or seven dozen from France in 1832, reared them in his pond, and then released them into the Hudson River, eight years after the completion of the Erie Canal. So there may have been carp in Lake Ontario as early as 1833, although it was not until 1880, when carp were brought into Ontario as a food fish by Messrs. S. and B.F. Reesor, of Markham (no doubt to sell to the growing Asian population in the province), near Toronto, that the carp was officially recorded this far north. In 1888, carp were introduced into Sandusky Bay, on Lake Erie, and in 1892, the Ontario Fish and Game Commission reported carp in the Grand River, which runs into Lake Ontario. The next year, 286,000 kilograms (631,000 pounds) of carp were caught in Lake Erie; in 1908 the harvest was 4 million kilograms (9 million pounds), and by then carp were common in every Great Lake except Superior.
The large, brownish, heavily scaled carp is a hearty fish and a prolific breeder. A 76-centimeter (30-inch) female can release more than 2 million eggs. They spawn in June and July in warm, shallow water, when their dorsal fins can sometimes be seen protruding, sharklike, above the surface of wetlands; the eggs adhere to submerged weeds and grasses and hatch three to six days later. The young grow to 23 centimeters (9 inches) in their first year and begin to spawn in their second year. They can survive with very little oxygen, and so they do well in heavily eutrophied lakes such as Lake Erie where native species, among them yellow perch and small-mouth bass, have difficulty breathing. But they are detrimental to many species because they stir up bottom mud when they feed on aquatic roots in shallow bays and wetlands, disturbing feeding and spawning sites and killing plants that are the main food supply of other fish and diving birds.
Although carp has some value as a food fish, especially among immigrants from Asia, its introduction into the Great Lakes is not regarded as a boon. Frederick H. Wooding, in his Book of Canadian Fishes (1959), observed, “Although quantities are marketed each year by commercial fishermen in live, iced or smoked forms, they are not generally popular.” This may be a good thing, since carp, with their high fat content and bottom-feeding ways, accumulate toxic pollutants at a great rate. A 1995 study of fish from five of the most polluted areas in the Great Lakes basin found that “the greatest carcinogenic risk for each exposure scenario resulted from consuming carp as opposed to pelagic species such as walleye or chinook salmon.” People eating carp from the Saginaw River, on the western side of Lake Huron, perhaps the most contaminated site on the Lakes, ran a 1-in-10,000 risk of developing cancer, compared with a 1-in-100,000 risk if they ate walleye. Carp from other, less contaminated areas, however, have been deemed safe for human consumption.

COMMON CARP
HITCHHIKERS FROM THE BA LTIC SEA
Following the appearance of sea lampreys, alewives, carp, and Pacific salmonids in the Great Lakes, the incidence of both accidental and deliberate introductions leveled off. The first fifty years of the twentieth century saw few new species; the white perch, which entered the Lakes via the Erie Canal in 1954 and is now an abundant food fish in Lake Ontario’s Bay of Quinte, is probably the most significant. With the completion of the St. Lawrence Seaway in 1959, however, allowing more and bigger saltwater vessels into the Lakes, the rate of introductions jumped significantly, from fewer than one per decade to almost one per year. In the past fifteen years, the rate of introductions has again shot up: in 2001 alone, fifteen new species were discovered to have invaded the Lakes.
There have been several vectors. Private aquariums, with their imported freshwater species, have been a major source. Approximately 10 percent of American households have aquariums and ornamental fish, and many of those—along with the snails, plants, and parasites that accompany them—end up in the Lakes, either through sewage systems or by direct dumping. Some of them do well. Goldfish, members of the carp family native to China, are now abundant in the lower Lakes. They spawn in warm, shallow waters in May, and the young are born sixty-five to seventy-six hours later, depending on the water temperature. Wild goldfish are greenish-brown, not gold, and are often mistaken for minnows and used as baitfish. Lesser-known organisms that are now common in the Lakes region and have come from private aquariums include aquatic plants such as the European frog-bit, the European water chestnut, and the Eurasian water milfoil, as well as the big-eared radix mollusk and the blue-spotted sunfish.
Most aquarium releases have not been detrimental to the Lakes ecosystem, but there is one—also a carp species—that could pose a major threat if it becomes established. A single specimen of the northern snakehead was netted by a fisher in Chicago’s Burnham Harbor in 2004. Nicknamed the Frankenfish, it can grow up to 92 centimeters (3 feet) long, has a mouthful of long, needlelike teeth, is an aggressive and impatient feeder on smaller fish, and can live up to three days out of water. Importation of snakeheads or their eggs into the United States is illegal, but breeding populations have nonetheless been found in Florida, in Virginia’s Potomac River, and in a tributary of the Delaware. When one was caught in a pond in Maryland, wildlife officials poisoned the pond to get rid of any others that might have lurked there. The Burnham Harbor specimen was probably an aquarium release, but electric-shock barriers have been placed across the Chicago Ship Canal in case any others decide to swim up from the Mississippi system.
A far more significant vector than aquarium releases, fishers’ bait pails, or restaurant fish tanks has been the ballast water of ships entering the Great Lakes from various European and Asian seaports. Ships charge their ballast tanks with water from, say, the Baltic Sea, then discharge them after entering the Great Lakes in preparation for taking on cargo. Even ships with empty ballast tanks (called NOBOBs, meaning “no ballast on board”) often clean out their tanks in Great Lakes ports, and microscopic invertebrates, protozoans, and aquatic macrophytes left over from previous trips thus enter the harbors. At the same time mollusks, algae, and crustaceans can easily adhere to ships’ hulls and anchor chains and be dropped or scraped off in inland waters. Thus the propagules, or seed colonists, of marine organisms from entirely different ecosystems are systematically and repeatedly injected into the Great Lakes. That’s how zebra mussels arrived.
THE DREADED DREISSENIDS
The first sighting of a zebra mussel was in Lake St. Clair in June 1985, a date that marks another tipping point in the natural history of the Great Lakes.
North America is the cradle of the world’s freshwater mussel population; the upper Mississippi basin and the rivers draining into it are probably where freshwater mussels originally evolved from marine oysters and clams. There are, or were, 297 species of them, one of the richest faunal diversities for an area that size in the world. The Great Lakes hosted 23 species, covering a wide range of shell shapes and sizes, from the larger pigtoes and threeridges to the delicate ladyfingers and the smaller lilliputs and tricorns.
Mussels are members of the Mollusca, a huge phylum that includes n early 5 0,000 m arine, f reshwater, a nd t errestrial s pe-cies— snails and slugs, limpets, whelks, clams, oysters, and even octopuses (in which the mollusk foot has been modified into arms).
Mussels, clams, and oysters belong to the class Bivalvia: soft-bodied organisms consisting of little more than a digestive system, a heart, a foot, and two hinged shells. The reduced head is a mere knot of ganglia that tell the muscular hinge when to shut the shell. North American mussels are of the family Unionidae. Unionid mussels lie half-buried on river or lake bottoms with their shells open, siphoning water over their mucus-covered gills. The gills filter out the algae, bacteria, and other organic matter suspended in the water column and propel the cleared water back into the environment. It is largely thanks to the natural filtration system of unionid mussels that, before European settlement, the Great Lakes and their tributaries were literally crystalline. Each individual mussel can filter up to 38 liters (10 gallons) of water a day, and there were millions upon millions of mussels. Wisconsin’s Lake Pepin was famous for its diverse mussel population; in the 1880s, its bottom was covered by an average of 50 large mussels per square meter (5 per square foot).

Zebra mussels filter phytoplankton, the food of such deepwater amphipods as Diporeia, from the water: before 1990, Diporeia formed 40 percent of the Lakes’ benthic biomass, but they have now all but disappeared.
The shells are composed of calcium carbonate and consist of a hard, outer crust called the periostracum, and a smooth, shiny, pinkish inner surface, the nacre or mother-of-pearl. Males push themselves through the sand or sediment with their single foot, up to 3 meters (10 feet) a day, looking for females. When they are near enough to one, they exude sperm into the water, and the females siphon it onto their eggs, which they have already deposited onto their gills. The eggs hatch and the larvae, or glochidia, are ejected into the water. This is the unionid’s parasitic phase, when its larvae encyst on the gill filaments of small fish (most mussel species parasitize a specific host fish), where they feed for one to twenty-five weeks before dropping onto the river or lake bottom to spend the next eight to ten years becoming sexually mature adults. Some freshwater mollusk species never grow much larger than a finger- nail and are eaten, despite their shelly exteriors, by small fish with big teeth. Others live for sixty or even a hundred years, growing to 31 centimeters (12 inches) or more and as much as 1.8 kilograms (4 pounds); these are eaten by gulls, shorebirds, raccoons, and humans.
People of the Woodland culture ate a lot of mussels and wore the pearls they found in the shells as ornamental and ritual jewelry. Louis Jolliet, the first European to see the Mississippi, recorded in 1672 that native people on that river were wearing pearl ear pendants and necklaces, and burial mounds in Ohio have been found to hold thousands of bored pearls that were evidently of great religious significance, not unlike the turquoise of the Hopi. But it was another two hundred years before a shoemaker in Paterson, New Jersey, bit into his dinner of mussels from nearby Notch Brook and found a pearl that, had he not cracked it with his teeth, would have been worth a small fortune. The Great Pearl Rush was on. Over the next two years, from 1857 to 1859, Notch Creek yielded $115,000 worth of pearls, some of them perfectly round and 2.5 centimeters (1 inch) in diameter. The frenzy soon spread north to the Great Lakes, into Ohio and Wisconsin, where the Pecatonia, Sugar, Apple, and Wisconsin rivers regularly swarmed with pearl harvesters.
The introduction of cultured pearls from Japan in the early 1900s put an end to the freshwater pearl industry, but not to mussel harvesting. In 1890, an American tariff on imported mother-of-pearl drove the price of buttons up so high—to $60 per 100 pounds (45 kilograms) of oyster shell—that a German button cutter who had moved to Muscatine, Iowa, began using freshwater mussel shells, which gave new life to the declining mussel fishery. By 1900, there were two hundred button factories in the northern United States, and supplying them with mussel shells was a huge industry, employing thousands of full-time and weekend harvesters on nearly every river, lake, pond, and wetland in the upper Mississippi and Great Lakes basins. In 1899, mussels were the most valuable fishery in Wisconsin; more than 7.3 million kilograms (16 million pounds) were harvested in that state alone, selling for 60 cents per 100 pounds (45 kilograms). Shell wasn’t as valuable as pearl, but there was a lot more of it.
So many mussels were taken that species began to disappear: the older, larger specimens were the best for buttons, as they had been for pearls, and since mussels don’t begin to reproduce until they are old and large, most of the sexually mature mussels were soon gone and the harvest switched to younger, smaller individuals. More recently pollution has also taken its toll. Today, of the almost 300 native freshwater mussel species that existed in North America 150 years ago, 30 have become extinct, 57 are endangered, and 70 have been designated “species of concern.” In Michigan, it is now illegal to possess a live unionid mussel or even a unionid shell.
It was into this underutilized unionid niche that the zebra mussel was introduced in 1985. Zebra mussels are native to the Caspian Sea but probably arrived in the Great Lakes aboard a ship from the Baltic Sea. The waters of the Ponto-Caspian basin—a three-lake system in eastern Asia that comprises the Caspian, Azov, and Black seas—are unlike those of the Great Lakes: they are high in salt content and low in oxygen in their deeper parts, having undergone rapid rates of eutrophication in the latter half of the twentieth century. Over their geological history, they have been subjected to a series of faunal invasions from the Mediterranean Sea, and many of their fish and mollusk species are therefore able to thrive in both saltwater and freshwater. When the Dnieper-Bug Canal linked the drainage basins of the Ponto-Caspian system and the Baltic Sea in the 1850s, many species, including zebra mussels, had no difficulty colonizing the latter and quickly spread into northern European rivers, lakes, and seaports. And the Baltic Sea is very much like the Great Lakes: northern, recently formed by glacial activity, oligotrophic, low in native species diversity, and with ready access to the North Atlantic. Most of the ships entering the Great Lakes come from northern European ports, and since 1959, when the St. Lawrence Seaway opened, 72 percent of the nonindigenous species introduced into the Great Lakes have been transported here in ballast tanks.
Like unionids, zebra mussels are filter-feeding bivalves; they have small (1.25-centimeter or 0.5-inch), razor-sharp shells with dark, zigzag markings, and prefer to adhere to hard substrates such as rocks, concrete piers, ships’ hulls, intake pipes, and other mus- sels, although they can also colonize sandy or muddy substrates. Their evolutionary survival strategy is rapid reproduction: the young reach sexual maturity in their first year and are capable of producing voluminous offspring. They fuel this rapid growth by having a high filtration rate, approximately ten times that of unionid mussels relative to their body size, which allows them to ingest up to 40 percent of their body carbon per day, mostly in the form of small units of phytoplankton. As a result, zebra mussels spread rapidly from Lake St. Clair; by 1986, they were in the Detroit River, and within five years they were in both Lakes Erie and Ontario. Their numbers are almost incalculable. In 1989, zebra mussels were clogging municipal water intake facilities around Lake Erie, having reached densities of more than 750,000 mussels per square meter (70,000 per square foot) of lake bottom.

Zebra mussels encrust a current meter in Lake Michigan.
Zebra mussels found a near-empty habitat and promptly began filling it, attaching themselves to rocks, cement, posts, and pipes, anywhere there was a bit of current. Because they are such efficient filterers and exist in such cosmic numbers, they have contributed greatly to clarifying the water in the lower Great Lakes. But there have been serious drawbacks, quite apart from clogged intake valves. Clearer water has meant increased light penetration, which in turn has led to greater and deeper algal production. The mussels feed only on certain types of green algae, which is what other aquatic organisms, including unionid mussels, amphipods, insect larvae, and small fish, also eat. This allows other phytoplankton species, such as blue-green algae, which is inedible, to proliferate. Thus while they themselves are well fed, they create a phytoplankton vacuum that is quickly filled by algae that other organisms cannot consume. As a result, Great Lakes basin residents who take their drinking water from lakes—even isolated, inland lakes—have noticed a distinct swampy taste, because their intake facilities are now exposed to sunlight and filled with blue-green algae.

Like many ducks, this female common eider feeds on mussels—including zebra mussels—and may be helping to keep the invasive population in partial check.
Within six years of the zebra mussel invasion, there were no live unionid mussels left in Lake St. Clair, and the Detroit River had undergone an 80 percent decline in unionid numbers and a 47 percent decline in unionid species. Other animals that eat phytoplankton were also adversely affected. By 1997 in Lake Ontario, the native deepwater amphipod Diporeia hoyi—the major food item of slimy sculpins, juvenile lake trout, bloaters, and whitefish, and a species tracked as a sentinel for lake-bottom ecosystem health— had declined from 2 ,100 individuals per square meter to 76 (from 1,756 per square yard to 64), with a consequent decline in those dependent fish populations. Diporeia had been starved out of its own pantry.
Possibly the only native species to benefit from the introduction of zebra mussels are eiders. The common eider male is a handsome sea duck, black at the waterline with a white back and neck and a black cap, that breeds in the Far North but migrates south during the winter. The closely related king eider is a more familiar visitor to the Lakes; it is similar to the common eider but slightly smaller, with the male showing less white on its back and a large orange patch above its bill. Both were once popular among the down-filled parka, pillow, and mattress set; in the Arctic, eiders nest in vast colonies and line their nests with their own down. Farther south, they feed in warm, shallow bays on mollusks and other bottom life. There is also some evidence that greater and lesser scaup are feeding on zebra mussels in the Great Lakes during migration, and some concern that they are therefore bioaccumulating toxins as a result. Even if eiders and scaup were to become as common on the Lakes as cormorants, however, it is unlikely they would make a serious dent in the zebra mussel infestation. Ironically, it has been two subsequent invaders, both also from the Ponto-Caspian basin, that have kept the zebra mussel in partial check.
The first is another dreissenid mussel. The quagga mussel is native to the Black Sea but has spread to North America the same way zebra mussels did, via ships arriving from the Baltic Sea, and especially in this case from the Dnieper River. They first appeared in the St. Clair River in 1989 and immediately began ousting zebra mussels from the deeper parts of Lakes St. Clair, Erie, and Ontario. Because the Black Sea is colder than the Caspian, quagga mussels are more cold-tolerant than zebra mussels, functioning well in water that is less than 4˚C (39˚F), although they also prosper in warmer, shallow water. In Lake Erie they are found in water up to 23˚C (73˚F). They prefer softer substrates, such as sand, silt, and mud, and can exist where there is limited food.
The pattern of dreissenid invasion seems to be that zebras move in first, dominate the shallows, especially those with rocky bottoms, and spread down to a depth of about 65 meters (213 feet). There, where the water is colder and the food supply becomes limited, the quagga mussels begin. There is some mixing of zebras and quag- gas between 65 and 85 meters (213 and 279 feet), but deeper than that it is all quaggas. In the Black and Baltic seas, quaggas are rarely found deeper than 100 meters (328 feet), but in Lake Ontario they dominate to 130 meters (426 feet) and flourish in the coldest parts of the lake.

The lesser scaup dives to shallow lake bottoms to feed on young mussels.
Another problem with an overabundance of filtering dreissenid mussels is that they concentrate chemical pollutants at the bottom of the water column. As they filter-feed on polluted phytoplankton, they defecate PCB-laden matter into the sediments, where the toxins build up in the food of benthic organisms, such as worms. High concentrations of PCBs, however, remain in the mussels’ body fat. Zebra mussels analyzed in 2000 were found to contain 100 parts per billion (PPB) of PCBs; small fish that ate the mussels contained up to four times that level, and larger fish such as smallmouth bass, which feed on small fish, had 1800 ppb of PCBs in their flesh. Far from blocking toxins out of the benthic environment, zebra and quagga mussels simply concentrate them in the sediments and then transfer them back into the food chain.
The only fish that feeds on zebra mussels is another nonindigenous invader from the Ponto-Caspian basin. In 1990, the round goby was discovered in the St. Clair River near Detroit, having evidently been transported there in the ballast tanks of a transoceanic freighter. A small, aggressive, deepwater fish, the round goby grows up to 25 centimeters (10 inches) in length but is typically less than 12 centimeters (5 inches). Gobies look like sculpins, except for a black spot on their anterior dorsal fins, and their round, bullish heads and protruding eyes make them look pugnacious, which they are.
The round goby is the very model of a conquering invader: tough, resilient, and a generalist par excellence. It can live in freshwater or saltwater, can tolerate low oxygen and high pollution, exists in a wide range of temperatures, from -1˚C to 33˚C (30˚F to 91˚F ), is found where the substrate is either sandy or rocky (although it prefers rocky or cobbled lake or stream beds), and enjoys a diverse diet, from young zebra mussels to zooplankton or the eggs of other fish species and even the fry of larger fish, such as smallmouth bass and perch. It has an advanced lateral line system—hair cells along its flanks that pick up anomalies in the water flow—that allows it to detect predators and prey in the dark, and its fused pelvic fin forms a quasi sucker that gives it a grip on rocks and substrates where the waters are choppy. The round goby uses these gifts to aggressive advantage, chasing other small fish away from food, nesting sites, and eggs. It also hides from potential predators, such as smallmouth bass, in the spaces between rocks and cobbles on the lakebed.

The female brown-headed cowbird lays her eggs in the nests of other birds, often warblers, who diligently raise her chicks at the expense of their own.
As a result, the round goby has spread rapidly into all five Great Lakes and now exists in vast numbers. There is evidence that it has entirely taken over habitat formerly occupied by such native species as the mottled sculpin and the logperch. A 2002 study determined that there were more than 10 billion round gobies in the western end of Lake Erie alone; some researchers estimate that they now constitute as much as 50 percent of the entire fish biomass of the Lower Great Lakes.

ROUND GOBY

SMALLMOUTH BASS
Just as alien plant species have drastically altered the composition of the visible landscape, so too have invading nonindigenous aquatic organisms almost completely taken over the less visible realm beneath the surface of the Great Lakes themselves. Whether this change is permanent has yet to be determined; the invasions have been recent, and it takes many generations for adaptation and colonization to take hold. But there is no doubt that many native species are in a bust cycle, and many nonindigenous species are booming. To a casual observer, a typical Great Lakes landscape— acres of rolling greenery reflected in the deep blue waters of Georgian Bay, for example—might appear much the same now as it did when Étienne Brûlé and Samuel de Champlain first skimmed down the French River in search of the Orient. But a closer look would reveal that nearly half the faces in the frame weren’t in the picture four hundred or even fifty years ago.
BOLD AS BRASSICA
Garlic mustard, a European wild brassica brought over by settlers as a potherb, has suddenly become an aggressive alien. Content as an edge plant for two centuries, growing abundantly but confined to the transition zone between forest and meadow, it has recently begun invading the deep woods in the Great Lakes region, where its tenaciousness and lack of natural enemies allow it to outcompete native flora. Recent research has found that its roots produce a toxic substance that kills the fungi that form underground symbiotic relationships with other plants. It is particularly damaging to trilliums and maple seedlings: its roots can kill maple seedlings two years after the garlic mustard itself has been eradicated. Stands of garlic mustard should be cut before the plants begin to form seeds, that is, in mid-May in Ontario and mid-June in Wisconsin.
A NEW TWIST
Of the 185 nonindigenous species known to have invaded the Great Lakes in the past two hundred years, the most recent is also the smallest. The virus that causes viral hemorrhagic septicemia (VHS), a disease commonly found in the Baltic Sea and the North Pacific, turned up in the Bay of Quinte on the north shore of Lake Ontario in 2005, but may have been in Lake St. Clair since 2003.
So far, VHS has killed tens of thousands of fish in Lakes Ontario, Erie, and St. Clair, affecting twelve species found in the Great Lakes, including muskellunge, northern pike, walleye, yellow perch, and rainbow trout. The disease destroys the linings of blood vessels, causing internal bleeding and liver failure; fish with VHS turn dark and have protruding eyes and pale gills. Those with the nervous form of the virus develop twisted bodies and tend to swim in circles. The virus is transmitted through bodily fluids and enters the fish’s internal organs through the gills or wounds. It is not harmful to humans, but people have been advised not to eat infected fish.
Scientists don’t know how the virus got into the Great Lakes, but there are two prime suspects: if it originated in Lake St. Clair, it probably arrived in the ballast tanks of a freighter that had recently sailed in the Baltic Sea; if in the Bay of Quinte, then the culprit may have been a local trout farm, since VHS is a common disease in hatchery-raised rainbow trout in Japan and in turbot farms on the Pacific Coast.
THE FIRST SPIKE
Purple loosestrife (Lythrum salicaria), a tall, perennial, magenta-spiked bog plant that is now choking native life out of our wetlands, was one of the earliest plant invaders in North America. It was first introduced in the early 1800s, either accidentally in sheep’s wool or deliberately, as a garden herb. Native to Eurasia, it is the Lysima-chia referred to by Pliny and was highly valued as a medicinal plant by the first-century herbalist Dioscorides, who describe it as “tart and astringent . . . good for stanching both inward and outward bleeding.” Its dried roots and leaves were made into a concoction to treat dysentery and diarrhea into the nineteenth century.
It is, however, highly invasive. Once arrived on the eastern seaboard, it quickly spread along canals and disturbed marshlands in the Great Lakes basin. Since the 1930s, it has invaded most shallow-water marshes, and now it is poised to invade deeper wetlands. With up to fifty 2-meter (6.5-foot) stems growing from a single rootstalk, purple loosestrife soon replaces native grasses, sedges, flowering plants, and bulrushes while providing no benefits to wildlife.
Some florists still sell purple loosestrife in what they call “guaranteed sterile” cultivars, but these are easily capable of hybridizing with the wild version as well as with any other of the thirteen Lythrum species in North America, such as thymeleaf loosestrife (L. thymofolia) and hyssop loosestrife (L. hyssopifolia). The sale of any purple loosestrife has been prohibited in Ontario, Wisconsin, Minnesota, and Illinois.

Purple loosestrife, an alien relative of the native swamp loosestrife, is known as Red-Sally in England.

A common loon and her chick. Loons build floating nests on “nursery pools,” where their fledged young are taught to dive for small fish.